Abstract
Parathyroid hormone-related protein (PTHrP) regulates cell fate and specifies the mammary mesenchyme during embryonic development. Loss of PTHrP or its receptor (Pthr1) abolishes the expression of mammary mesenchyme markers and allows mammary bud cells to revert to an epidermal fate. By contrast, overexpression of PTHrP in basal keratinocytes induces inappropriate differentiation of the ventral epidermis into nipple-like skin and is accompanied by ectopic expression of Lef1, β-catenin and other markers of the mammary mesenchyme. In this study, we document that PTHrP modulates Wnt/β-catenin signaling in the mammary mesenchyme using a Wnt signaling reporter, TOPGAL-C. Reporter expression is completely abolished by loss of PTHrP signaling and ectopic reporter activity is induced by overexpression of PTHrP. We also demonstrate that loss of Lef1, a key component of the Wnt pathway, attenuates the PTHrP-induced abnormal differentiation of the ventral skin. To characterize further the contribution of canonical Wnt signaling to embryonic mammary development, we deleted β-catenin specifically in the mammary mesenchyme. Loss of mesenchymal β-catenin abolished expression of the TOPGAL-C reporter and resulted in mammary buds with reduced expression of mammary mesenchyme markers and impaired sexual dimorphism. It also prevented the ectopic, ventral expression of mammary mesenchyme markers caused by overexpression of PTHrP in basal keratinocytes. Therefore, we conclude that a mesenchymal, canonical Wnt pathway mediates the PTHrP-dependent specification of the mammary mesenchyme.
Keywords: Epidermal appendage, Mammary, PTHrP, Wnt, Mouse
INTRODUCTION
Murine mammary gland development begins at embryonic day (E) 10.5 with the formation of bilateral mammary lines, which are multilayered ridges of columnar epidermal cells extending between the fore- and hindlimb buds on the ventral surface of the embryo (Veltmaat et al., 2003; Cowin and Wysolmerski, 2010). Between E11 and E13, cells within the mammary lines migrate and condense into five pairs of placodes, which then invaginate into the underlying mesenchyme to form the ten mammary buds (Hens and Wysolmerski, 2005; Cowin and Wysolmerski, 2010). Throughout this process, epithelial and stromal cells engage in a series of sequential and reciprocal interactions that specify the proper differentiation of both cell types as well as the subsequent morphogenesis of the mammary gland. The mammary-specific mesenchyme consists of three to five concentric layers of fibroblasts that compact around the epithelial bud in a radial fashion and express specific molecular markers, including androgen receptor (AR), estrogen receptor (ER), tenascin C, Lef1 and β-catenin (Dunbar et al., 1999; Cowin and Wysolmerski, 2010). Functionally, the mammary mesenchyme is required to maintain the commitment of the bud epithelial cells to a mammary fate, to trigger nipple formation and to initiate the outgrowth and morphogenesis of the epithelial ducts. However, in male mice, fetal androgens cause the mammary mesenchyme cells to constrict around the stalk of the epithelial bud, disrupting its connection with the overlying epidermis and inhibiting the formation of the epithelial ducts. As the ducts grow out, mammary mesenchyme cells differentiate into the dense stroma associated with the nipple, whereas the epithelial duct system grows into a separate adipocyte-rich stromal compartment known as the mammary fat pad (Boras-Granic and Wysolmerski, 2008). The end result of embryonic mammary development is the formation of a nipple and a rudimentary ductal tree with eight to ten branches (Veltmaat et al., 2003; Cowin and Wysolmerski, 2010). After birth, the ducts grow slowly and with the onset of puberty they enter a second phase of active branching morphogenesis that is stimulated by circulating hormones (Boras-Granic and Wysolmerski, 2008).
Embryonic mammary development is regulated by complex interactions between multiple growth factors, including parathyroid hormone-related protein (PTHrP; Pthlh – Mouse Genome Informatics) and Wnts. PTHrP was initially discovered as a cause of hypercalcemia in cancer patients and it contributes to the development of multiple organs (Burtis et al., 1987; Wysolmerski, 2010). During embryonic mammary development, PTHrP is expressed in the epithelial cells of the mammary bud, whereas its cognate G protein-coupled receptor, the type I Pth/PTHrP receptor (Pthr1; Pth1r – Mouse Genome Informatics), is expressed in the mesenchyme surrounding the invaginating mammary bud (Jüppner et al., 1991; Wysolmerski and Stewart, 1998). Both PTHrP and Pthr1 are required for mammary development as evidenced by the lack of mammary epithelial ducts in PTHrP−/− and Pthr1−/− mice (Wysolmerski and Stewart, 1998). Conversely, transgenic overexpression of PTHrP in the basal epidermis (under control of the keratin 14 promoter, K14-PTHrP mice) leads to conversion of the ventral dermis into mammary mesenchyme, suppression of hair follicle development and differentiation of the entire ventral surface into nipple-sheath epidermis (Foley et al., 2001). These and other results have demonstrated that PTHrP signals from the mammary bud epithelium to the mesenchyme to induce the proper differentiation of the specialized mammary mesenchyme, which, in turn, supports further epithelial morphogenesis (Hens et al., 2007; Cowin and Wysolmerski, 2010).
Wnts are glycosylated short-range secreted morphogens that bind to a co-receptor complex composed of a member of the frizzled (Frz) family of seven-pass transmembrane receptors and either low density lipoprotein receptor-related protein (Lrp) 5 or Lrp6. Canonical Wnt signaling involves a triad of intracellular proteins (adenomatous polyposis coli, axin 1 and glycogen synthase kinase-3β) that phosphorylate cytoplasmic β-catenin and target it for destruction in the proteasome (Incassati et al., 2010). Binding of Wnts to their receptor complex inhibits this process and stabilizes cytoplasmic β-catenin, which then accumulates and translocates to the nucleus to act as a transcription factor. Wnt signaling is negatively regulated at the cell surface by secreted proteins that competitively bind Wnts or by antagonists, such as dickkopf and kremen, that prevent assembly of ligand-receptor complexes (Gordon and Nusse, 2006; Niehrs, 2006). Wnts can also signal through β-catenin-independent non-canonical pathways, such as the planar polarity pathway or the Wnt/Ca2+ pathway (Gordon and Nusse, 2006; Niehrs, 2006).
Several lines of evidence show that canonical Wnt signaling is required for normal embryonic mammary development. First, factors involved in this signaling pathway, such as Wnt10b, Lef1 and β-catenin, are expressed in the developing mammary line and buds (Chu et al., 2004; Veltmaat et al., 2004). Second, Wnt signaling activity has been observed in embryonic mammary epithelium and mesenchyme in vivo using transgenic Wnt-reporter mice (Chu et al., 2004; Boras-Granic et al., 2006). Finally, genetic alterations in the canonical Wnt pathway have been shown to disrupt normal embryonic mammary development. Transgenic mice overexpressing the Wnt-inhibitor Dkk1 in the embryonic epidermis do not form a mammary line or placodes (Chu et al., 2004). Lef1−/− mice lack the second and third pairs of mammary buds altogether, and only develop rudimentary first, fourth and fifth pairs, which subsequently degenerate (van Genderen et al., 1994; Boras-Granic et al., 2006). Finally, ablation of the Wnt co-receptors Lrp5 and Lrp6, or the canonical Wnt modifier pygopus, leads to delayed and stunted outgrowth of the mammary ducts (Lindvall et al., 2006; Gu et al., 2009; Lindvall et al., 2009).
Lef1 and β-catenin are expressed in both the mammary mesenchyme and the mammary epithelium (Dunbar et al., 1999). Previous work has demonstrated that loss of PTHrP or Pthr1 inhibits Lef1 and β-catenin expression selectively in the mammary mesenchyme (Dunbar and Wysolmerski, 1999). Moreover, overexpression of PTHrP in the epidermis results in ectopic upregulation of these molecules in the ventral mesenchyme (Foley et al., 2001). In this study, we use a Wnt-reporter mouse to demonstrate that PTHrP is required for canonical Wnt/β-catenin signaling in the mammary mesenchyme. We find that although Lef1 contributes to the actions of PTHrP on the mammary mesenchyme, it is not required to induce Wnt signaling. Finally, we show that mesenchymal β-catenin is required for Wnt-reporter activity and for the specification and function of the mammary mesenchyme.
MATERIALS AND METHODS
Mouse strains
K14-PTHrP and PTHrP−/− mice have been described previously (Wysolmerski et al., 1995; Dunbar et al., 1999). These were crossed to Wnt reporter mice, TOPGAL-C (Boras-Granic et al., 2006) or TOPGAL-F mice on a CD-1 background (DasGupta and Fuchs, 1999). The appearance of the vaginal plug was considered to be day 0 of gestation. Lef1−/−, Dermo1-cre and β-cateninlox/lox mice were obtained from Dr P. Hamel, Dr D. Ornitz and the Jackson Laboratories, respectively (van Genderen et al., 1994; Brault et al., 2001; Yu et al., 2003). tdTomato mice were obtained from Dr V. Greco (Yale University, New Haven, CT, USA). All experiments were approved by Yale University's Institutional Animal Care and Use Committee.
β-Galactosidase staining of whole embryos
Whole embryos or embryonic skin were fixed in 2% paraformaldehyde (PFA)/0.02% glutaraldehyde for 30 minutes (E13.5) or 1 hour (E15.5) at room temperature and stained overnight in 1 mg/ml 4-chloro-5-bromo-3-indoyl-β-d-galactopyranoside (X-gal), 2 mM MgCl2, 5 mM ethylene glycol tetra acetic acid, pH 8.0, 0.02% IGEPAL, 5 mM K3Fe(CN)6 and 5 mM K4Fe(CN)6.6H2O at 37°C for 12 hours. Tissues were rinsed in 1× PBS and post-fixed in 4% PFA at 4°C (Dunbar et al., 2001) Paraffin-embedded sections were counterstained with Eosin (Foley et al., 2001)
Histology and immunohistochemistry
Whole embryos were fixed in 4% PFA at 4°C for 12 hours. Ventral skins containing mammary buds were dissected and embedded. Mammary buds were identified by serial sectioning, as described previously (Dunbar et al., 1999). At least four separate buds from two mutant embryos were examined at E13.5. Immunohistochemistry was performed using standard techniques (Foley et al., 2001). Antigen retrieval was accomplished by heating sections in 7 mM or 10 mM citrate, under pressure. Sections were incubated overnight at 4°C with antibodies directed against ER (SC-56836, Santa Cruz Biotechnology, Santa Cruz, CA, USA), AR (PG-21, Millipore, Billerica, MA, USA), Lef1 (a kind gift from Dr R. Grosschedl, Max-Planck Institute of Immunobiology and Epigenetics, Freiburg, Germany), tenascin C (a kind gift from Dr T. Yoshida, Mie University School of Medicine, Japan), GFP (A10263, Invitrogen, Carlsbad, CA, USA), RFP (R10367, Invitrogen, Carlsbad, CA, USA), Ki-67 (MIB-5, DAKO, Carpinteria, CA, USA) or β-catenin (a kind gift from Dr D. Rimm, Yale University, New Haven, CT, USA). Staining was detected using Vector Elite ABC Kits (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine or NovaRED as chromogen (Vector Laboratories), or Alexa Fluor 488-conjugated goat anti-mouse, Alexa Fluor 555-conjugated goat anti-rabbit and streptavidin-conjugated Alexa Fluor 647 secondary antibodies (Invitrogen) for immunofluorescence. Analyses of mesenchyme marker expression and cre-mediated recombination were performed on serial sections from the same mammary bud.
Proliferation and TUNEL analysis
To detect proliferation, 13-day pregnant mice were injected with 100 μl of 5 mg/ml 5-ethynyl-2′-deoxyuridine (EdU) in saline per 10 g of mouse body weight, two hours before sacrifice. Embryos were fixed in 4% PFA for 4 hours or overnight. Paraffin-embedded sections were incubated with ‘Click It’ reagent at room temperature, according to manufacturer's instructions (C10339, Invitrogen). Fluorescence was detected using a Cy3 filter. EdU-positive and total cells were counted in three concentric layers of mammary mesenchyme adjacent to mammary buds from wild-type (WT) and Dermo1-cre;β-cateninlox/lox (n=8 buds, two embryos for each genotype) embryos. The percentage of proliferating mesenchymal cells was calculated and statistical significance was determined by performing Student's t-test. Tissues were processed for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) using the Roche Fluorescein Kit (11684795910, Roche, Indianapolis, IN, USA).
Measurement of hair follicle density, epidermal thickness and proliferation
Ventral skin was dissected from female E18.5 K14-PTHrP (n=4) and K14-PTHrP;Lef1−/− (n=3) embryos and fixed in 4% PFA overnight. Paraffin-embedded transverse sections (7 μm) were stained with Hematoxylin and Eosin. The 20× objective was used to count the number of hair follicles in six fields per slide, in three sections, at least 70 μm apart. To determine epidermal thickness, the sections were photographed using a Zeiss Axiovision microscope and analyzed using Axiovision v4.5 software. Epidermal thickness was measured at three equidistant points that were determined by overlaying the images with a grid, to ensure random sampling. Measurements were made from the bottom of the basal layer to the top of the granular layer along an axis perpendicular to the plane of the basal layer. Average epidermal thickness was calculated for each image and the significance of the results was determined by performing Student's t-test using Prism v4.0b software (GraphPad Software, La Jolla, CA, USA). Immunohistochemistry was performed in six sections per genotype, using the Ki67 antibody. Mammary mesenchyme marker expression occurs in the six topmost layers of the dermis of the ventral skin of K14-PTHrP mice, designated as the ectopic mammary mesenchyme. The average ratio of proliferating (Ki67-positive) cells to total cells was determined for each genotype and the significance was determined as above.
RNA isolation and RT-PCR
Mammary buds were harvested from female E15 WT (n=210 buds from 23 embryos) and PTHrP−/− (n=189 buds from 21 embryos) embryos and stored in RNAlater (Invitrogen). Ventral skins were dissected from female E15 WT and K14-PTHrP embryos, pooled into two groups (WT: n=7 embryos each group; K14-PTHrP: n=7, n=4) and stored in RNAlater. RNA was isolated from pooled samples using the Qiagen RNeasy Kit with QiaShredder column and purified by DNase1 digestion using the Message Clean Kit (GenHunter Corporation, Nashville, TN, USA). RNA integrity was confirmed on a non-denaturing 1% agarose gel, and 5 μg of RNA were used with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) to make cDNA. RT-PCR reactions were performed by assaying each pool in triplicate using the Full Velocity or Brilliant SYBR Green qPCR Kit (Agilent Technologies, Santa Clara, CA, USA) using the following primers: Rspo1F, 5′-CGACATGAACAAATGCATCA-3′; Rspo1R, 5′-CTCCTGACACTTGGTGCAGA-3′; WNT11F, 5′-CCACCATCAGTCACACCATC-3′; and WNT11R, 5′-TTTATTGGCTTGGGATCCTG-3′. Samples were normalized for relative quantification of expression by the 2−ΔΔCT method using Gapdh as an internal control (Hens et al., 2009). For each assay, gene expression in the WT samples was arbitrarily set to 1. Gene expression in mutant samples was calculated as the fold difference relative to WT. The significance of the results was determined by performing a one-tailed Student's t-test using Prism v4.0b software (GraphPad Software).
RESULTS
PTHrP activates mesenchymal Wnt/β-catenin signaling during mammary development
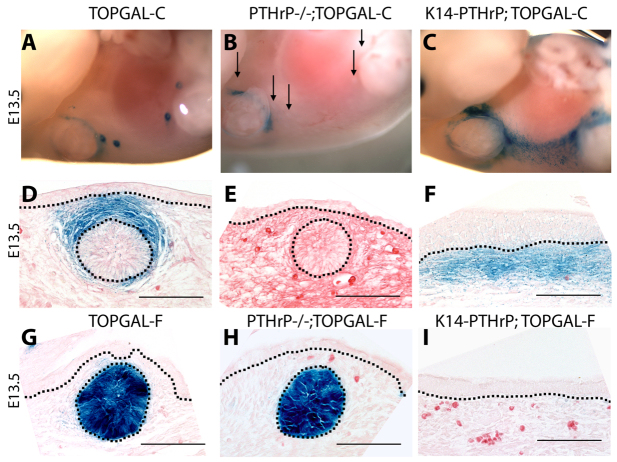
PTHrP signaling induces Lef1 and β-catenin expression in the mammary mesenchyme, suggesting that PTHrP might modulate canonical Wnt signaling in these cells (Dunbar et al., 1999). To examine whether PTHrP directly regulates Wnt signaling in vivo, we utilized a Wnt-sensitive transgene (TOPGAL-C) consisting of three TCF/β-catenin sites driving expression of bacterial β-galactosidase (Cheon et al., 2002). Staining of the tissues from these reporter mice for β-galactosidase activity using X-gal produces a blue color in the presence of active canonical Wnt signaling and a prior study using TOPGAL-C mice demonstrated Wnt signaling specifically in the mammary mesenchyme (Boras-Granic et al., 2006). In agreement with this previous work, we found that in E13.5 TOPGAL-C embryos, X-gal staining was observed in the mammary mesenchyme, but not in the epithelial cells within the bud (Boras-Granic et al., 2006) (Fig. 1A,D). By contrast, X-gal staining was not observed in the mammary mesenchyme of PTHrP−/−;TOPGAL-C embryos, indicating that PTHrP is required to activate mesenchymal Wnt reporter gene expression (Fig. 1B,E). Histological sections confirmed that TOPGAL-C activity was limited to the mammary mesenchyme in WT buds (Fig. 1D) but was lost from these cells when the Pthlh gene is disrupted (Fig. 1E). We also generated K14-PTHrP;TOPGAL-C embryos, to determine whether ectopic PTHrP overexpression could induce canonical Wnt signaling in the ventral dermal mesenchyme. As shown in Fig. 1C, K14-PTHrP;TOPGAL-C embryos had a zone of ectopic TOPGAL-C activity running in an arc from the first to the fifth mammary bud in addition to the TOPGAL-C activity that is normally present around the developing buds. As development progresses, this zone of ectopic Wnt activity broadens and spreads medially to encompass much of the ventral-lateral surface of the embryo (Fig. 2C). Histological sections of these embryos demonstrate that this ectopic Wnt activity is restricted to the mesenchymal cells just beneath the epidermis (Fig. 1F), the same cells previously shown to express ectopic mammary mesenchyme markers in these mice (Foley et al., 2001). These data confirm that PTHrP modulates canonical Wnt activity in mammary mesenchyme cells.
Fig. 1.
PTHrP activates Wnt signaling in mouse mammary mesenchyme in vivo. (A-F) X-gal staining of wholemounts (A-C) and sections (D-F) of TOPGAL-C (A,D), PTHrP−/−;TOPGAL-C (B,E) and K14-PTHrP;TOPGAL-C (C,F) embryos at E13.5. Note reporter expression in the mammary mesenchyme surrounding the epithelial buds in TOPGAL-C embryos (A,D), but loss of Wnt reporter expression in the mammary mesenchyme when the Pthlh gene is disrupted (B,E). Black arrows point to mammary buds. Epidermal overexpression of PTHrP in K14-PTHrP mice induces ectopic mesenchymal Wnt reporter activity in the ventral dermal mesenchyme (C,F). (G-I) X-gal-stained sections of TOPGAL-F (G), PTHRP−/−;TOPGAL-F (H) and K14-PTHRP;TOPGAL-F (I) embryos demonstrating Wnt reporter activity in the mammary epithelium (G) that is unaffected by loss of PTHrP (H). Epidermal overexpression of PTHrP in K14-PTHrP mice does not induce ectopic β-galactosidase activity using the TOPGAL-F reporter. Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
Fig. 2.
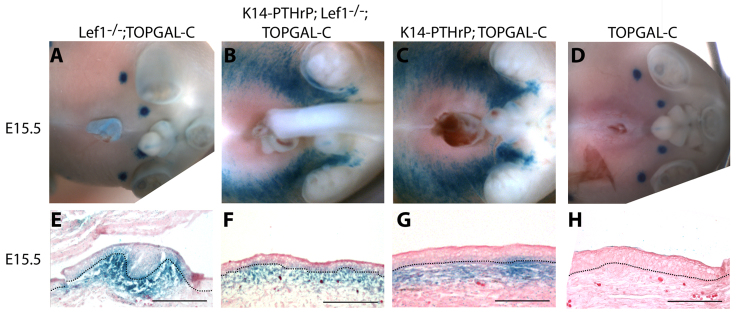
Lef1 is not required for Wnt signaling in mouse mammary mesenchyme. (A,E) X-gal staining of Lef1−/−;TOPGAL-C embryos demonstrating normal Wnt signaling in the mammary mesenchyme in buds 4 and 5 at E15.5. (B,F) Loss of Lef1 does not alter the ectopic Wnt signaling that is induced by PTHrP-overexpression. (C,G) Ectopic Wnt signaling induced by epidermal overexpression of PTHrP in K14-PTHrP mice at E15.5; for comparison with B and F. (D,H) Ectopic Wnt reporter activity is not observed in the ventral skin of TOPGAL-C embryos. Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
Previously, we had used another TOPGAL transgenic mouse (TOPGAL-F), generated in the Fuchs laboratory, to demonstrate the importance of epithelial Wnt activity for embryonic mammary development (Chu et al., 2004). As demonstrated by Chu and colleagues, TOPGAL-F reports Wnt activity in the mammary bud epithelium (Fig. 1G) (Chu et al., 2004). It is not clear why TOPGAL-C and TOPGAL-F mice have different patterns of β-galactosidase activity (Barolo, 2006), but we took advantage of this different expression pattern to determine whether loss of PTHrP signaling affected epithelial Wnt activity. As shown in Fig. 1G,H, PTHrP−/−;TOPGAL-F embryos retained epithelial Wnt activity. Moreover, overexpression of PTHrP in the basal epidermis in K14-PTHrP mice, did not affect epithelial Wnt reporter activity (Fig. 1I). Therefore, PTHrP signaling is necessary for mesenchymal but not epithelial Wnt/β-catenin activity.
PTHrP-induced mesenchymal Wnt signaling is independent of Lef1
PTHrP signaling induces Lef1 expression specifically in the mammary mesenchyme and, given that Lef1 participates in canonical Wnt signaling, we investigated whether Lef1 is required for PTHrP-induced Wnt signaling. In order to examine this, we bred the K14-PTHrP and TOPGAL-C transgenes onto an Lef1−/− background and performed X-gal staining on Lef1−/−:TOPGAL-C embryos. The second and third pairs of mammary buds do not form in the absence of Lef1, so we focused our studies on the fourth and fifth pairs of buds, which are present in the developing embryo but degenerate before birth (van Genderen et al., 1994). Despite the loss of Lef1, TOPGAL-C reporter activity was identical to that seen in control TOPGAL-C embryos (compare Fig. 2A and 2D) (van Genderen et al., 1994; Boras-Granic et al., 2006). We also examined whether loss of Lef1 would abolish the ability of PTHrP to induce ectopic TOPGAL-C activity in K14-PTHrP;Lef1−/−;TOPGAL-C embryonic skin. As shown in Fig. 2B, X-gal staining was essentially the same as in K14-PTHrP;TOPGAL-C mice (Fig. 2C). Histological analyses indicated that Wnt activity was restricted to the ventral ectopic mesenchyme, as expected (Fig. 2F,G). Wnt reporter activity was not observed in the ventral dermal mesenchyme of TOPGAL-C mice (Fig. 2D,H), which do not overexpress PTHrP in basal keratinocytes. Together, these studies demonstrate that Lef1 is not required to activate mesenchymal Wnt signaling downstream of PTHrP.
Lef1 partially mediates the actions of PTHrP on mammary mesenchyme
We next investigated whether Lef1 was necessary for PTHrP to specify the mammary mesenchyme even though it was not necessary for PTHrP to activate canonical Wnt signaling. We first examined the expression of a panel of molecular markers of the differentiated mammary mesenchyme, including Lef1, AR, β-catenin and ER in WT, PTHrP−/− and Lef−/− mammary buds at E13. As shown in Fig. 3B,E,H,K, the expression of each of these markers was reduced in the mesenchymal cells surrounding the PTHrP−/− mammary buds compared with WT buds (Fig. 3A,D,G,J). Loss of Lef1 expression only partially phenocopied loss of PTHrP. As expected, Lef1 staining was absent from both the epithelial and mesenchymal cells in the Lef1−/− buds (Fig. 3C). AR staining was reduced in the mesenchyme around Lef1−/− buds, but not to the same degree as it was reduced in PTHrP−/− buds (Fig. 3F). Although expression of β-catenin and ER was significantly reduced in the mammary mesenchyme of PTHrP−/− buds (Fig. 3H,K), these markers appeared to be expressed at similar levels in Lef1−/− and WT mammary buds (Fig. 3I,L). We also examined the ectopic expression of mammary mesenchyme markers in the ventral skin of K14-PTHrP;Lef1−/− embryos compared with WT and K14-PTHrP embryos. As with the results in the mesenchyme surrounding the mammary buds, we observed that loss of Lef1 resulted in a reduction in β-catenin (Fig. 4C), tenascin C (Fig. 4F) and AR (Fig. 4I) staining in K14-PTHrP;Lef1−/− ventral skin compared with K14-PTHrP ventral skin (Fig. 4B,E,H). Expression of ER (Fig. 4L) also appeared to be reduced in the K14-PTHrP;Lef1−/− ventral skin although not to the same degree as the other markers.
Fig. 3.
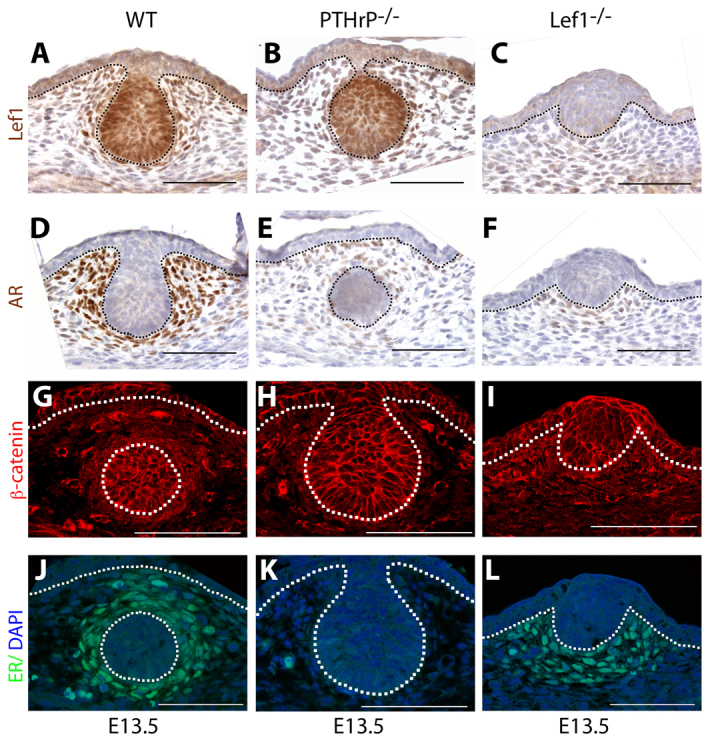
Loss of Lef1 partially phenocopies the loss of PTHrP. (A,D,G,J) In WT mice, Lef1 (A), AR (D), β-catenin (G) and ER (J) are expressed in the mammary mesenchyme. (B,E,H,K) Loss of PTHrP results in reduced expression of all the mesenchyme markers tested. (C,F,I,L) By comparison, loss of Lef1 results in complete loss of Lef1 protein expression (C) and reduced mesenchymal expression of AR (F). By contrast, β-catenin (I) and ER (L) are still expressed in the mammary mesenchyme even in the absence of Lef1. Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
Fig. 4.
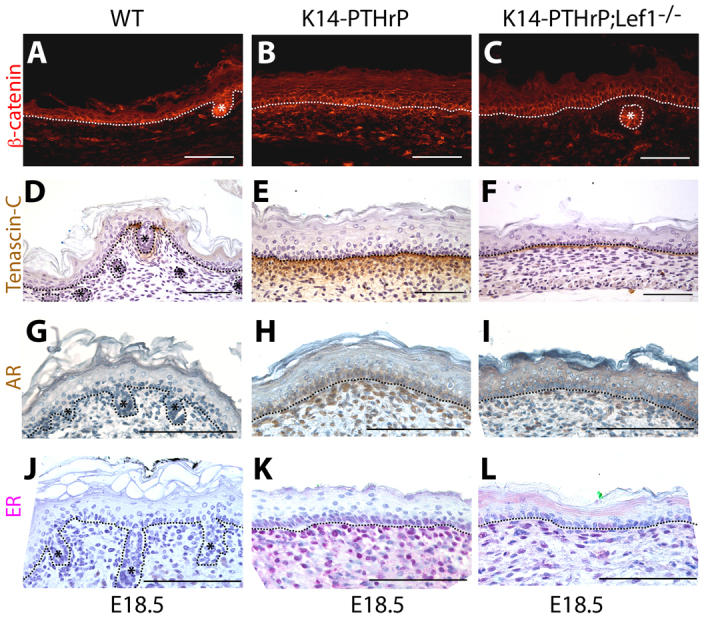
Loss of Lef1 affects expression of mesenchymal markers in the ectopic mammary mesenchyme induced by overexpression of PTHrP. (A-L) Immunostaining for β-catenin (A-C), tenascin C (D-F), AR (G-I) and ER (J-L) on sections of ventral skin from E18.5 WT (n=3; A,D,G,J), K14-PTHrP (n=4; B,E,H,K) and K14-PTHrP; Lef1−/− (n=3; C,F,I,L) mice. Note that overexpression of PTHrP induces the ectopic expression of β-catenin, tenascin C, AR and ER in the ventral dermis. Asterisks denote hair follicles. Dotted lines delineate the dermo-epidermal junction. Scale bars: 100 μm.
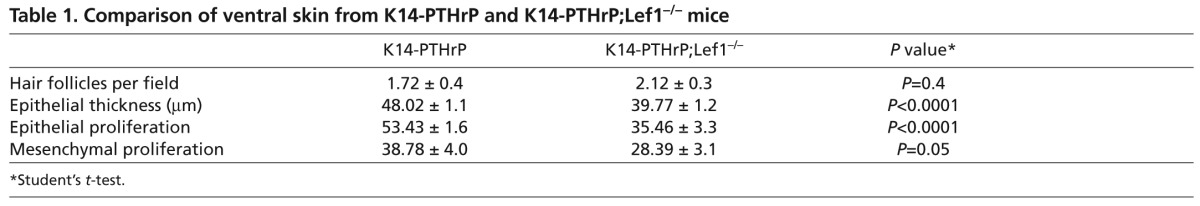
The ventral epidermis in K14-PTHrP mice takes on characteristics of nipple skin such as an increase in epidermal thickness and a suppression of hair follicle development (Foley et al., 2001). Although loss of Lef1 expression did not result in the return of hair follicles in K14-PTHrP;Lef1−/− embryos compared with K14-PTHrP embryos, we did note a significant reduction in epidermal thickness (Table 1). This was associated with a reduction in both epithelial and mesenchymal cell proliferation (Table 1), but no change in apoptosis (data not shown). Taken together, these data demonstrate that Lef1 contributes to the differentiation of the mammary-specific mesenchyme as well as nipple skin differentiation downstream of PTHrP signaling.
Table 1.
Comparison of ventral skin from K14-PTHrP and K14-PTHrP;Lef1−/− mice
Wnt11 and Rspo1 might mediate the actions of PTHrP on mesenchymal Wnt signaling
A previous study characterizing Wnt family gene expression in the embryonic mammary bud had shown that Wnt11 expression was specific to the mammary mesenchyme (Chu et al., 2004). In addition, a prior gene array study suggested that PTHrP might regulate the expression of Rspo1 in the mammary mesenchyme (Hens et al., 2009). Rspo1 is a member of the R-spondin superfamily of proteins that have been shown to enhance Wnt signaling at sites of native Wnt expression. Friedman and colleagues have shown that R-spondin 2 can amplify canonical Wnt signaling induced by Wnt11 in osteoblasts (Friedman et al., 2009). For these reasons, we wondered whether PTHrP might enhance mesenchymal Wnt signaling by increasing the expression of Wnt11 and/or Rspo1. Therefore, we compared expression of Wnt11 and Rspo1 in the ventral skin of E15 WT and K14-PTHrP embryos and in mammary buds from WT and PTHrP−/− embryos. Our results show that expression of both Rspo1 and Wnt11 is significantly increased in K14-PTHrP compared with WT ventral skin (Fig. 5A,C) and is reduced in PTHrP−/− mammary buds compared with their WT counterparts (Fig. 5B,D). Therefore, Wnt11 and Rspo1 are regulated by PTHrP in the mammary mesenchyme in vivo, providing a potential mechanism through which PTHrP might activate canonical Wnt signaling in these cells.
Fig. 5.
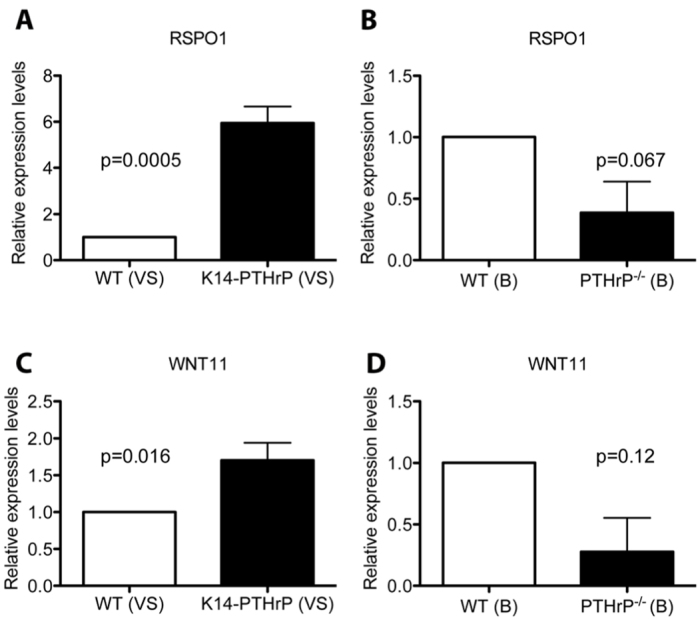
PTHrP regulates expression of Wnt11 and Rspo1 in the mammary mesenchyme. (A-D) Real-time quantitative PCR demonstrating a sixfold increase in Rspo1 (A) and a 1.7-fold increase in Wnt11 expression (C) in cDNA obtained from ventral skins (VS) of E15 WT and K14-PTHrP embryos. Reduced expression of Rspo1 (B) and Wnt11 (D) is observed in mammary buds from PTHrP−/− (KO) embryos compared with their WT counterparts. Gene expression normalized to Gapdh and fold expression changes normalized to WT.
Mesenchymal β-catenin is required for Wnt reporter activity in the mammary mesenchyme
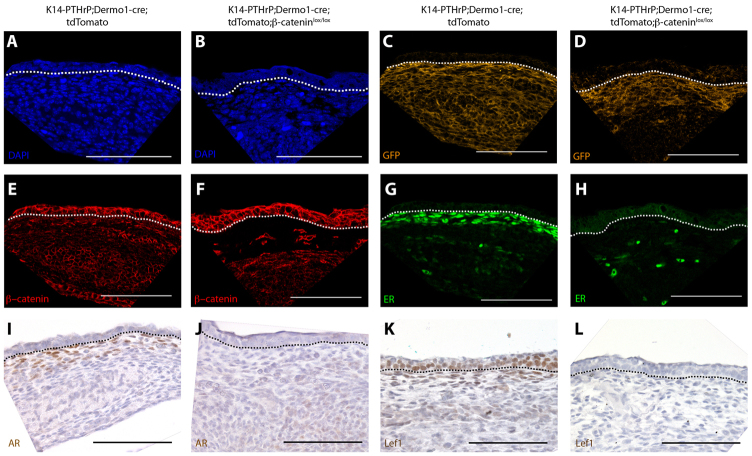
In order to determine whether canonical Wnt signaling mediates the effects of PTHrP on the mammary mesenchyme, we deleted β-catenin specifically in the mammary stromal compartment using Dermo1-cre knock-in mice. Yu and colleagues have previously shown that the Dermo1 (Twist2) promoter directed cre recombinase expression in many condensing mesenchymes during development (Yu et al., 2003). Additionally, De Langhe and colleagues demonstrated that Dermo1-cre;β-cateninlox/lox embryos had widespread developmental defects including syndactyly, failure of ventral body wall closure and altered lung development that led to embryonic lethality (De Langhe et al., 2008). However, neither group specifically examined the mammary mesenchyme. Therefore, we first crossed Dermo1-cre mice to R26R reporter mice and examined cre recombinase activity by X-gal staining of embryos between stages E10.5 and E15.5. X-gal staining was detected in the mammary mesenchyme of Dermo1-cre;R26R embryos as early at E11.5 and it became more prominent between E12.5 and E15.5 (Fig. 6A, arrows). Histological examination revealed X-gal staining only in the mesenchymal cells and not in the epithelial cells in the developing mammary buds (Fig. 6B, arrows). However, Dermo1-cre activity was not specific to the mammary buds and, as previously reported, we also observed X-gal staining in other sites such as the developing skeleton and vasculature (data not shown). We performed similar experiments using the tdTomato reporter mouse at E13.5. In these mice, all cells express the red fluorescent tdTomato gene. However, when cre recombinase is active, expression of the tdTomato reporter is silenced and expression of a GFP transgene is activated. As shown in Fig. 6D, in mammary buds, GFP expression was limited to the condensed mesenchyme in Dermo1-cre;tdTomato embryos. These data suggested that breeding the Dermo1-cre transgene onto a ‘floxed’ β-catenin background would result in removal of β-catenin from mesenchymal cells, but not epithelial cells, in the mammary buds.
Fig. 6.
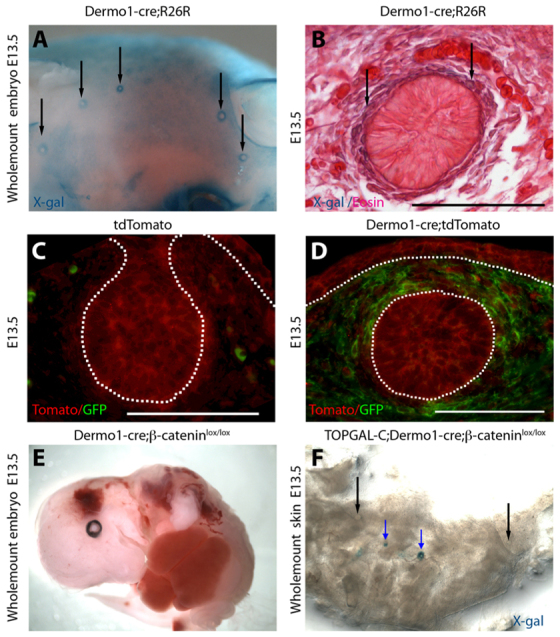
Dermo1-cre directs recombination in mouse mammary mesenchyme. (A,B) X-gal staining of a wholemount (A) and section (B) of a mammary bud from an E13.5 Dermo1-cre;R26R embryo demonstrating lacZ reporter expression in the developing dermis and in mammary mesenchyme (arrows) but not in the mammary epithelium. (C,D) Cre activity as determined by immunofluorescence staining for GFP and RFP expression in mammary buds from E13.5 tdTomato (C) and Dermo1-cre;tdTomato (D) mice. Cre activity induces expression of the GFP reporter (D). (E) Wholemount of an E13.5 Dermo1-cre;β-cateninlox/lox embryo showing multiple deformities including the ventral body wall defect. (F) Expression of the Wnt reporter TOPGAL-C in an E13.5 TOPGAL-C;Dermo1-cre;β-cateninlox/lox embryo showing variable retention of reporter activity. Some buds are completely devoid of Wnt signaling (black arrows) whereas others retain some Wnt-reporter activity (blue arrows). Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
We generated TOPGAL-C;Dermo1-cre;β-cateninlox/lox mice to determine whether loss of mesenchymal β-catenin would ablate TOPGAL-C activity. As expected, in TOPGAL-C;Dermo1-cre;β-cateninlox/lox embryos, Wnt reporter activity, as assessed by X-gal staining, was absent in the mammary mesenchyme (Fig. 6F, black arrows). However, loss of TOPGAL-C expression was not uniform and some mammary buds in these embryos retained Wnt reporter activity (Fig. 6F, blue arrows). The variability in Wnt reporter activity correlated with variability in loss of β-catenin expression (Fig. 7; data not shown). We also examined expression of TOPGAL-F in Dermo1-cre;β-cateninlox/lox mice and found that epithelial Wnt signaling was unaffected by loss of mesenchymal β-catenin (supplementary material Fig. S1).
Fig. 7.
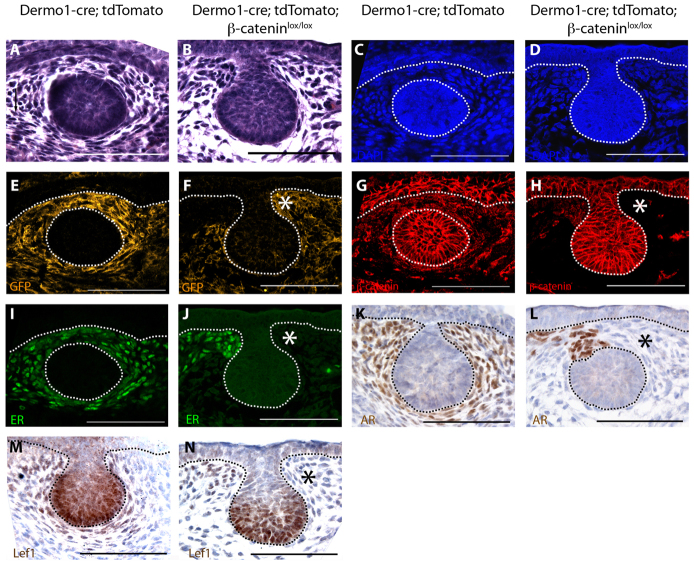
Effects of β-catenin deletion on mouse mammary mesenchyme. (A-N) Histology (A,B), DAPI staining to demonstrate muclei (C,D), efficiency of cre-mediated recombination (E,F) and analyses of mesenchyme marker expression (G-N) in a representative mammary bud from a Dermo1-cre;tdTomato (A,C,E,G,I,K,M) and a Dermo1-cre;tdTomato;β-cateninlox/lox (B,D,F,H,J,L,N) embryo at E13.5. Note paucity and lack of organization in the mesenchyme in B compared with compacted, concentrically arranged fibroblasts in A. Also note mosaic expression of Dermo1-cre as demonstrated by the GFP reporter (F) and corresponding reduction of β-catenin (H) and all tested mesenchyme markers (J,L,N) in the regions in which Dermo1-cre efficiently deletes β-catenin (asterisks). Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
Loss of β-catenin inhibits the development and function of the mammary mesenchyme
Given the variability in loss of β-catenin within the mesenchyme of Dermo1-cre;β-cateninlox/lox mammary buds (Fig. 6F), we restricted further analysis to buds in which we first documented cre activity by the switch from Tomato to GFP expression and loss of β-catenin protein by immunofluorescence. Consistent with previous reports, Dermo1-cre;tdTomatoβ-cateninlox/lox mice had a spectrum of developmental defects that led to death between E13.5 and E15.5, further restricting our analysis to early time points in embryonic mammary development. In control Dermo1-Cre;tdTomato buds at E13.5, the mammary mesenchyme consists of three to five layers of compacted fibroblast-like cells that are arranged around the mammary epithelial cells (Fig. 7A). However, in the absence of β-catenin, the mammary mesenchyme consisted of fewer layers of cells with more rounded nuclei, suggesting incomplete compaction (Fig. 7B). In addition to their typical shape, mammary mesenchyme cells are characterized by the expression of specific molecular markers. In control mice, ER and AR are expressed exclusively in the mesenchyme (Fig. 7I,K), whereas β-catenin and Lef1 are expressed in both the bud epithelium and the mammary mesenchyme (Fig. 7G,M). In Dermo1-cre;tdTomato β-cateninlox/lox embryos, loss of mesenchymal β-catenin (Fig. 7H) correlated with reduction of both ER and AR (Fig. 7J,L). Moreover, Lef1 expression was reduced selectively in the mammary mesenchyme but not in the mammary epithelium (Fig. 7N). These changes were seen only in cells in which expression of GFP signified active cre expression and recombination (Fig. 7F), suggesting that loss of β-catenin had cell-autonomous effects on the expression of mammary mesenchyme markers. We could not assess the expression of tenascin C, as its expression is extremely variable in WT mammary buds before E15 and the Dermo1-cre;tdTomato;β-cateninlox/lox embryos that survived until E15 had less efficient deletion of β-catenin in the mammary mesenchyme. These data demonstrate that β-catenin is crucial for the proper differentiation of the mammary mesenchyme.
In these experiments, we noted that expression of ER was a very sensitive indicator of the loss of β-catenin expression and the activation of GFP expression in Dermo1-cre; tdTomato;β-cateninlox/lox embryos. Therefore in subsequent experiments, we used the presence or absence of ER expression as an indicator of efficient β-catenin deletion in Dermo1-cre;β-cateninlox/lox mice. We investigated next whether the reduction in the numbers of mesenchymal cells in buds from Dermo1-cre;β-cateninlox/lox embryos was a result of decreased proliferation or increased apoptosis. We enumerated dividing mesenchymal cells by measuring EdU (a bromodeoxyuridine analog) incorporation in buds with partial or complete loss of mesenchymal ER expression. Compared with control embryos (35.31±3.76%) proliferation was significantly reduced (P=0.01) in the mammary mesenchyme of Dermo1-cre;β-cateninlox/lox embryos (23.46±3.59%) (Fig. 8). Using a TUNEL assay, we observed almost no apoptosis in either epithelial or mesenchymal cells of mammary buds from Dermo1-cre;β-cateninlox/lox embryos or their control littermates. These data demonstrate that β-catenin is required for the proliferation of cells comprising the mammary mesenchyme.
Fig. 8.
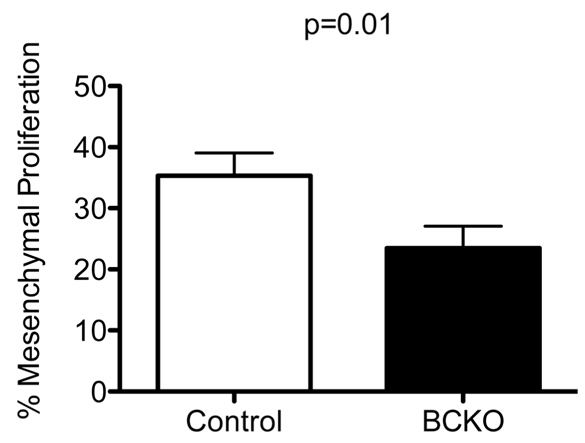
Loss of β-catenin impairs mesenchymal proliferation. Percentage of proliferating mesenchymal cells was significantly reduced in mammary buds from E13.5 Dermo1-cre;β-cateninlox/lox (BCKO, black bar) embryos compared with control (white bar) embryos. Student's t-test, P=0.01. Error bars represent s.e.m.
One test of the biological function of the mammary mesenchyme is its ability to pinch off the mammary rudiment beneath the epidermis in male mice. Given the loss of AR expression in the mammary mesenchyme of Dermo1-cre;β-cateninlox/lox mammary buds, we examined whether the androgen-dependent male response was impaired. We examined the histology of male WT and Dermo1-cre;β-cateninlox/lox embryos at E14.5. As seen in the WT embryos, the typical ‘male’ phenotype was defined by condensation of the mammary mesenchyme around the stalk of the bud leading to its obliteration (Fig. 9A). This is accompanied by widespread apoptosis within the condensed mesenchyme and some apoptosis within the mammary epithelium as well (Fig. 9C). However, in male E14.5 Dermo1-cre;β-cateninlox/lox embryos the buds retained a stalk connecting them to the overlying epidermis (Fig. 9B) and lacked apoptosis (Fig. 9D), demonstrating impaired biological function of the mammary mesenchyme. These data support the conclusion that β-catenin signaling is required for mammary mesenchyme function.
Fig. 9.
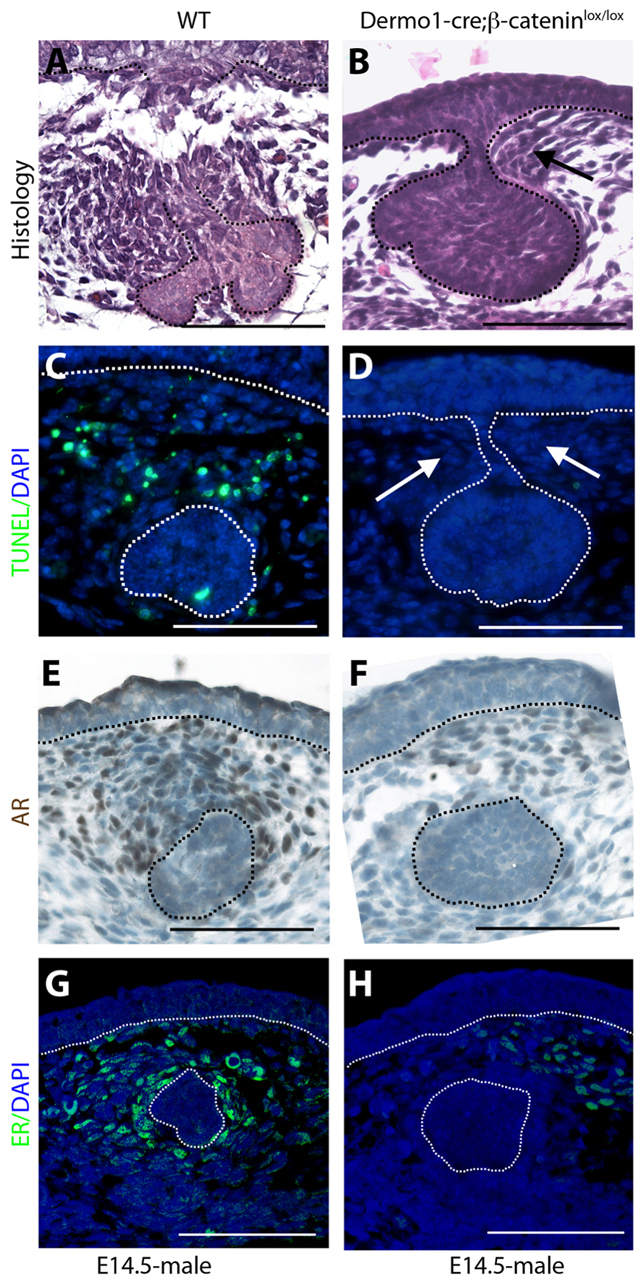
Loss of mesenchymal β-catenin impairs sexual dimorphism. (A-H) Histology (A,B), TUNEL staining (C,D) and immunohistochemistry for AR (E,F) and ER (G,H) on mammary buds from E14 male WT (A,C,E,G) and Dermo1-cre;β-cateninlox/lox (B,D,F,H) embryos. Note that although some mammary mesenchyme is present in Dermo1-cre;β-cateninlox/lox (B, arrow), the bud remains connected to the epidermis via a stalk, there is a lack of mesenchymal apoptosis (D, white arrows) and reduction in AR and ER staining (F,H). Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
Overexpression of PTHrP in K14-PTHrP transgenic mice has been shown to induce the ectopic expression of mammary mesenchyme markers in the ventral dermal mesenchyme. We generated K14-PTHrP;Dermo1-cre;tdTomato;β-cateninlox/lox embryos in order to determine whether β-catenin was necessary for the induction of ectopic mammary mesenchyme. First, we observed that the overexpression of PTHrP in K14-PTHrP;Dermo1-cre;tdTomato embryos was associated with induction of Dermo1-Cre expression in the ectopic mammary mesenchyme (Fig. 10C). As shown in Fig. 10E,G,I,K, E13 K14-PTHrP;Dermo1-cre;tdTomato control embryos had ectopic expression of β-catenin, ER, AR and Lef1 in the ventral mesenchyme. By contrast, loss of β-catenin in K14-PTHrP;Dermo1-Cre;tdTomato;β-cateninlox/lox embryos blocked the ectopic expression of these markers (Fig. 10F,H,J,L). These data demonstrate that β-catenin signaling is required for PTHrP to induce the differentiation of ectopic mammary mesenchyme.
Fig. 10.
β-catenin is required for K14-PTHrP-mediated ectopic mammary mesenchyme specification. (A-L) Ventral skin harvested from E13.5 K14-PTHrP;Dermo1-cre;tdTomato (A,C,E,G,I,K) and K14-PTHrP;Dermo1-cre;tdTomato;β-cateninlox/lox (B,D,F,H,J,L) embryos was examined by DAPI staining (A,B), immunofluorescence staining for expression of GFP (C,D), β-catenin (E,F), ER (G,H) and immunohistochemistry for expression of AR (I,J) and Lef1 (K,L). Ectopic expression of markers is visible beneath the ventral epidermis in PTHrP-overexpressing mice (E,G,I,K) but is reduced when β-catenin is conditionally deleted in the ectopic mesenchyme via Dermo1-cre expression (F,H,J,L). Dotted lines delineate the dermo-epidermal junction and the mammary bud. Scale bars: 100 μm.
DISCUSSION
Our results demonstrate that canonical Wnt/β-catenin signaling is activated in the condensing mesenchyme during the formation of the embryonic mammary buds. Mesenchymal Wnt signaling requires the actions of PTHrP and β-catenin, but not Lef1, and contributes to proper mammary mesenchyme proliferation, differentiation and function. Ablation of mesenchymal β-catenin phenocopies critical elements of the loss of PTHrP and the Pthr1, and blocks the effects of PTHrP overexpression. Our previous work has documented that PTHrP is secreted from mammary epithelial cells as soon as the mammary placode begins to form. It acts on its receptor (Pthr1) expressed on the mammary mesenchyme to trigger the full differentiation of these cells (Wysolmerski et al., 1995; Wansbury et al., 2011). In response, these cells support the mammary fate of the epithelial cells, induce nipple formation and promote the outgrowth of the primary mammary duct from the bud. Our current results demonstrate that PTHrP regulates mammary mesenchyme differentiation and function, in part, by activating Wnt signaling specifically within mesenchymal cells.
Previous studies have documented the importance of the canonical Wnt pathway to the formation of mammary placodes and the development of the embryonic mammary duct system. Work from our laboratory and others' has shown that multiple Wnts, Wnt receptors and Wnt modulators/inhibitors are expressed by the epithelial and/or mesenchymal cells of the mammary bud (Boras-Granic et al., 2006; Chu et al., 2004; Gu et al., 2009). In addition, transgenic Wnt reporter genes have demonstrated active Wnt signaling in both epithelial cells (TOPGAL-F, BATGAL and Axin2 reporters) and mesenchymal cells (TOPGAL-C and Axin2 reporters) (Chu et al., 2004; Boras-Granic et al., 2006) (M.H., unpublished observations). Genetic manipulations have demonstrated that canonical Wnt signaling is necessary for the formation of mammary placodes, the normal outgrowth of the primary duct system, the proper development of the mammary fat pad and the establishment/maintenance of mammary epithelial progenitor cells (Gu et al., 2009; Lindvall et al., 2009). These genetic experiments have either targeted epithelial cells or have targeted both cell types and little attention has been paid to the relative contribution of epithelial versus mesenchymal Wnt signaling to the resulting phenotypes. Our data demonstrate that Wnt signaling is activated in the condensing mesenchyme in response to PTHrP, which is secreted from the epithelium. Furthermore, activation of Wnt signaling in mesenchymal cells is necessary for them to differentiate fully in response to PTHrP signaling. Loss of β-catenin only in the mammary mesenchyme results in a mammary bud with fewer surrounding mesenchymal cells. This appears to be the result of reduced proliferation of the mesenchymal cells and not an increased rate of apoptosis. In addition, loss of β-catenin inhibits ER, AR and Lef1 expression, all of which normally respond to PTHrP signaling. Deletion of β-catenin using Dermo1-Cre causes significant embryonic mortality between E13 and E16. Therefore, we were unable to examine bud outgrowth and formation of the primary duct system. Instead, we used sexual dimorphism as a measure of the functional capacity of the mammary mesenchyme. Loss of mesenchymal β-catenin interfered with the ability of the mesenchyme to support the androgen-mediated destruction of the mammary buds in male embryos. Therefore, we conclude that Wnt signaling acts downstream of PTHrP to stimulate mesenchymal cell proliferation, differentiation and function.
Epistasis experiments suggest that Lef1 is not necessary for PTHrP to activate Wnt signaling but, rather, acts downstream of Wnt/β-catenin signaling to mediate some, but not all, of the actions of PTHrP on the mammary mesenchyme. This role of Lef1 is consistent with the fact that loss of Lef1 reverts some aspects of the ventral nipple-skin of K14-PTHrP mice, but does not lead to a full return to the WT state. Lef1 might be part of a Wnt auto-amplification loop in the mammary mesenchyme, as it is known to play this role in numerous other systems, including hair follicles and sebaceous glands (DasGupta et al., 2002; Niemann et al., 2002). Nevertheless, our data also demonstrate differential requirements for Lef1 and β-catenin in stimulating expression of specific mammary mesenchyme markers. For example, both β-catenin and Lef1 modulate AR expression, whereas ER regulation appears to be independent of Lef1. Interestingly, Lef1 is known to drive AR expression in prostate tissue and contributes to the formation of androgen-independent prostate cancer (Li et al., 2009). By contrast, Lef1 is an ER target in the uterus and is upregulated in response to estradiol treatment and estradiol-induced epithelial-mesenchymal transition (Eger et al., 2000; Ray et al., 2008).
Interactions between Pthr1 and Wnt signaling have been previously noted in bone cells and vascular smooth muscle. Treatment of osteoblasts with Pth or PTHrP has been shown to activate Wnt reporter genes and regulate the expression of Wnt ligands, Frizzled proteins, Lrp5, Lrp6 and Wnt antagonists, such as Dkk1 and sclerostin (Kulkarni et al., 2005; Portal-Núñez et al., 2010). Studies in MC3T3E1 preostoblastic cells have suggested that Pthr1 signaling enhances the activation of canonical Wnt signaling through protein kinase A (PKA)-mediated phosphorylation of β-catenin (Tian et al., 2011). Recent studies have also suggested that Pthr1 can activate downstream β-catenin signaling in a Wnt ligand-independent fashion. Wan and colleagues demonstrated direct interactions between Pthr1 and Lrp6 that lead to the recruitment of the Gs α subunit to Pthr1, PKA-mediated phosphorylation of Lrp6, recruitment of Axin to the Pthr1-Lrp6 complex and activation of β-catenin signaling (Wan et al., 2008; Wan et al., 2011). In addition, Romero and colleagues have shown that, upon activation, Pthr1 can recruit dishevelled and initiate downstream β-catenin signaling in a Wnt- and Lrp-independent fashion (Romero et al., 2010). Importantly, similar to our data in the mammary mesenchyme, inhibition of Wnt signaling has been shown to inhibit some functions of Pth in osteoblasts both in vitro and in vivo (Shi et al., 2011). In contrast to the data in osteoblasts and the mammary mesenchyme, Cheng and colleagues have shown that activation of the Pthr1 in vascular smooth muscle cells blocks Wnt/β-catenin signaling in a cell-autonomous fashion (Cheng et al., 2010). This appears to be important for Pth to inhibit vascular calcification in a model of diabetic vasculopathy. Although the details may vary with cellular context, collectively, the experiments in osteoblasts, vascular smooth muscle cells and our results in mammary mesenchyme cells suggest that Wnt and Pthr1 signaling are likely to be intertwined in many different cell types and that β-catenin signaling is broadly involved in mediating the biological effects of Pth and PTHrP.
The molecular mechanisms by which PTHrP activates β-catenin signaling in the condensing mammary mesenchyme remain unclear but might involve autocrine or paracrine activation of the canonical Wnt pathway. Our results demonstrate upregulation of both Rspo1 and Wnt11 in response to PTHrP. Previous studies have demonstrated that both of these genes are expressed specifically by mesenchymal cells in the embryonic mammary buds (Chu et al., 2004; Nam et al., 2007). R-spondin proteins potentiate canonical Wnt signaling by interacting with Kremen to prevent its binding with Dickkopf, allowing Lrp to bind with Wnts and Frizzleds instead (Kim et al., 2006; Binnerts et al., 2007; Kim et al., 2008). Although Wnt11 is often considered a ‘non-canonical’ Wnt ligand, it can activate the canonical pathway as well (Gong et al., 2004; Cha et al., 2008; Cha et al., 2009; Friedman et al., 2009). In fact, in osteoblasts, Pth upregulates R-spondin 2 expression, which facilitates canonical Wnt signaling by Wnt11 (Friedman et al., 2009). Therefore, it is possible that PTHrP activates Wnt signaling in the mammary bud through the upregulation of Wnt11 and Rspo1 in the mammary mesenchyme. In addition, it is possible that the activation of Wnt signaling in the mammary mesenchyme by PTHrP involves Lrp6. Although disruption of either Lrp5 or Lrp6 impairs mammary ductal development, the phenotype of the Lrp6−/− mice is more severe, demonstrating minimal ductal outgrowth from the mammary buds (Lindvall et al., 2006; Lindvall et al., 2009). This phenotype is reminiscent of the failure of bud outgrowth in PTHrP−/− and Pthr1−/− mice, although differentiation of the mammary mesenchyme was not assessed in Lrp6−/− mice. Further genetic studies will be required to define the exact mechanisms by which Pthr1 activates β-catenin signaling in the mammary mesenchyme in vivo and to determine whether it interacts with Lrp6 in either a Wnt-dependent or Wnt-independent fashion.
In summary, we have established that mesenchymal β-catenin signaling is crucial for the normal development of the embryonic mammary bud and for the expression of key molecular markers of the mammary mesenchyme. Furthermore, activation of Wnt/β-catenin signaling in these cells is downstream of the PTHrP/Pthr1 signaling pathway. We propose a working model (Fig. 11) in which PTHrP secreted from the mammary epithelium activates Wnt/β-catenin signaling via its receptor in the mammary mesenchyme. This results in Wnt-reporter activity accompanied by expression of Rspo1 and Wnt11, as well as the expression of ER that defines the early mammary mesenchyme. Subsequent Wnt signaling appears to drive mesenchymal Lef1 expression, which contributes to amplification of the Wnt signal, the expression of other mesenchyme markers such as AR and the acquisition of crucial mammary mesenchyme functions necessary for further development of the mammary gland.
Fig. 11.
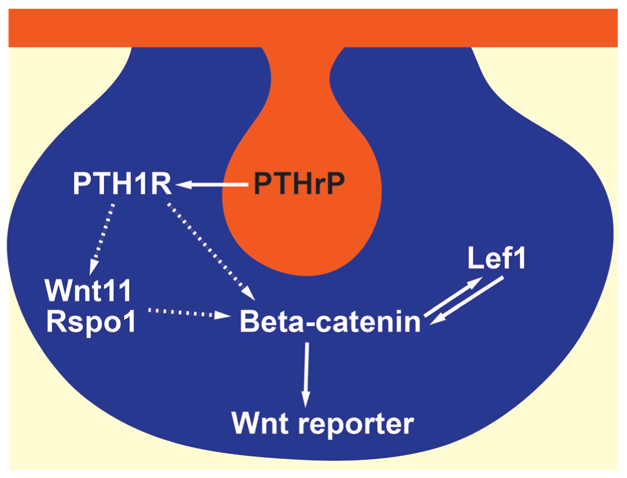
Model of PTHrP-mediated Wnt signaling in the mammary mesenchyme. Mammary epithelium (orange) secretes PTHrP, which acts on Pthr1 expressed in the mammary mesenchyme. Downstream β-catenin signaling may be activated either directly or indirectly, possibly via upregulation of Wnt11 and Rspo1 (dotted arrow). This results in activation of the Wnt reporter in the mammary mesenchyme (blue) and the expression of ER and Lef1. Lef1, in turn, acts downstream of PTHrP and β-catenin to amplify Wnt signaling and contribute to full differentiation of the mammary mesenchyme.
Supplementary Material
Acknowledgements
We thank Dr David Ornitz for the Dermo1-Cre mice; Dr Paul Hamel for Lef1−/− and TOPGAL-C mice; and Dr V. Greco for the tdTomato mice.
Footnotes
Funding
This work was supported by National Institutes of Health (NIH) grants [DK055501 and CA153702 to J.W.; NIH DK045735 to the Yale Diabetes and Endocrine Research Core; HL068597 and HL109932 to W.S] and the Idaho INBRE Program [P20 RR016454 and P20GM103408]. M.H. is supported by a Department of Defense Breast Cancer Research program postdoctoral fellowship [W81XWH-10-1-0032] and by the Mountain States Tumor and Medical Research Institute. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.080671/-/DC1
References
- Barolo S. (2006). Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25, 7505-7511 [DOI] [PubMed] [Google Scholar]
- Binnerts M. E., Kim K. A., Bright J. M., Patel S. M., Tran K., Zhou M., Leung J. M., Liu Y., Lomas W. E., 3rd, Dixon M., et al. (2007). R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. USA 104, 14700-14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras-Granic K., Wysolmerski J. J. (2008). Wnt signaling in breast organogenesis. Organogenesis 4, 116-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boras-Granic K., Chang H., Grosschedl R., Hamel P. A. (2006). Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev. Biol. 295, 219-231 [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253-1264 [DOI] [PubMed] [Google Scholar]
- Burtis W. J., Wu T., Bunch C., Wysolmerski J. J., Insogna K. L., Weir E. C., Broadus A. E., Stewart A. F. (1987). Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase-stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J. Biol. Chem. 262, 7151-7156 [PubMed] [Google Scholar]
- Cha S. W., Tadjuidje E., Tao Q., Wylie C., Heasman J. (2008). Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135, 3719-3729 [DOI] [PubMed] [Google Scholar]
- Cha S. W., Tadjuidje E., White J., Wells J., Mayhew C., Wylie C., Heasman J. (2009). Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr. Biol. 19, 1573-1580 [DOI] [PubMed] [Google Scholar]
- Cheng S. L., Shao J. S., Halstead L. R., Distelhorst K., Sierra O., Towler D. A. (2010). Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/β-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ. Res. 107, 271-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon S. S., Cheah A. Y., Turley S., Nadesan P., Poon R., Clevers H., Alman B. A. (2002). β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl. Acad. Sci. USA 99, 6973-6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. Y., Hens J., Andl T., Kairo A., Yamaguchi T. P., Brisken C., Glick A., Wysolmerski J. J., Millar S. E. (2004). Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 131, 4819-4829 [DOI] [PubMed] [Google Scholar]
- Cowin P., Wysolmerski J. (2010). Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb. Perspect. Biol. 2, a003251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557-4568 [DOI] [PubMed] [Google Scholar]
- DasGupta R., Rhee H., Fuchs E. (2002). A developmental conundrum: a stabilized form of β-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J. Cell Biol. 158, 331-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe S. P., Carraro G., Tefft D., Li C., Xu X., Chai Y., Minoo P., Hajihosseini M. K., Drouin J., Kaartinen V., et al. (2008). Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by β-catenin signaling. PLoS ONE 3, e1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar M. E., Wysolmerski J. J. (1999). Parathyroid hormone-related protein: a developmental regulatory molecule necessary for mammary gland development. J. Mammary Gland Biol. Neoplasia 4, 21-34 [DOI] [PubMed] [Google Scholar]
- Dunbar M. E., Dann P. R., Robinson G. W., Hennighausen L., Zhang J. P., Wysolmerski J. J. (1999). Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development 126, 3485-3493 [DOI] [PubMed] [Google Scholar]
- Dunbar M. E., Dann P., Brown C. W., Van Houton J., Dreyer B., Philbrick W. P., Wysolmerski J. J. (2001). Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J. Endocrinol. 171, 403-416 [DOI] [PubMed] [Google Scholar]
- Eger A., Stockinger A., Schaffhauser B., Beug H., Foisner R. (2000). Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of β-catenin and upregulation of β-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J. Cell Biol. 148, 173-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J., Dann P., Hong J., Cosgrove J., Dreyer B., Rimm D., Dunbar M., Philbrick W., Wysolmerski J. (2001). Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development 128, 513-525 [DOI] [PubMed] [Google Scholar]
- Friedman M. S., Oyserman S. M., Hankenson K. D. (2009). Wnt11 promotes osteoblast maturation and mineralization through R-spondin 2. J. Biol. Chem. 284, 14117-14125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Mo C., Fraser S. E. (2004). Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689-693 [DOI] [PubMed] [Google Scholar]
- Gordon M. D., Nusse R. (2006). Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 281, 22429-22433 [DOI] [PubMed] [Google Scholar]
- Gu B., Sun P., Yuan Y., Moraes R. C., Li A., Teng A., Agrawal A., Rhéaume C., Bilanchone V., Veltmaat J. M., et al. (2009). Pygo2 expands mammary progenitor cells by facilitating histone H3 K4 methylation. J. Cell Biol. 185, 811-826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens J. R., Wysolmerski J. J. (2005). Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 7, 220-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens J. R., Dann P., Zhang J. P., Harris S., Robinson G. W., Wysolmerski J. (2007). BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development 134, 1221-1230 [DOI] [PubMed] [Google Scholar]
- Hens J., Dann P., Hiremath M., Pan T. C., Chodosh L., Wysolmerski J. (2009). Analysis of gene expression in PTHrP−/− mammary buds supports a role for BMP signaling and MMP2 in the initiation of ductal morphogenesis. Dev. Dyn. 238, 2713-2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incassati A., Chandramouli A., Eelkema R., Cowin P. (2010). Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res. 12, 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M., et al. (1991). A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254, 1024-1026 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Zhao J., Andarmani S., Kakitani M., Oshima T., Binnerts M. E., Abo A., Tomizuka K., Funk W. D. (2006). R-Spondin proteins: a novel link to β-catenin activation. Cell Cycle 5, 23-26 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Wagle M., Tran K., Zhan X., Dixon M. A., Liu S., Gros D., Korver W., Yonkovich S., Tomasevic N., et al. (2008). R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni N. H., Halladay D. L., Miles R. R., Gilbert L. M., Frolik C. A., Galvin R. J., Martin T. J., Gillespie M. T., Onyia J. E. (2005). Effects of parathyroid hormone on Wnt signaling pathway in bone. J. Cell. Biochem. 95, 1178-1190 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang L., Zhang M., Melamed J., Liu X., Reiter R., Wei J., Peng Y., Zou X., Pellicer A., et al. (2009). LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 69, 3332-3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall C., Evans N. C., Zylstra C. R., Li Y., Alexander C. M., Williams B. O. (2006). The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J. Biol. Chem. 281, 35081-35087 [DOI] [PubMed] [Google Scholar]
- Lindvall C., Zylstra C. R., Evans N., West R. A., Dykema K., Furge K. A., Williams B. O. (2009). The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS ONE 4, e5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J. S., Turcotte T. J., Yoon J. K. (2007). Dynamic expression of R-spondin family genes in mouse development. Gene Expr. Patterns 7, 306-312 [DOI] [PubMed] [Google Scholar]
- Niehrs C. (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469-7481 [DOI] [PubMed] [Google Scholar]
- Niemann C., Owens D. M., Hülsken J., Birchmeier W., Watt F. M. (2002). Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129, 95-109 [DOI] [PubMed] [Google Scholar]
- Portal-Núñez S., Lozano D., de Castro L. F., de Gortázar A. R., Nogués X., Esbrit P. (2010). Alterations of the Wnt/beta-catenin pathway and its target genes for the N- and C-terminal domains of parathyroid hormone-related protein in bone from diabetic mice. FEBS Lett. 584, 3095-3100 [DOI] [PubMed] [Google Scholar]
- Ray S., Xu F., Wang H., Das S. K. (2008). Cooperative control via lymphoid enhancer factor 1/T cell factor 3 and estrogen receptor-alpha for uterine gene regulation by estrogen. Mol. Endocrinol. 22, 1125-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G., Sneddon W. B., Yang Y., Wheeler D., Blair H. C., Friedman P. A. (2010). Parathyroid hormone receptor directly interacts with dishevelled to regulate β-Catenin signaling and osteoclastogenesis. J. Biol. Chem. 285, 14756-14763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Li J., Wang W., Cao W., Cao X., Wan M. (2011). Antagonists of LRP6 regulate PTH-induced cAMP generation. Ann. N. Y. Acad. Sci. 1237, 39-46 [DOI] [PubMed] [Google Scholar]
- Tian Y., Xu Y., Fu Q., He M. (2011). Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol. Cell. Biochem. 355, 211-216 [DOI] [PubMed] [Google Scholar]
- van Genderen C., Okamura R. M., Fariñas I., Quo R. G., Parslow T. G., Bruhn L., Grosschedl R. (1994). Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 8, 2691-2703 [DOI] [PubMed] [Google Scholar]
- Veltmaat J. M., Mailleux A. A., Thiery J. P., Bellusci S. (2003). Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71, 1-17 [DOI] [PubMed] [Google Scholar]
- Veltmaat J. M., Van Veelen W., Thiery J. P., Bellusci S. (2004). Identification of the mammary line in mouse by Wnt10b expression. Dev. Dyn. 229, 349-356 [DOI] [PubMed] [Google Scholar]
- Wan M., Yang C., Li J., Wu X., Yuan H., Ma H., He X., Nie S., Chang C., Cao X. (2008). Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 22, 2968-2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M., Li J., Herbst K., Zhang J., Yu B., Wu X., Qiu T., Lei W., Lindvall C., Williams B. O., et al. (2011). LRP6 mediates cAMP generation by G protein-coupled receptors through regulating the membrane targeting of Gα(s). Sci. Signal. 4, ra15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansbury O., Mackay A., Kogata N., Mitsopoulos C., Kendrick H., Davidson K., Ruhrberg C., Reis-Filho J. S., Smalley M. J., Zvelebil M., et al. (2011). Transcriptome analysis of embryonic mammary cells reveals insights into mammary lineage establishment. Breast Cancer Res. 13, R79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski J. J. (2010). Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann. N. Y. Acad. Sci. 1192, 161-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski J. J., Stewart A. F. (1998). The physiology of parathyroid hormone-related protein: an emerging role as a developmental factor. Annu. Rev. Physiol. 60, 431-460 [DOI] [PubMed] [Google Scholar]
- Wysolmerski J. J., McCaughern-Carucci J. F., Daifotis A. G., Broadus A. E., Philbrick W. M. (1995). Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development 121, 3539-3547 [DOI] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063-3074 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.