Abstract
Vertebrate kidney tissue exhibits variable morphology that in general increases in complexity when moving from anterior to posterior along the body axis. The nephric duct, a simple unbranched epithelial tube, is derived in the avian embryo from a rudiment located in the anterior intermediate mesoderm (IM) adjacent to somites 8 to 10. Using quail-chick chimeric embryos, the current study finds that competence to form nephric duct is fixed when IM precursor cells are still located in the primitive streak, significantly before the onset of duct differentiation. In the primitive streak, expression of the gene HoxB4 is associated with prospective duct IM, whereas expression of the more posterior Hox gene HoxA6 is associated with more posterior, non-duct-forming IM. Misexpression of HoxA6, but not of HoxB4, in prospective duct-forming regions of the IM resulted in repression of duct formation, suggesting a mechanism for the restriction of duct formation to the anterior-most IM. The results are discussed with respect to their implications for anterior-posterior patterning of kidney tissue and of mesoderm in general, and for the loss of duct-forming ability in more posterior regions of the IM that has occurred during vertebrate evolution.
Keywords: Cell type specification, Chick embryo, Hox genes, Kidney, Mesoderm patterning, Nephric duct
INTRODUCTION
The kidney is the primary organ responsible for maintaining the internal body environment in vertebrates. Up to three separate kidneys are formed sequentially during embryonic development (see Fig. 1G). The first to form and the most anterior is the pronephros, which is the functional embryonic kidney in most fish and amphibians, and a transient embryonic structure in amniotes. Next to form and in a more posterior location is the mesonephros, the adult kidney in anamniotes and the main embryonic/fetal kidney in amniotes. The metanephros, which is the last kidney to form and the most posterior, is formed only in amniote organisms, where it serves as the definitive adult kidney (Saxen, 1987).
Fig. 1.
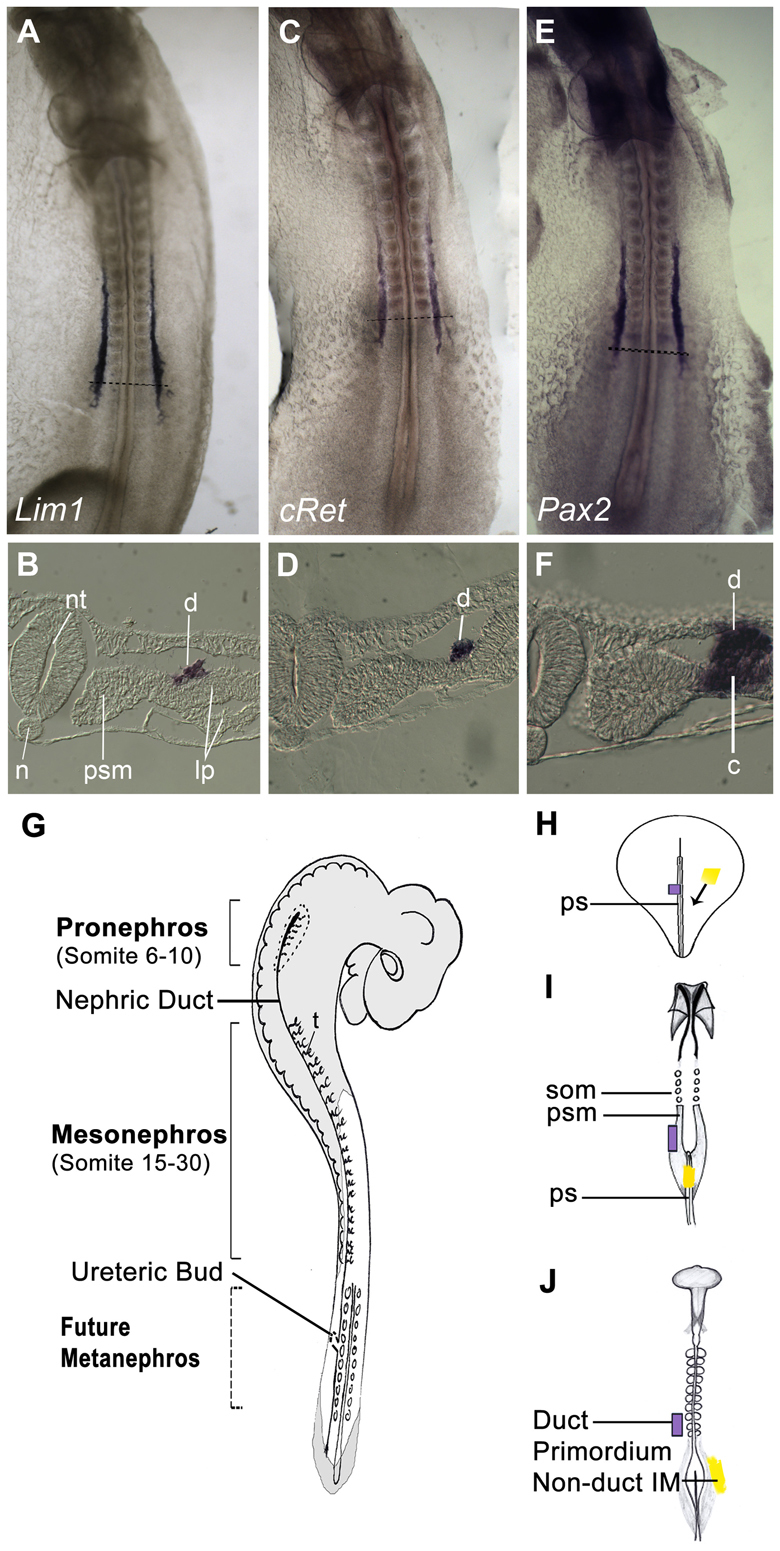
Nephric duct markers. (A-F) Stage 11 chick embryos stained by whole-mount in situ hybridization for Lim1 (A,B), cRet (C,D) and Pax2 (E,F). B,D,F are sections of A,C,E taken at the indicated axial levels. Note that Lim1 and cRet mark the nephric duct specifically, whereas Pax2 is expressed in both the nephric duct and nephrogenic cord. (G) Diagram of chick embryo indicating locations of components of the nephric system. (H-J) Summary of fate map of the intermediate mesoderm (IM) at stages 5 (H), 8 (I) and 10 (J). Anterior (future head) is upwards. IM migrates through the mid-portion of the primitive streak. Tissue that gastrulates earlier (blue) ends up in more anterior regions of the IM, including the nephric duct, whereas tissue that gastrulates later (yellow) ends up in more posterior, non-duct regions of the IM. c, nephrogenic cord; d, nephric duct; IM, intermediate mesoderm; lp, lateral plate; n, notochord; nt, neural tube; ps, primitive streak; psm, presomitic mesoderm; som, somite; t, tubule.
All three kidneys are built from basic units called nephrons. Each nephron contains a glomerulus, which filters the blood; a tubule, which processes the glomerular filtrate; and a collecting system, which drains the tubular products to the outside. According to their different functional requirements, kidneys differ significantly in the number and arrangement of their nephrons. Anterior kidney tissue tends to have a simple arrangement, with only several tubules connected to an unbranched collecting system. More posterior kidney tissue, which emerged later during vertebrate evolution, is more complex, with large numbers of nephrons connecting in an intricate pattern to a collecting system that is highly branched (Saxen, 1987; Schultheiss, 2007; Vize et al., 2003).
Kidney tissue is derived from the intermediate mesoderm (IM), a strip of mesoderm that lies just lateral to the somites. While the glomeruli and tubules of each kidney type are formed from IM at the axial level where that kidney will form, the collecting system of all the kidneys is derived from the nephric duct, which originates exclusively from the IM of the pronephric region (Schultheiss et al., 2003). In chick embryos, a nephric duct rudiment forms from IM in the pronephric region at the axial level of somites 8 to 10 and subsequently extends posteriorly until it empties into the cloaca (Obara-Ishihara et al., 1999; Schultheiss et al., 2003). As the duct passes through the prospective mesonephric and metanephric regions, mutual inductive interactions between the duct and the IM lead to the differentiation of tubules and glomeruli from the IM and to the formation of a collecting system from the duct (Grobstein, 1955; Gruenwald, 1943; Waddington, 1938). An exclusively pronephric source for the nephric duct and its derivatives has been documented for several species, including chick (Obara-Ishihara et al., 1999) and Xenopus (Lynch and Fraser, 1990), although there is some uncertainty as to whether in some species there may also be contributions from more posterior regions of the IM (Serluca and Fishman, 2001).
The nephric system, with its formation of different types of kidney tissue along the anterior-posterior (AP) axis, is a potentially useful model for studying AP patterning of the mesoderm. However, in practice this has proved difficult to carry out, as the differences between kidney types are primarily those of quantity and arrangement of nephrons, and it has been difficult to identify molecular markers that cleanly distinguish between specific kidney types. One study has documented several molecular differences between the mesonephric and metanephric tubules (Mugford et al., 2008), although most molecular markers are co-expressed in both the mesonephros and metanephros. The nephric duct may prove to be a more tractable model for studying A-P patterning of the IM, as it is a unique product of the pronephric IM and expresses several distinctive molecular markers, including the transcription factors Lim1 (Taira et al., 1994) and Sim1 (Ema et al., 1996; Fan et al., 1996; Obara-Ishihara et al., 1999) the receptor c-Ret (Pachnis et al., 1993), as well as the antigen recognized by the antibody 4A6 (Brennan et al., 1998). However, knowledge of the factors that regulate initial formation of the nephric duct is still quite incomplete (Schultheiss et al., 2003).
Hox genes are well-documented for their involvement in anterior-posterior patterning of the embryo (Graham et al., 1989; Kessel and Gruss, 1990; Krumlauf, 1994). Hox genes are arranged in four clusters in the vertebrate genome and are expressed in a nested pattern in which the more 5′ members of each cluster have anterior borders of expression that are in general located at successively more posterior axial levels. In the mesoderm, most attention has been focused on the role of Hox genes in formation of skeletal elements of the axial skeleton and the limbs. Alterations in Hox gene expression can lead to changes in vertebrate and limb morphology, often in accordance with a rule of ‘posterior prevalence’ in which morphology is strongly influenced by the identity of the most ‘posterior’ (i.e. 5′) Hox gene expressed in that tissue (Carapuço et al., 2005; Kessel et al., 1990; Wellik, 2009). Evidence is accumulating that proper Hox gene expression is also required for normal development of IM derivatives (Patterson and Potter, 2003; Wellik, 2011; Wellik et al., 2002), and misexpression of Hox genes has been reported to affect expression of region-specific IM markers (Mugford et al., 2008; Preger-Ben Noon et al., 2009).
In the current study, we investigated nephric duct formation in the avian embryo. Through a series of quail-chick transplantation studies we found that specification of the nephric duct occurs already during gastrulation, while the duct precursors still reside in the primitive streak, and that duct formation does not require a unique ‘duct-inducing environment’ associated with the place of its normal differentiation at the somite 8-10 axial level. We further found that the gene HoxB4 is expressed in nephric duct precursors from the primitive streak stage onwards, whereas the more posterior Hox gene HoxA6 is expressed in non-duct IM. HoxA6 was found to specifically repress duct formation when misexpressed in duct-forming regions of the IM. These results are discussed with respect to their implications for the regulation of IM and general mesodermal patterning along the AP axis, and for the evolution of distinct types of kidney tissue.
MATERIALS AND METHODS
Transplantation
Tissues were excised from donor quail embryos, labeled with Cell tracker DiI (Molecular Probes) and washed with Tyrode's saline. A similar-sized tissue was removed from recipient chick embryos and donor pieces were placed into the vacant location in the host using tungsten needles. Embryos were cultured in modified New culture as previously described (James and Schultheiss, 2003), fixed in 4% paraformaldehyde for 30 minutes and stored in PBS until analysis.
Immunofluorescence
Fixed embryos were embedded in 7.5% gelatin/15% sucrose/PBS and cryosectioned at 10 μm intervals. After permeabilization in 0.25% Triton X-100/PBS for 15 minutes and blocking (1% BSA, 1% goat serum, 1% horse serum and 0.02% Tween 20 in PBS) for 15 minutes, sections were incubated in the following primary antibodies: mouse anti-quail cell (1:250) (Developmental Studies Hybridoma Bank), mouse anti-Lim1/2 (DSHB), rabbit anti-Pax2 (1:250) (Covance) and rabbit anti-Gfp (1:1000) (Molecular Probes). Alexa Fluor 488, Cy3 and DyLight 649 nm secondary antibodies (Jackson ImmunoResearch) were used at a 1:250 dilution in blocking solution. DAPI at 1 μg/ml was used to visualize nuclei. Images were collected on a Zeiss Axioimager microscope with a Qimaging ExiBlue digital camera and Image Pro plus imaging software.
Direct labeling of QCPN monoclonal antibody
QCPN hybridoma (DSHB) was grown in DCCM2 Serum Free Medium (Biological Industries), and IgG was isolated from the culture medium using a Protein G column (Amersham) on a Profinia chromatography system (BioRad). Purified QCPN-IgG was directly labeled using a Dylight 649 Antibody Labeling Kit (Pierce). After labeling, immunofluorescence was carried out as described above with the following adjustments: first the unlabeled antibody (Lim1) and the appropriate secondary antibody were applied. After the secondary antibody, sections were soaked in 5% mouse serum, 0.02% Tween 20 in PBS for 1 hour in order to block any remaining mouse IgG-binding sites on the secondary antibody. Sections were then incubated with the labeled QCPN antibody at a 1:1000 dilution in mouse blocking solution overnight at 4oC.
Expression plasmids
Chicken full-length HoxA6 cDNA was obtained from ARK genomics (Pubmed Clone AY307380), amplified using PCR (primers 5′-ggggagagcaaatgagttcc-3′, 5′-cggatccacacggttctactcccctga-3′), and subcloned into pMES (Swartz et al., 2001), which drives gene expression from the chick β-actin enhancer/CMV promoter and which contains an Internal Ribosomal Entry Sequence (IRES) driving green fluorescence protein (GFP). Chicken HoxB4 in pCIZ was a kind gift from O. Pourquié (Iimura and Pourquié, 2006).
Electroporation
Embryos were incubated for 24 hours to reach stage 3+, attached to a paper ring (Whatman), and electroporated as previously described (Wilm et al., 2004). Embryos were incubated endoderm side up on albumin-agar at 38°C and fixed in 4% paraformaldehyde.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed as previously described (Schultheiss et al., 1995; Wilkinson and Nieto, 1993). Probes included chick Lim1 (Tsuchida et al., 1994), Pax2 (Burrill et al., 1997), cRet (Schuchardt et al., 1994), HoxA6 (ARK Genomics, Pubmed Clone AY307380), and HoxB4 (Iimura and Pourquié, 2006). Following signal development, embryos were embedded in sucrose/gelatin as described above, cryosectioned at 20 μm intervals and examined using a Zeiss Axioimager microscope with DIC optics.
Combined in situ hybridization and immunohistochemistry on paraffin sections
Embryos were fixed with 4% PFA in PBS for 30 minutes, embedded in paraffin and sectioned at 8 μm. Slides were baked on a hot plate at 60oC for 1 hour, dewaxed in xylene for 2 washes and rehydrated to PBS. Endogenous peroxidase was quenched with 0.6% hydrogen peroxidase in PBS for 10 minutes, followed by 3 washes in PBT, fixation with PFA for 10 minutes and washing again in PBT. Sections were treated with 1 μg/ml Proteinase K (Sigma) for 5 minutes, washed in PBT, fixed again in PFA for 5 minutes and washed in PBT. Acetylation was performed using 625 μl acetic anhydride in 250 ml triethanolamine for 10 minutes, followed by a PBT wash and rinse in H2O. After air drying the sections, cRet probe (1:100) (Schuchardt et al., 1994) in Hyb solution [10 ml Tris (pH 7.5), 600 mM NaCl, 1 mM EDTA, 0.24% SDS, 10% dextran sulfate, 1× Denhardt's reagent, 200 μg/ml yeast RNA, 50% formamide] was added to the sections. Slides were covered with polypropylene coverslips and incubated overnight at 65°C in a humidified chamber. Post-hybridization washes, performed at 60°C, consisted of rinses in 5×SSC, 1×SCC/50% formamide for 30 minutes, 2×SCC for 20 minutes and 0.2×SCC for 20 minutes twice. Slides were then washed in MABT [100 mM maleic acid, 150 mM NaCl, 0.1% Tween-20 (pH=7)], blocked in 20% heat-inactivated sheep serum/2% Boehringer blocking reagent in MABT for 1 hour and incubated with αDIG-HPR (POD) (Roche) (1:300) in 5%HISS/MABT at 4°C overnight in a humidified chamber. Slides were then washed three times with MABT, incubated for 10 minutes with Fluorescein Tyramide (PerkinElmer) and washed with MABT. Subsequently, immunofluorescence for quail nuclear antigen (using QCPN primary and Dylight649-conjugated secondary antibodies) was performed as described above.
RESULTS
Molecular markers of the nephric duct
The avian nephric duct is a single-cell-thick epithelial tube that forms from a mesenchymal duct rudiment that arises in the pronephric IM (Obara-Ishihara et al., 1999). The duct rudiment can first be recognized morphologically at ~10-somite stage [stage 10 (Hamburger and Hamilton, 1951)], when cells from the IM that extends between the axial levels of the 8th to 10th somites start to bulge dorsally (James and Schultheiss, 2003). By the 12-somite stage (stage 11−), the duct rudiment constitutes a distinct mesenchymal structure between the IM and the overlying ectoderm (James and Schultheiss, 2003; Obara-Ishihara et al., 1999). Beginning at stage 11, the duct rudiment extends caudally, migrating through the mesonephric and metanephric regions and eventually connecting to the cloaca at approximately stage 18 (33 somites) (Schultheiss et al., 2003). In parallel with its caudal extension, the cells of the duct rudiment undergo a mesenchymal-to-epithelial transition (MET) to generate the epithelialized nephric duct.
In this study, we have used the transcription factor Lim1 (Lhx1) (Taira et al., 1994) and the receptor cRet (Pachnis et al., 1993; Schuchardt et al., 1995) as nephric duct markers. Both genes are expressed specifically in the duct and not in surrounding tissues during the initial stages of formation of the nephric duct (Fig. 1A-D). This is in contrast to the transcription factor Pax2 (Dressler et al., 1990), which is expressed throughout the IM, including both the duct and the non-duct components (Fig. 1E,F). Note that although Lim1 and cRet extend anteriorly until the 6th somite axial level, fate-mapping studies have found that the nephric duct rudiment itself is found at axial levels 8-10 (Obara-Ishihara et al., 1999; Schultheiss et al., 2003). The IM at axial levels 6-7 does not take place in duct formation and subsequently degenerates.
Duct progenitors are determined already in the primitive streak
Previous fate-mapping studies from our lab and others (James and Schultheiss, 2003; Psychoyos and Stern, 1996) have found that precursors to the nephric duct are located in the mid-region of the primitive streak (one-third to one-half of the streak length from the anterior tip of the streak) at stage 5. Cells that leave the mid primitive streak region at stage 5 migrate to form the IM adjacent to somites 6 and posterior and thus contribute to the nephric duct rudiment. Cells from the same position in the streak (one-third to one-half streak length) from older embryos give rise to IM of more posterior regions, and thus do not normally contribute to the duct (James and Schultheiss, 2003) (see Fig. 1H-J).
Based on these fate maps, we investigated the state of commitment of prospective nephric duct cells while they were still in the primitive streak. Small grafts were taken from the mid-streak of stage 4 quail embryos and transplanted into the equivalent position in stage 8 chick embryos. (Because transplantation causes a slight delay in the emergence of cells from the primitive streak, transplantations were conducted at stage 4 in order to have graft cells consistently contribute to the IM of axial levels 6-10.) The use of quail embryos as the donor tissue allowed for the detection of transplanted cells using a quail-specific monoclonal antibody. The grafts were also marked with DiI so that the extent of graft migration could be followed in vivo. Embryos were grown until stage 12 or later and assayed for co-expression of duct markers and the quail-specific marker.
Grafts from stage 4 to stage 8 embryos gave rise to cells that migrated to axial levels of somite 12 or posterior (as judged by DiI fluorescence at the end of the experiment, Fig. 2F,I, Fig. 3A). Thus, the grafted cells migrated only into regions that were outside of the normal zone of the nephric duct primordia. It should be noted that we did observe that the transplanted stage 4 streak cells reached more anterior regions than would be expected from stage 8 primitive streak host tissue (compare Fig. 2B with 2F,I and Fig. 3A), consistent with the report of Iimura and Pourquié (Iimura and Pourquié, 2006) who observed that transplanted cells from younger embryos ingressed and moved through the primitive streak earlier than their counterparts from older embryos. However, this enhanced anterior migration did not result in the graft cells reaching axial levels anterior to somite 10.
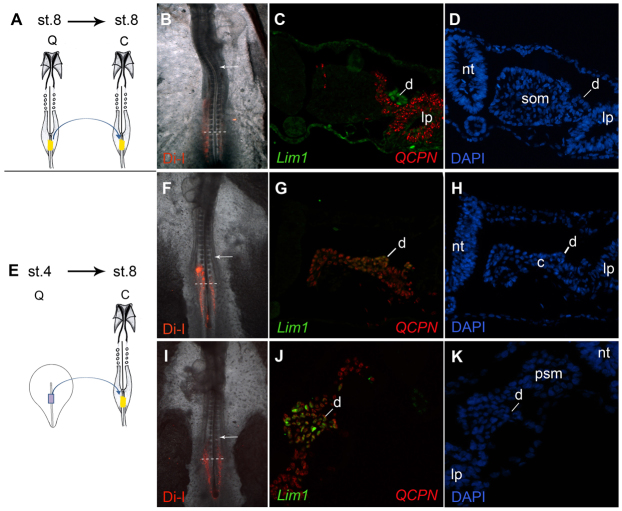
Fig. 2.
The nephric duct primordium is determined already in the primitive streak. (A-K) Explants from stage 8 (A-D) or stage 4 (E-K) quail mid-primitive streak were stained with DiI and transplanted into the equivalent location in the primitive streak of stage 8 chick embryos. DiI images in B, F and I indicate that the grafted cells remained below the region of the nephric duct primordia at axial level of somites 8-10 (arrow indicates somite 10). Embryos were sectioned and stained for Lim1 (C,G,J, green), the quail marker QCPN (C,G,J, red) and DAPI (D,H,K). Stage 4 donors formed nephric duct tissue (E-K), whereas stage 8 donors did not (A-D). c, nephrogenic cord; d, nephric duct; lp, lateral plate; nt, neural tube; psm, presomitic mesoderm; som, somite.
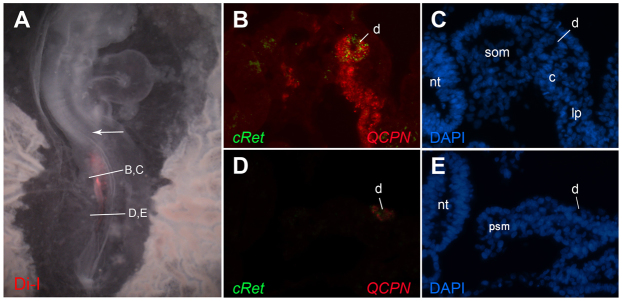
Fig. 3.
Analysis of cRet expression in transplants of prospective duct tissue into non-duct regions. Explants from stage 4 quail mid-primitive streak were stained with DiI and transplanted into the equivalent location in the primitive streak of stage 8 chick embryos, as in Fig. 2. DiI image in A indicate that the grafted cells remained below the region of the nephric duct primordia at axial level of somites 8-10 (arrow indicates somite 10). Embryos were sectioned and analyzed by combined fluorescent in situ hybridization for cRet (B,D, green) and immunofluorescence for the quail marker QCPN (B,D, red) and for DAPI (C,E). cRet-expressing graft cells are found in a morphologically normal nephric duct. c, nephrogenic cord; d, nephric duct; lp, lateral plate; nt, neural tube; psm, presomitic mesoderm; som, somite.
Duct formation in grafted embryos was analyzed by examination of co-expression of the quail marker QCPN with duct markers Lim1 or cRet. Because an antibody to avian Lim1 is available, co-expression of Lim1 and QCPN could be detected by double immunofluorescence staining (Fig. 2). For detecting co-expression of cRet and QCPN, we developed a protocol for combined immunofluorescence (to QCPN) and in situ hybridization (to cRet, Fig. 3; see Materials and methods).
Despite their location posterior to the normal nephric duct progenitor region, stage 4-grafted primitive streak cells differentiated into Lim1- and cRet-expressing structures located in the normal location of the nephric duct on the dorsal side of the nephric cord (Fig. 2E-K; Fig. 3; 8/8 embryos). On some occasions, the graft cells formed a completely morphologically normal nephric duct with epithelial morphology (Fig. 3B,C), whereas in other instances the Lim1 or cRet-expressing graft cells did not exhibit complete duct morphological differentiation and the separation between the duct and nephrogenic cord was not completely distinct (Fig. 2G,J). Interestingly, hybrid ducts were often seen, with a seamless integration of both quail and chick cells (Fig. 3B), indicating that as the host duct migrated into the region of the graft, graft cells joined the host duct and became integrated into a single structure. These hybrid ducts tended to have more fully developed duct morphologies than duct-like tissue formed solely from graft cells, perhaps indicating that the host duct (which migrated from the pronephric region) served as a template for forming the epithelialized duct. In control experiments in which stage 8 mid-streak grafts were transplanted into stage 8 hosts, the grafted cells did not differentiate into duct tissue (0/4). As shown in Fig. 2A-D, graft cells filled the IM and surrounding regions, but the duct exclusively comprised cells of host origin that had migrated from the normal duct-forming region in the pronephros.
Slack defines a tissue as ‘determined’ if ‘it will continue to develop autonomously after grafting to any other region of the embryo’ (Slack, 1991). The current results indicate that nephric duct precursors in the primitive streak are determined with respect to grafting into non-duct regions of the IM.
Posterior IM precursor cells cannot form nephric duct even if exposed to the environment in which the nephric duct normally differentiates
In the next set of experiments, we investigated whether cells that normally would not participate in formation of the duct could be induced to differentiate into duct tissue if grafted into duct-forming regions of the embryo. In the reciprocal to the experiments described above, grafts were taken from the mid-streak region of stage 8 embryos (which normally contributes to the IM of the mesonephric region) and transplanted into the equivalent mid-streak position of stage 4 embryos (which normally contributes to the pronephros and the nephric duct). Although grafted tissue migrated into the pronephric region at the axial levels of somites 8-10 (Fig. 4F), it never differentiated into duct tissue (Fig. 4G,H, 0/4); in all cases, the duct tissue comprised exclusively host chicken cells. In control experiments of grafts from stage 4 to stage 4 primitive streak, the grafted cells contributed robustly to the duct (Fig. 4A-D, 5/5).
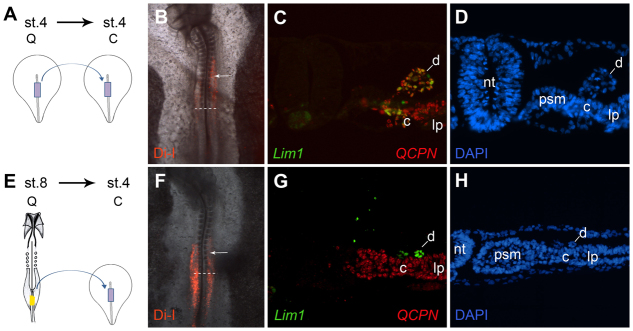
Fig. 4.
The prospective IM region of the stage 8 primitive streak is not competent to form nephric duct. (A-H) Transplants of quail stage 4 (A-D) or stage 8 (E-H) mid-primitive streak were labeled with DiI and grafted into the equivalent area of the primitive streak of stage 4 chick embryos. As indicated by the DiI localization (B,F), graft cells migrated into the duct formation region (arrows indicate somite 10). Embryos were sectioned and stained for Lim1 (C,G, green), the quail marker QCPN (C,G, red) and DAPI (D,H). Stage 8 grafts did not differentiate into Lim1-expressing duct tissue (E-H), whereas stage 4 control grafts exhibited robust duct differentiation (A-D). c, nephrogenic cord; d, nephric duct; lp, lateral plate; nt, neural tube; psm, presomitic mesoderm.
In these experiments, the stage 8 grafts were exposed to the same environment as the normal duct progenitors from the time of their exit from the primitive streak until their arrival in the pronephric IM adjacent to somites 8-10, and yet they did not differentiate into duct tissue. This result indicates that the stage 8 primitive streak cells were not competent to respond to whatever duct-promoting signals that might have been present in the environment of the nephric duct precursors from the time of their exit from the primitive streak until their differentiation.
In order to determine more precisely the time at which competence to make duct is lost, experiments were conducted with grafts taken from the stage 6 embryos. The stage 6 primitive streak normally gives rise to IM cells that lie just posterior to the duct-forming region. Transplants from stage 6 to stage 6 embryos ended up posterior to somite 10 and rarely gave rise to duct tissue (Fig. 5A-D, 1/5 embryos). By contrast, when stage 6 mid-streak tissue was transplanted into the stage 4 mid-streak region, the grafted cells localized to more anterior axial levels, including the normal duct-forming region, and differentiated robustly into nephric duct tissue (Fig. 5E-H, 7/9 embryos). Thus, the competence to form nephric duct appears to be lost in the primitive streak between stages 6 and 8.
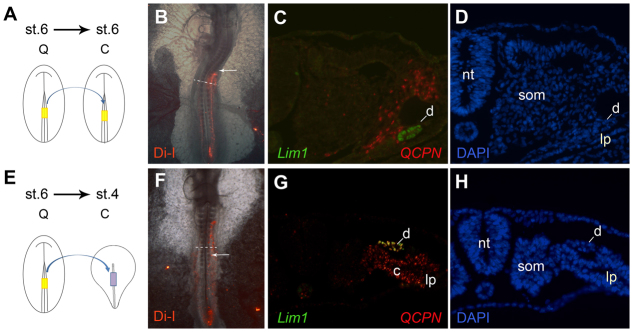
Fig. 5.
Stage 6 primitive streak cells can differentiate into duct tissue if placed into a duct-forming region. (A-H) Stage 6 mid-primitive streak was labeled with DiI and grafted into the equivalent region of stage 6 (A-D) or stage 4 (E-H) embryos. The control grafts migrated to positions posterior to the normal duct forming region (B, arrow indicates somite 10), whereas when transplanted into stage 4 embryos the graft cells reached the duct-forming region (F; arrow indicates somite 10). Embryos were sectioned and stained for Lim1 (C,G, green), the quail marker QCPN (C,G, red) and DAPI (D,H). Grafts transplanted into stage 4 embryos (E-H) differentiated into Lim1-expressing duct tissue, in contrast to the controls grafted into stage 6 embryos (A-D). c, nephrogenic cord; d, nephric duct; lp, lateral plate; nt, neural tube; som, somite.
A posterior Hox gene can repress nephric duct formation
Hox genes have been well-documented to regulate development along the anterior-posterior (AP) embryonic axis (McGinnis and Krumlauf, 1992; Pearson et al., 2005). Hox genes are expressed in nested patterns along the AP axis to generate a ‘Hox code’ that is thought to regulate many aspects of region-specific tissue formation. As discussed above, the nephric duct forms only at a specific AP level: the IM adjacent to somites 8-10. As the nephric duct rudiment does not form in IM posterior to somite 10, we hypothesized that Hox genes with an anterior border posterior to somite 10 might play a role in preventing duct formation in more posterior locations.
We surveyed several Hox genes that were candidates for having an anterior border in the IM near the 10th somite axial level (Burke et al., 1995), including HoxA5, HoxA6 and HoxC5. Of these, HoxA6 had the most suggestive expression pattern with respect to possibly being involved in the regulation of duct formation. As seen in Fig. 6A-E, HoxA6 expression is not detected at stage 4. At stage 6, HoxA6 is detected in a salt-and-pepper pattern in the primitive streak, which strengthens by stage 8 to expression throughout the primitive streak. At stage 11+ (14 somites), HoxA6 expression has a clear anterior border at the axial level of the 12th somite. Cross-sections of stage 11+ (14 somites) embryos reveal that HoxA6 is expressed in the IM, somites lateral plate and neural tube, with little to no expression in the nephric duct (Fig. 6E) (although at later stages HoxA6 expression can be seen in posterior regions of the duct, data not shown). Thus, from the stage 4 primitive streak until the formation of the nephric duct primordia at stage 10-11, HoxA6 is not expressed in tissues containing duct precursor cells. Instead, HoxA6 is expressed in more posterior IM and its precursors, which do not contribute to duct formation.
Fig. 6.
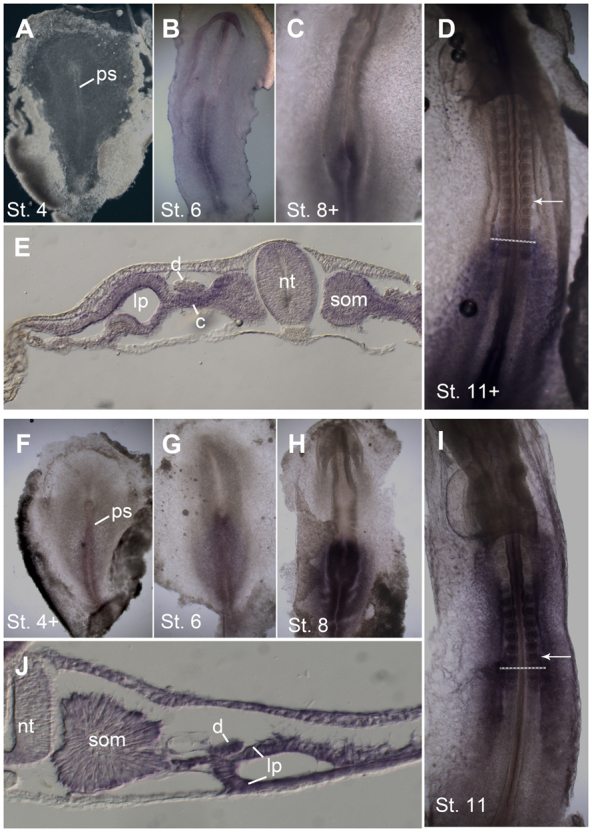
Expression of HoxA6 and HoxB4. (A-J) Whole-mount in situ hybridization for HoxA6 (A-E) and HoxB4 (F-J) in chick embryos. (A) No HoxA6 expression is detectable at stage 4. (B,C) At stage 6 (B) and stage 8 (C), expression is seen in the primitive streak. (D,E) Stage 11 whole mount shows expression up to the axial level of somite 12 (D, arrow indicates somite 10), and in cross-sections expression is seen primarily in the somite, nephrogenic cord lateral plate and neural tube, with little expression in the nephric duct (E). (F-H) Hox B4 expression initiates at stage 4 in the primitive streak (F) and expands to surrounding tissues at stages 6 (G) and 8 (H). (I) At stage 11, HoxB4 is expressed up to the axial level of somite 6. (J) Section of stage 11 embryo shows HoxB4 expression throughout the mesoderm, including the nephric duct. Dashed lines in D and I indicate level of sections in E and J, respectively. c, nephrogenic cord; d, nephric duct; lp, lateral plate; nt, neural tube; ps, primitive streak; som, somite.
We also examined expression of the more 3′ Hox gene HoxB4 (Fig. 6F-J). In agreement with published results (Preger-Ben Noon et al., 2009), we found that HoxB4 is expressed in the stage 4 primitive streak. At stage 11, HoxB4 has an anterior expression border at the 6th somite axial level, and on cross-sections can be seen to be expressed in the nephric duct as well as surrounding tissues (Fig. 6J). Thus, starting from the time of their location in the primitive streak, nephric duct precursor cells express HoxB4.
As HoxA6 was expressed only in IM posterior to the duct-forming region, we investigated whether misexpression of HoxA6 in more anterior regions would influence nephric duct formation. A vector co-expressing HoxA6 and eGFP was electroporated into the mid-primitive streak at stage 3 and embryos were grown until at least stage 12, when the duct had formed and had begun to elongate. Control electroporations were performed with the same vector expressing eGFP alone. eGFP fluorescence showed that both vectors were expressed in a broad region, including the prospective duct-forming region at the somite 6-10 axial level (Fig. 7 A,F). In situ hybridization for Lim1 showed that the duct did form in most HoxA6-eGFP-electroporated embryos, but the intensity of Lim1 staining was significantly lower than in eGFP-electroporated controls (Fig. 7B,G; HoxA6-eGFP and eGFP-electroporated embryos were developed in parallel and photographed under identical conditions. Embryos in Fig. 7 are representative of 11 analyzed HoxA6-GFP and eight eGFP-electroporated embryos). In sections, the nephric ducts in HoxA6-electroporated embryos were seen to be thinner, but the intensity of Lim1 expression per cell was similar to that in control embryos (Fig. 7C,H). Thus, the weaker Lim1 expression seen in HoxA6-electroporated embryos seems to be attributable to fewer duct cells rather than to a lower amount of Lim1 expression in each duct cell.
Fig. 7.
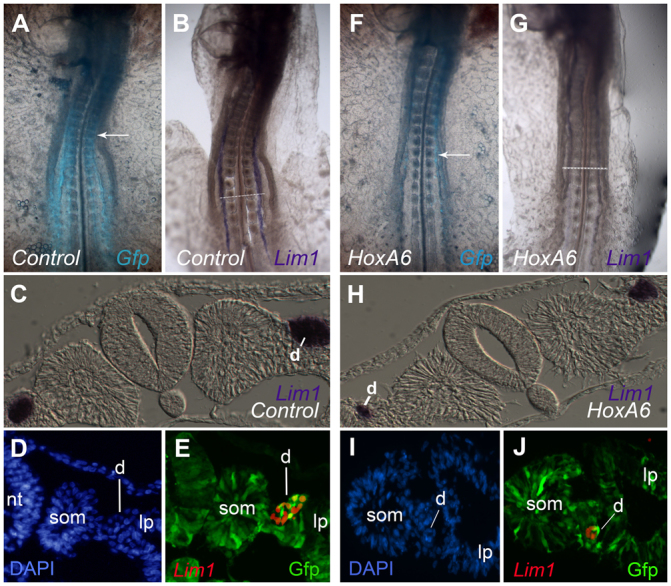
HoxA6 misexpression represses nephric duct formation. (A-J) Embryos were electroporated with an empty pMes-GFP vector (A-E) or pMes-HoxA6-GFP expression vector (F-J) and analyzed by whole-mount in situ hybridization for Lim1 (B,C,G,H) or by immunofluorescence for Lim1 and Gfp (D,E,I,J). In HoxA6-electroporated embryos, Lim1 expression was weaker than controls (compare B with G) and the nephric duct was typically smaller (compare C with H). When analyzed by immunofluorescence, GFP-expressing cells (indicative of electroporation) were found with lower frequency in the nephric duct of HoxA6-electroporated embryos (I,J) when compared with controls (D,E), despite the fact that they are abundant in the surrounding tissues in both situations. Arrows indicate somite 10. d, nephric duct; lp, lateral plate; nt, neural tube; som, somite.
One possible explanation for the reduction of the nephric duct in HoxA6-electroporated embryos is that cells that received HoxA6 were prevented from forming duct, and that the duct in these embryos was formed from cells that did not receive the HoxA6 expression plasmid (electroporation introduces DNA into only a fraction of cells in the targeted region, up to 50% in our experience). In order to test this hypothesis, HoxA6-electroporated embryos were examined by immunofluorescence for presence of the HoxA6-expression plasmid in the nephric duct. As see in Fig. 7I,J, the nephric ducts of HoxA6-electoporated embryos contained a lower percentage of GFP-expressing cells than those of control embryos (Fig. 7D,E), suggesting an incompatibility of HoxA6 expression with duct formation. The exclusion of HoxA6 expression plasmid from duct structures was not absolute, as some GFP-expressing cells could be found in the ducts of HoxA6-electroporated embryos (Fig. 7J).
As a further control, we examined the effects of electroporating HoxB4, which is normally expressed in the duct-forming region adjacent to somites 6-10 (Fig. 6I) (Preger-Ben Noon et al., 2009). As seen in Fig. 8, electroporation of HoxB4 did not lower intensity of Lim1 expression, or the morphology of the nephric duct in sections, when compared with eGFP-electroporated control embryos. Thus, the duct-repressing effects of HoxA6 misexpression do not appear to be a general consequence of Hox gene overexpression, but rather a specific effect of misexpressing HoxA6.
Fig. 8.
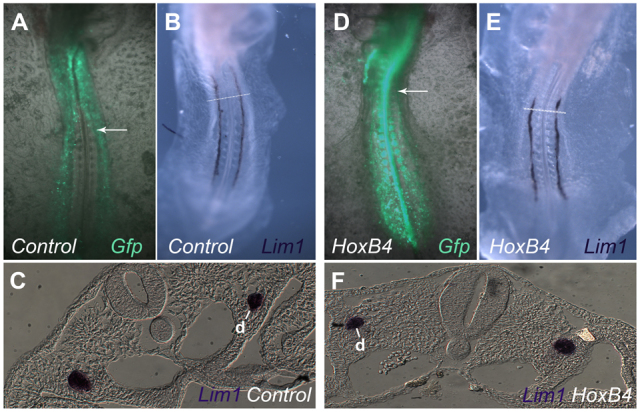
HoxB4 misexpression does not repress nephric duct formation. (A-F) Embryos were electroporated with an empty pCIZ-GFP vector (A-C) or pCIZ-HoxB4-GFP expression vector (D-F) and analyzed by whole-mount in situ hybridization for Lim1 (B,C,E,F). The nephric duct appeared similar under the two conditions. Arrows indicate somite 10. d, nephric duct.
DISCUSSION
Timing of nephric duct specification and implications for mesodermal patterning
Although the molecular signs of overt nephric duct differentiation are first detectable in the IM of the pronephric region (adjacent to future somites 8-10 at stage 9 in the chick embryo), the current study found that duct precursors are already determined much earlier, before they have migrated away from the primitive streak (Figs 2, 3). Conversely, prospective IM from the primitive streak of older embryos (which normally contribute only to more posterior, non duct-forming regions of the IM) did not differentiate into duct tissue even if it was exposed to the same environment as the endogenous duct precursors from the time of its exit from the primitive streak until its coming to lie in the duct-forming region adjacent to somites 8-10 (Fig. 4). Thus, the patterning of the prospective IM with respect to whether or not it is competent to give rise to nephric duct is already fixed before cells have left the primitive streak.
The design of the transplantation experiments in these studies was isotopic/heterochronic (Garcia-Martinez and Schoenwolf, 1992; Schoenwolf et al., 1992), i.e. tissues were taken from the prospective IM region of the primitive streak of one embryo and moved to the same position in the primitive streak of an older or younger embryo. At the end of the experiment, the transplanted tissues ended up in more posterior or anterior regions of the IM, respectively, but always within the IM and surrounding regions. We and others have previously reported the results of the converse type of experiment – heterotopic/isochronic – wherein cells were transplanted between the IM-forming region and other regions of the streak in embryos of the same age (Garcia-Martinez and Schoenwolf, 1992; James and Schultheiss, 2003). In those transplantations, the fate of the transplanted tissues was found to be changeable. Prospective IM regions of the primitive streak differentiated into somites or lateral plate if transplanted into more anterior or posterior parts, respectively, of the primitive streak of embryos of the same stage. Conversely, prospective somite or lateral plate regions of the primitive streak differentiated into IM if placed into the IM region of the primitive streak. Taking the current and past results together, one is led to the conclusion that the regionalization of the IM (whether to make duct or non-duct types of IM) is fixed before cells are even committed to make IM at all.
Although this conclusion may seem somewhat counterintuitive, it is consistent with previous studies of the patterning of the skeleton in avian embryos. Transplants of the paraxial presomitic mesoderm between thoracic and lumbar axial levels resulted in vertebral morphologies (e.g. presence or absence of ribs) that were characteristic of the donor tissue and not the host (Kieny et al., 1972). However, studies from our lab (James and Schultheiss, 2003; James and Schultheiss, 2005) and others (Tonegawa et al., 1997) have found that at this stage of development the presomitic mesoderm can still be diverted to an intermediate mesoderm or lateral plate fate by transplantation or by manipulation of levels of BMP signaling. Thus, in agreement with the current findings with respect to the IM, the potential of the paraxial mesoderm to generate one or another type of vertebral morphology has already been fixed before the identity of the mesoderm as paraxial mesoderm has been determined. In addition, Iimura and Pourquié have reported that the timing of ingression of prospective paraxial mesoderm cells into the primitive streak, and hence their eventual position along the AP axis, is already fixed before ingression (Iimura and Pourquié, 2006), which is before the fixation of their identity as paraxial mesoderm (James and Schultheiss, 2003).
These results can be considered in the framework of mesodermal patterning along the anterior-posterior and medial-lateral axes. Moving cells between different positions within the streak at a particular developmental stage results in their eventual occupation different places on the medial-lateral axis (equivalent to the dorsal-ventral axis in round embryos such as Xenopus), while moving cells between embryos of different ages but in the same relative streak position results in their occupying different places along the anterior-posterior axis but in the same medial-lateral position (Garcia-Martinez and Schoenwolf, 1992; James and Schultheiss, 2003; Psychoyos and Stern, 1996). Integrating the current and past results, one comes to the conclusion that fixation of the anterior-posterior pattern of the mesoderm appears to occur prior to fixation of its medial-lateral pattern. In other words, it appears that in the primitive streak it is not yet determined whether a cell will become intermediate mesoderm; however, if a cell does become intermediate mesoderm, then the type of intermediate mesoderm that it will form (duct or non-duct) appears to be already determined. The same framework would also seem to apply to the patterning of the paraxial mesoderm (Iimura and Pourquié, 2006; Kieny et al., 1972; Tonegawa et al., 1997).
This early fixation of the anterior-posterior character of the mesoderm may be connected to the mechanism of the establishment of Hox gene expression patterns. In the model of Durston and co-workers (Wacker et al., 2004), sequential waves of Hox gene expression are activated in the non-axial mesoderm and are stabilized by signals from the organizer. This model is consistent with the data in the current report: the Hox pattern of stage 4 or 8 primitive streak donor tissues would have already been stabilized and thus refractory to repatterning by transplantation into stage 8 or 4 embryos, respectively. This fixation of the Hox gene expression pattern would in turn influence the competence of the primitive streak tissue to differentiate into different types of IM derivatives.
Regulation of IM regionalization by Hox genes
There is accumulating evidence that proper Hox expression is crucial for kidney development and that Hox genes play a role in regulating AP patterning of the IM. Genes of the Hox11 paralogous group are expressed in the metanephric but not more anterior regions of the IM (Mugford et al., 2008) and have been found to be essential for formation of the metanephros (Wellik et al., 2002). Misexpression of HoxD11 in the mesonephros results in mesonephros expansion and activation of some features of metanephric-type gene expression, including expression of Six2 and distal nephric markers (Mugford et al., 2008). Swapping of the homeodomain of HoxA11 with that of HoxA4 resulted in severe metanephric kidney malformations, further pointing to the importance of proper Hox gene activity for normal kidney development (Zhao and Potter, 2002). In addition, HoxB4 has been reported to regulate the position of the anterior border of the pronephros (between somites 6 and 7) in avian embryos (Preger-Ben Noon et al., 2009).
The current study found that a Hox gene (HoxA6) with an anterior border of expression just posterior to the duct-forming region of the IM could repress duct formation if misexpressed in more anterior regions of the IM. In the framework of the large literature on Hox gene function, this could be considered a posterior transformation (Kessel and Gruss, 1990), wherein an anterior region of the IM, which normally forms a nephric duct, acquires a characteristic of more posterior IM (the inability to form a duct). One would therefore conjecture that HoxA6, and perhaps other posterior Hox genes, repress expression of a nephric duct differentiation program, either directly or indirectly. Reports of mouse loss-of-function mutations of HoxA6 and other paralogous group 6 Hox genes have not described alterations in nephric duct formation (Chisaka and Capecchi, 1991; McIntyre et al., 2007). However, this is not surprising, as Hox gene loss of function would be predicted to possibly cause an anteriorization and thus a potential broadening of the duct-forming rudiment posteriorly into regions where the duct does not normally form. As the duct rudiment extends posteriorly as part of its normal development, any posterior broadening of the duct rudiment as a result of Hox gene loss of function would be likely obscured by the normal posterior extension of the duct rudiment. Misexpression studies of HoxA6 or other Hox group 6 genes in more anterior regions in mice have not been reported. Preger-Ben Noon et al. have reported that misexpression of HoxB4 in regions anterior to the pronephros causes an expansion of Lim1 expression into the region anterior to somite 6 (Preger-Ben Noon et al., 2009). Although that study did not examine expression of other duct markers or duct morphology, this result suggests that anterior Hox genes may play an active role in specifying the duct-forming region of the IM.
The specific molecular mechanism whereby expression of HoxA6 represses duct formation is not currently clear. The observation that HoxA6-expressing cells tend not to be included in the nephric duct in HoxA6-electroporated embryos (Fig. 7J) indicates that ectopic HoxA6 may inhibit duct formation on a cell-autonomous basis. However, this does not rule out additional non-cell autonomous effects, such as alterations in environmental signals that are crucial for duct formation. The fate of HoxA6-electroporated duct precursors that are prevented from forming duct is also not clear. It is possible that a proportion of these cells died, although an excess of apoptotic cells was not observed in the IM of HoxA6-electroporated embryos (data not shown). One interesting possibility is that they may be capable of differentiating into more posterior IM derivatives. However, with the current embryo culture system it was not possible to grow the embryos long enough to determine whether HoxA6-electroporated cells contributed to mesonephric tubules or other posterior IM structures.
Factors that regulate nephric duct formation
Although HoxA6 appears to inhibit duct formation, little is known about specific factors that promote duct formation. The current findings indicate that the region adjacent to somites 8-10 where duct markers are first activated does not provide specific information that is essential to promote duct formation, as transplanted duct precursors will differentiate into duct in more posterior locations. It is of interest that the transplanted duct precursors differentiated into duct only in the position where the duct normally forms, i.e. in the IM region and not more medially or laterally. This suggests that there may be environmental factors present in the region of the IM in general that are required for activation of a duct differentiation program. The difference between prospective duct cells and prospective non-duct cells may be in their competence to activate duct genes in response to this general IM environment. HoxA6 and other Hox genes may modulate the response to such IM factors, such that only presumptive IM from the stage 4 primitive streak can initiate duct differentiation, analogous to the concept developed in Drosophila of selector genes that modify cellular responses to embryonic signals (García-Bellido, 1975). A similar situation has been reported in the studies of the border between the kidney and non-kidney-forming regions of the IM (located at the border between somites 6 and 7), where HoxB4 has been proposed to regulate the competence of cells to activate IM genes in response to general signals originating from axial embryonic structures (Barak et al., 2005; Preger-Ben Noon et al., 2009). Notch signaling has also been found to play a role in the decision to differentiate as nephric duct versus tubule in the Xenopus pronephros (McLaughlin et al., 2000). Future studies of the molecular differences between the prospective IM regions of the stage 4 and stage 8 primitive streak may help to shed light on the molecular mechanisms whereby Hox genes regulate competence to form the nephric duct.
Nephric duct formation and A-P patterning of the IM in evolutionary context
Repression of duct formation may have played an important role during vertebrate kidney evolution. In the embryo of the hagfish, considered to be the most primitive extant vertebrate, the IM does not become regionalized into a pro-, meso- and metanephros, but instead produces a single holonephros, which consists of one nephron per body segment (Dean, 1899; Schultheiss, 2007; Torrey, 1965). Each of these nephrons is thought to generate a small part of the nephric duct, which then connects to the duct regions from the immediately anterior and posterior body segments to generate a single continuous nephric duct. Thus, in hagfish embryos, duct formation is not confined to the most anterior part of the intermediate mesoderm, but instead occurs throughout a much wider anterior-posterior extent of the IM. In more derived vertebrates, the IM is typically more regionalized: the ability to form duct is confined to the pronephros and appears to have been lost from more posterior regions of the IM. This change may have come about in response to the need to reserve posterior IM in order to generate different types of kidney tissue to meet the metabolic needs of different phases of the life cycle, e.g. aquatic versus terrestrial environments (Schultheiss, 2007). It will be interesting to determine whether repression of duct-forming ability in posterior regions during vertebrate evolution is regulated by the acquisition of duct-repressing functions by posterior Hox genes.
Acknowledgements
We thank Or Ben Shaul for technical assistance, Rami Reshef for critical reading of the manuscript and Olivier Pourquié for the generous gift of plasmids.
Footnotes
Funding
This work was supported by grants to T.M.S. from the Israel Science Foundation [1328/08], the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases) [DK071041] and the Rappaport Faculty of Medicine and Research Institute, Technion-Israel Institute of Technology. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Barak H., Rosenfelder L., Schultheiss T. M., Reshef R. (2005). Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev. Dyn. 232, 901-914 [DOI] [PubMed] [Google Scholar]
- Brennan H. C., Nijjar S., Jones E. A. (1998). The specification of the pronephric tubules and duct in Xenopus laevis. Mech. Dev. 75, 127-137 [DOI] [PubMed] [Google Scholar]
- Burke A. C., Nelson C. E., Morgan B. A., Tabin C. (1995). Hox genes and the evolution of vertebrate axial morphology. Development 121, 333-346 [DOI] [PubMed] [Google Scholar]
- Burrill J. D., Moran L., Goulding M. D., Saueressig H. (1997). PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development 124, 4493-4503 [DOI] [PubMed] [Google Scholar]
- Carapuço M., Nóvoa A., Bobola N., Mallo M. (2005). Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 19, 2116-2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisaka O., Capecchi M. R. (1991). Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature 350, 473-479 [DOI] [PubMed] [Google Scholar]
- Dean B. (1899). On the embryology of bdellostoma stouti. Verlag von Gustav Fischer in Jena, pp. 221-276
- Dressler G. R., Deutsch U., Chowdhury K., Nornes H. O., Gruss P. (1990). Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109, 787-795 [DOI] [PubMed] [Google Scholar]
- Ema M., Morita M., Ikawa S., Tanaka M., Matsuda Y., Gotoh O., Saijoh Y., Fujii H., Hamada H., Kikuchi Y., et al. (1996). Two new members of the murine Sim gene family are transcriptional repressors and show different expression patterns during mouse embryogenesis. Mol. Cell. Biol. 16, 5865-5875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. M., Kuwana E., Bulfone A., Fletcher C. F., Copeland N. G., Jenkins N. A., Crews S., Martinez S., Puelles L., Rubenstein J. L., et al. (1996). Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol. Cell. Neurosci. 7, 1-16 [DOI] [PubMed] [Google Scholar]
- García-Bellido A. (1975). Genetic control of wing disc development in Drosophila. Ciba Found. Symp. 0, 161-182 [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V., Schoenwolf G. C. (1992). Positional control of mesoderm movement and fate during avian gastrulation and neurulation. Dev. Dyn. 193, 249-256 [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. (1989). The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell 57, 367-378 [DOI] [PubMed] [Google Scholar]
- Grobstein C. (1955). Inductive interactions in the devlopment of the mouse metanephros. J. Exp. Zool. 130, 319-339 [Google Scholar]
- Gruenwald P. (1943). Stimulation of nephrogenic tissue by normal and abnormal inductors. Anat. Rec. 86, 321-339 [Google Scholar]
- Hamburger V., Hamilton H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49-92 [PubMed] [Google Scholar]
- Iimura T., Pourquié O. (2006). Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature 442, 568-571 [DOI] [PubMed] [Google Scholar]
- James R. G., Schultheiss T. M. (2003). Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev. Biol. 253, 109-124 [DOI] [PubMed] [Google Scholar]
- James R. G., Schultheiss T. M. (2005). Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev. Biol. 288, 113-125 [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. (1990). Murine developmental control genes. Science 249, 374-379 [DOI] [PubMed] [Google Scholar]
- Kessel M., Balling R., Gruss P. (1990). Variations of cervical vertebrae after expression of a Hox-1.1 transgene in mice. Cell 61, 301-308 [DOI] [PubMed] [Google Scholar]
- Kieny M., Mauger A., Sengel P. (1972). Early regionalization of somitic mesoderm as studied by the development of axial skeleton of the chick embryo. Dev. Biol. 28, 142-161 [DOI] [PubMed] [Google Scholar]
- Krumlauf R. (1994). Hox genes in vertebrate development. Cell 78, 191-201 [DOI] [PubMed] [Google Scholar]
- Lynch K., Fraser S. E. (1990). Cell migration in the formation of the pronephric duct in Xenopus laevis. Dev. Biol. 142, 283-292 [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. (1992). Homeobox genes and axial patterning. Cell 68, 283-302 [DOI] [PubMed] [Google Scholar]
- McIntyre D. C., Rakshit S., Yallowitz A. R., Loken L., Jeannotte L., Capecchi M. R., Wellik D. M. (2007). Hox patterning of the vertebrate rib cage. Development 134, 2981-2989 [DOI] [PubMed] [Google Scholar]
- McLaughlin K. A., Rones M. S., Mercola M. (2000). Notch regulates cell fate in the developing pronephros. Dev. Biol. 227, 567-580 [DOI] [PubMed] [Google Scholar]
- Mugford J. W., Sipilä P., Kobayashi A., Behringer R. R., McMahon A. P. (2008). Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev. Biol. 319, 396-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara-Ishihara T., Kuhlman J., Niswander L., Herzlinger D. (1999). The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126, 1103-1108 [DOI] [PubMed] [Google Scholar]
- Pachnis V., Mankoo B., Costantini F. (1993). Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119, 1005-1017 [DOI] [PubMed] [Google Scholar]
- Patterson L. T., Potter S. S. (2003). Hox genes and kidney patterning. Curr. Opin. Nephrol. Hypertens. 12, 19-23 [DOI] [PubMed] [Google Scholar]
- Pearson J. C., Lemons D., McGinnis W. (2005). Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 6, 893-904 [DOI] [PubMed] [Google Scholar]
- Preger-Ben Noon E., Barak H., Guttmann-Raviv N., Reshef R. (2009). Interplay between activin and Hox genes determines the formation of the kidney morphogenetic field. Development 136, 1995-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychoyos D., Stern C. D. (1996). Fates and migratory routes of primitive streak cells in the chick embryo. Development 122, 1523-1534 [DOI] [PubMed] [Google Scholar]
- Saxen L. (1987). Organogenesis of the Kidney. London: Cambridge University Press; [Google Scholar]
- Schoenwolf G. C., Garcia-Martinez V., Dias M. S. (1992). Mesoderm movement and fate during avian gastrulation and neurulation. Dev. Dyn. 193, 235-248 [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380-383 [DOI] [PubMed] [Google Scholar]
- Schuchardt A., Srinivas S., Pachnis V., Costantini F. (1995). Isolation and characterization of a chicken homolog of the c-ret proto-oncogene. Oncogene 10, 641-649 [PubMed] [Google Scholar]
- Schultheiss T. M. (2007). Topics in vertebrate kidney formation: a comparative perspective. In Principles of Developmental Genetics (ed. Moody S.), pp. 778-804 San Diego: Academic Press; [Google Scholar]
- Schultheiss T. M., Xydas S., Lassar A. B. (1995). Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203-4214 [DOI] [PubMed] [Google Scholar]
- Schultheiss T. M., James R. G., Listopadova A., Herzlinger D. (2003). Formation of the nephric duct. In The Kidney, (ed. Vize P., Woolf A. S., Bard J. B. L.). Amsterdam: Academic Press; [Google Scholar]
- Serluca F. C., Fishman M. C. (2001). Pre-pattern in the pronephric kidney field of zebrafish. Development 128, 2233-2241 [DOI] [PubMed] [Google Scholar]
- Slack J. M. W. (1991). From Egg to Embryo. Cambridge: Cambridge University Press; [Google Scholar]
- Swartz M. E., Eberhart J., Pasquale E. B., Krull C. E. (2001). EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development 128, 4669-4680 [DOI] [PubMed] [Google Scholar]
- Taira M., Otani H., Jamrich M., Dawid I. B. (1994). Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development 120, 1525-1536 [DOI] [PubMed] [Google Scholar]
- Tonegawa A., Funayama N., Ueno N., Takahashi Y. (1997). Mesodermal subdivision along the mediolateral axis in chicken controlled by different concentrations of BMP-4. Development 124, 1975-1984 [DOI] [PubMed] [Google Scholar]
- Torrey T. (1965). Morphogenesis of the vertebrate kidney. In Organogenesis (ed. DeHaan R., Ursprung H.), pp. 559-579 New York: Holt, Rinehart and Winston; [Google Scholar]
- Tsuchida T., Ensini M., Morton S. B., Baldassare M., Edlund T., Jessell T. M., Pfaff S. L. (1994). Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell 79, 957-970 [DOI] [PubMed] [Google Scholar]
- Vize P. D., Woolf A. S., Bard J. B. L. (2003). The Kidney, from Normal Development to Congenital Disease. San Diego: Academic Press; [Google Scholar]
- Wacker S. A., Jansen H. J., McNulty C. L., Houtzager E., Durston A. J. (2004). Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev. Biol. 268, 207-219 [DOI] [PubMed] [Google Scholar]
- Waddington C. H. (1938). The morphogenetic function of a vestigal organ in the chick. J. Exp. Biol. 15, 371-377 [Google Scholar]
- Wellik D. M. (2009). Hox genes and vertebrate axial pattern. Curr. Top. Dev. Biol. 88, 257-278 [DOI] [PubMed] [Google Scholar]
- Wellik D. M. (2011). Hox genes and kidney development. Pediatr. Nephrol. 26, 1559-1565 [DOI] [PubMed] [Google Scholar]
- Wellik D. M., Hawkes P. J., Capecchi M. R. (2002). Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 16, 1423-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Nieto M. A. (1993). Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 225, 361-373 [DOI] [PubMed] [Google Scholar]
- Wilm B., James R. G., Schultheiss T. M., Hogan B. L. (2004). The forkhead genes, Foxc1 and Foxc2, regulate paraxial versus intermediate mesoderm cell fate. Dev. Biol. 271, 176-189 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Potter S. S. (2002). Functional comparison of the Hoxa 4, Hoxa 10, and Hoxa 11 homeoboxes. Dev. Biol. 244, 21-36 [DOI] [PubMed] [Google Scholar]