Abstract
Recent improvements in ultrasound (US) software and hardware have markedly increased the role of this imaging modality in the evaluation of the musculoskeletal system. US is currently one of the main imaging tools used to diagnose and assess most tendon, muscle, and ligament disorders. Compared with magnetic resonance imaging, US is much less expensive; it has no contraindications and is also widely available. Diseases affecting the digital flexor system (DFS) require early diagnosis if treatment is expected to limit functional impairment of the hand. US scans performed with high-resolution, broad-band transducers allows superb visualization of the flexor tendons of the hand and the annular digital pulleys. In addition, dynamic US can be used to assess movement of the tendon within the pulleys during passive or active joint movements. This article examines the anatomy and US appearance of the normal DFS and reviews the US findings associated with the most common disorders affecting it.
Keywords: Pulleys, Ultrasound, Flexor tendons, Hand
Sommario
I recenti miglioramenti nell'hardware e software delle apparecchiature ecografiche hanno aumentato in maniera importante il ruolo dell'ecografia nella valutazione del sistema muscolo-scheletrico. Attualmente l'ecografia rappresenta un'importante metodica d'imaging per la valutazione delle malattie dei tendini, muscoli e legamenti. L'ecografia non è invasiva ed è meno cara e più disponibile della risonanza magnetica.
Le malattie del sistema flessorio delle dita richiedono una diagnosi precoce al fine di instaurare un trattamento corretto capace di prevenire la limitazione funzionale della mano.
Se realizzata con apparecchiature ad alta risoluzione operanti con trasduttori a larga banda, l'ecografia consente un'eccellente valutazione dei tendini flessori della mano e delle pulegge anulari. L'ecografia consente, inoltre, un accurato esame dinamico dello scorrimento dei tendini nei tunnel osteofibrosi digitali durante i movimenti di flesso-estensione delle dita.
In quest'articolo presentiamo l'anatomia normale del sistema flessorio delle dita della mano e l'anatomia ecografica normale, seguite da una presentazione dell'aspetto ecografico nelle più comuni malattie interessanti i tendini flessori della mano.
Introduction
The role of ultrasound (US) in the assessment of musculoskeletal disorders is steadily increasing thanks to its low cost, widespread availability, and noninvasivity together with the possibility it offers for dynamic examination [1]. Hand and wrist disorders, including those affecting the digital flexor system (DFS) can be accurately assessed with high-frequency transducers [2–6]. Early diagnosis and appropriate treatment are important in these cases to limit functional impairment. The aim of this article is to illustrate the US appearance of the normal DFS and to describe the US findings of its most common disorders.
Normal anatomy
The flexor pollicis longus tendon runs between the superficial and deep bellies of the flexor pollicis brevis muscle and then passes through the osteofibrous canal of the thumb to its insertion at the base of the distal phalanx. At the level of the metacarpal head, the tendon passes between two sesamoid bones, the lateral one included in the combined tendon of the flexor pollicis brevis and abductor pollicis longus, and the medial one in the adductor pollicis tendon.
Each of the four medial digits has two flexor tendons, the flexor digitorum superficialis (FDS) and profundus (FDP). At the base of the finger the tendons enter the osteofibrous tunnel formed by the palmar aspect of the digital bones and the fibrous tendon sheath composed of the annular and cruciform pulleys (Fig. 1). Inside the tunnel the tendons are surrounded by a single synovial sheath that allows them to slide freely over each other during movements of the fingers. The fibrous sheath plays an essential role in preventing anteroposterior and lateral divergence of the tendons from the finger axis. It also provides the points where the force of the tendons is exerted during flexion movements [7]. The soft, flexible cruciform pulleys allow flexion of the interphalangeal joints while the thicker annular pulleys are more efficient for keeping the flexor tendons close to the palmar surfaces of the bone during joint motions and muscle activity. The first annular pulley (A1) extends from the palmar plate of the metacarpophalangeal joint to the base of the proximal phalanx. The second (A2) runs from the cranial part of the proximal phalanx to the junction of the proximal two thirds and the distal third of the proximal phalanx. The third annular pulley (A3), which is small, is located over the proximal interphalangeal joint. The fourth annular pulley (A4) lies in the middle third of the intermediate phalanx, and the fifth (A5) passes over the distal interphalangeal joint. Of the five, the A2-pulley is the strongest, and the A4 is the least flexible [8,9]. From the pathophysiologic point of view, these two pulleys provide critical resistance against displacement of the palmar tendons [10,11]. They attach to the bone whereas the A1-, A3- and A5-pulleys arise from the palmar plate of the joint. As noted earlier, the main function of the pulleys is to stabilize the flexor tendons during flexion of the fingers and to prevent ulnar and radial displacement or palmar bowstringing. Displacement of the tendons diminishes their mechanical efficiency and reduces digital performance.
Fig. 1.
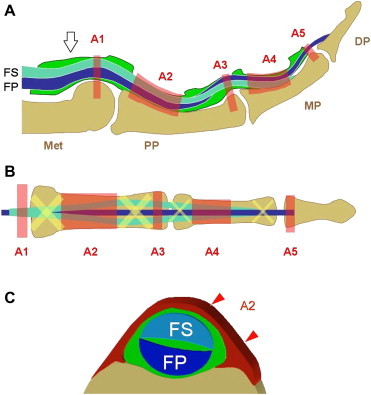
(A, B) Schematic drawing showing the normal anatomy of the flexor tendons of the fingers. FS = flexor digitorum superficialis tendon, FP = flexor digitorum profundus tendon. White arrowhead = synovial sheath, A1–A5 = annular digital pulleys. Met = metacarpal, PP = proximal phalanx, MP = middle phalanx, DP = distal phalanx. (C) Axial schematic drawing showing the relationship between the A2 pulley and the flexor digitorum superficialis/profundus tendons (FS, FP).
At the base of the proximal phalanx, the tendon of the FDS divides into two slips that pass on either side of the FDP tendon to reach its deep face and insert into the middle phalanx. After crossing the superficialis, the FDP continues its straight course and inserts into the base of the distal phalanx.
Normal US anatomy
The flexor tendons can be accurately assessed with either a longitudinal or transverse US scan. They appear as hyperechoic, fibrillar structures running immediately superficial to the bones and overlying the joints [12]. The FDS and FDP tendons can be distinguished by their anatomic locations. They can be clearly depicted on high-resolution US all the way to their distal insertions. Dynamic examination can be useful in distinguishing the two tendons and in assessing their integrity. Selective movements of the FDP can be evaluated during passive extension and flexion of the distal phalanx, while the examiner holds the middle phalanx in an extended position. Concomitant flexion of the distal and middle phalanges permits evaluation of the gliding movements of the two flexor tendons. In normal states, the synovial envelope surrounding the flexor tendons inside the fibrous digital sheath and the thin film of internal synovial fluid are difficult to visualize at US.
Normal annular pulleys can now be demonstrated with high-resolution US. They appear as thin, anisotropic bands covering the flexor tendons [11–13] (Fig. 2), and they are best visualized on transverse scans. In general, the volar portion of the pulleys has a hyperechoic, fibrillar appearance due to the perpendicular incidence of the US beam, whereas their lateral aspects are often hypoechoic as a result of anisotropy and can be seen diverging at both sides of the flexor tendons. Dynamic scanning in the transverse planes during passive and active movements of the fingers can help to distinguish the fixed pulley system from the gliding flexor tendons that underlie it.
Fig. 2.
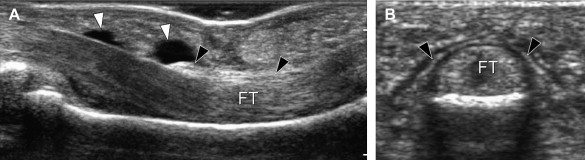
(A, B) Longitudinal and transverse US images of the A2 pulley. The pulley appears as a hyperechoic band (black arrowheads) surrounding the flexor tendons (FT). In (A) there is a small effusion (white arrowheads) located inside the synovial tendon sheath.
Pathologic changes
Pulley disorders
Annular pulleys can be damaged by acute injuries or chronic microtrauma.
Acute tears
Acute tears of the annular pulleys are among the most frequent lesions in elite climbers. They most commonly involve the ring and middle fingers. Tears are commonly seen in free climbers who use hand grips in which the entire weight of the body is borne by a single finger [14]. Excessive traction on the flexor tendons when the fingers are flexed tears the pulleys and causes anterior bowstringing of the tendons, which no longer lie against the bone plane. The A2-pulley ruptures more frequently than the A4-pulley, and A2 tears may be associated with rupture of the A3-pulley. If they are not diagnosed, complete pulley tears can lead to flexion contractures of the proximal interphalangeal joint with secondary osteoarthritis. In patients presenting with an acute tear, local swelling and pain frequently limit the physical examination. The differential diagnosis, which includes other post-traumatic conditions, such as tenosynovitis or a sprain of proximal interphalangeal joint, can be difficult in these cases, and imaging findings play a primary role. However, if the torn pulleys are difficult to detect by imaging, the diagnosis of complete pulley rupture can be made indirectly by demonstrating palmar bowstringing of the flexor tendons [13,15–18]. US, MRI [11,19,20] and CT can be used to evaluate digital pulley ruptures.
When used correctly, US has 98% sensitivity, 100% specificity, and 99% accuracy for the detection of finger-pulley injuries [17]. The scan is performed in the longitudinal planes with the dorsal aspect of the hand and the affected finger completely extended on the examination table. The transducer is first placed on the proximal phalanx to image the A2 pulley and then moved distally over the middle phalanx to assess the A3 and A4 pulleys. In most patients, the tonus of the powerful flexor muscles allows bowstringing of the tendons even in the resting position. US scanning during active forced flexion can enhance visualization of the subluxation of tendons, but this is rarely required to establish a definite diagnosis. During dynamic longitudinal US scanning, the patient is asked to keep the finger slightly flexed with the metacarpophalangeal joint extended while the examiner tries to extend it by gently pushing the fingertip [17]. Transverse sonograms confirm volar bowstringing of the flexor tendons but usually add no significant information.
The site of maximal bowstringing helps identify which pulley is ruptured. In A2-pulley rupture, maximal volar displacement occurs over the proximal phalanx (Fig. 3), whereas in A4 pulley tears, the bowstringing is chiefly observed over the middle phalanx. A hypoechoic rim surrounding the tendons (due to the accumulation of sheath fluid) can be seen in the acute phase as an expression of secondary traumatic tenosynovitis. Dynamic sonograms obtained during flexion/extension movements of the finger show smooth gliding of the flexor tendons and are useful in the assessment of the tendons.
Fig. 3.
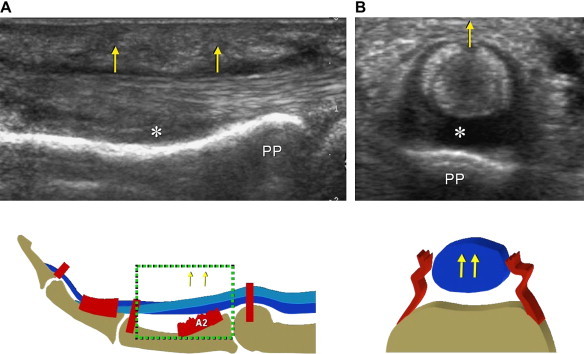
(A, B) Longitudinal and transverse US images of a complete tear of the A2 pulley. The pulley in no longer detectable. The flexor tendons present a palmar dislocation (arrows). The space (asterisks) between the tendons and the anterior cortex of the proximal phalanx (PP) is filled with fibrous tissue in (A) (old tear) and with an anechoic effusion in (B) (acute tear).
Partial pulley ruptures are difficult to diagnose because they do not lead to tendon displacement. The partially torn pulley appears swollen and hypoechoic [13] (Fig. 4).
Fig. 4.
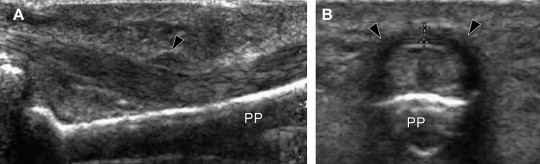
(A, B) Longitudinal and transverse US images of a partial tear of the A2 pulley. The pulley (black arrowheads) appears swollen and hypoechoic. PP = proximal phalanx.
Although MR and CT can be used to diagnose DAP tears [11,19,20], US is less expensive, and non-traumatic, and it allows dynamic evaluation. One of the advantages of US with respect to CT is that it can reveal a synovial sheath effusion. US is just as valuable as MRI in diagnosing complete tears of the digital pulleys [15]. It can easily rule out other diseases, such as tenosynovitis of the flexor tendons or joint diseases, reveal the numbers of pulleys disrupted, and distinguish between partial and complete tears.
Chronic lesions
Chronic repetitive movements of the finger during occupational or recreational activities may lead to thickening of the A1-pulley. Impaired movement of the flexor tendons below the thickened pulley can cause local friction that leads to tenosynovitis, tendon swelling, and further tendon impingement inside the narrowed digital tunnel. Clinically patients present with the so-called “trigger finger”, a term that refers to the transient locking of the finger in a flexed position followed by a painful snapping sensation during extension. This condition usually affects middle-aged women, but it can also be seen in subjects of both sexes who are suffering from diabetes mellitus.
US reveals diffuse hypoechoic thickening of the A1-pulley that may or may not be associated with abnormalities of the underlying flexor tendons (Fig. 5). Comparison with the adjacent unaffected fingers on axial images can be useful in establishing the correct diagnosis, particularly when evaluating the thickness of the pulley. The affected tendons are typically swollen. On axial scans they have a more rounded cross-section than the unaffected adjacent tendons [21,22]. In the acute phases, synovial sheath effusion can be demonstrated proximal to the pulley. In the longitudinal planes, dynamic changes in the shape of the synovial sheath can be observed with US during flexion and extension of the finger, but this test is rarely required to establish the diagnosis. Color and power Doppler often demonstrate flow signals within the inflamed pulley (Fig. 6). Although the diagnosis of trigger finger is essentially clinical, US can be a helpful guide for deciding an appropriate treatment. A markedly thickened A1-pulley usually requires surgical release, while less evident thickening can be treated conservatively with splinting, NSAIDs, and local injections of steroids. The latter are usually administered without imaging guidance, but US can also be used for this purpose (Fig. 7). Surgical section of the A1-pulley generally relieves triggering quickly and permanently, and it has no significant functional and clinical repercussions on finger movements. US is also useful for diagnosing surgical complications, such as section of the proximal part of the A2 tear or infective tenosynovitis.
Fig. 5.
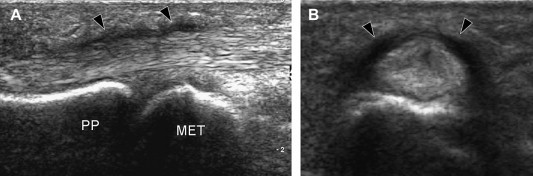
(A, B) Longitudinal and axial color US images in a patient with trigger finger. Note the massive thickening of the A1 pulley (black arrowheads). PP = proximal phalanx, MET = metatarsal head.
Fig. 6.
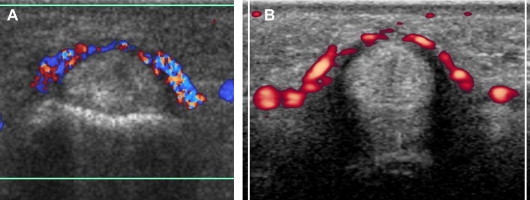
(A, B) Two axial images (color and power Doppler) of a trigger finger clearly show internal flow signals in the involved pulley.
Fig. 7.
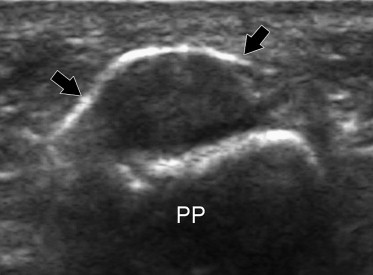
Axial US image obtained after US-guided steroid infiltration shows the steroid solution (black arrows) in close contact with the affected pulley. PP = proximal phalanx.
Tendon disorders
Tears
Flexor tendon tears can be partial or complete, and they are usually the result of penetrating injuries [23]. More rarely tendon tears are caused by rheumatoid arthritis or closed trauma.
A partial tear presents as a fusiform hypoechoic swelling of the tendon with focal interruption of the internal fibrillar pattern. It may be difficult to differentiate from focal tendinopathy based on US findings alone. Clinical findings and history are essential for this purpose. When doubts arise, dynamic US scanning should be done during passive and active movements of the finger to evaluate the continuity of tendons and to differentiate between partial and complete ruptures. In complete tears, the ruptured tendon will not be visualized at the site of injury (Fig. 8). The retracted tendon ends appear irregular and diffusely hypoechoic with loss of fibrillar echoes and in many cases posterior acoustic shadowing. Acute lesions are frequently associated with a tendon-sheath effusion, while in chronic tears the retracted tendon is surrounded by a hypoechoic area that reflects perilesional adhesions and fibrosis. The amount of proximal retraction depends on the size of the muscle and the mechanism of injury. US plays a dual role in assessing complete tendon tears: it serves not only to confirm the clinical diagnosis, but also to demonstrate and quantify retraction of the proximal tendon stump. This is particularly helpful since tendon retraction is difficult, if not impossible, to assess clinically.
Fig. 8.
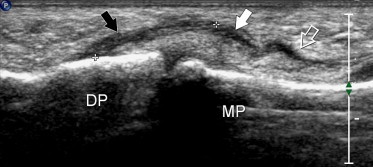
Longitudinal US image shows a complete tear (white arrow) of the flexor digitorum profundus tendon of the third finger. Note the irregular hypoechoic distal stump (black arrow) and the empty proximal synovial sheath (void arrow). DP = distal phalanx, MP = middle phalanx. The distal interphalangeal joint is normal.
After reconstructive surgery, US is an effective modality for verifying the success of flexor tendon repair [24]. When a re-rupture is suspected on the basis of reduced or absent tendon function, US can identify the point of disruption of the suture. Difficulties may arise in differentiating a normal repaired tendon from one with what is known as “callus elongation,” that is a fibrous zone connecting the two tendon ends. US evaluation of the internal echotexture of previously sutured tendons is often unreliable. Dynamic US examinations performed during repetitive flexion and extension of the involved finger can reveal post-surgical adhesions. Attention should be focused not only on the tendon movements but also on the soft tissues immediately adjacent to the tendon. Synchronous movements of these tissues with those of the tendons allows a definitive diagnosis.
Tenosynovitis
Tenosynovitis of the flexor digitorum tendons may be the result of systemic disorders, such as rheumatoid arthritis and related conditions, mechanical stress, or infectious processes. The hallmarks of acute tenosynovitis of the flexor digitorum tendons (as well as of other tendons of the body) are an increased amount of sheath fluid, which is seen as a hypo-/anechoic collection surrounding the tendons [25,26], and/or thickening of the synovium.
Careful scanning is required not to miss mild fluid effusions inside the tendon sheaths. Effusions are easier to identify when they are proximal to the metacarpal heads, where the fluid collects inside the “cul-de-sac” of the sheath. In the fingers, effusions assume a typical lobulated appearance created by the discontinuous array of the pulleys (Fig. 9). In subacute and chronic tenosynovitis, thickening of the synovial sheath may also be seen (Fig. 10).
Fig. 9.
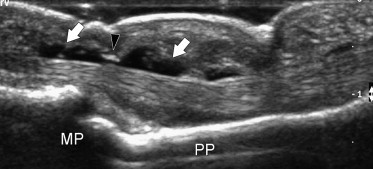
Sagittal US image in a patient with serous tenosynovitis. Note the hypoechoic fluid effusion located inside the tendon sheath (white arrows). The tendons present a normal internal echostructure. MP = middle phalanx, PP = proximal phalanx, black arrowhead = A4 pulley.
Fig. 10.
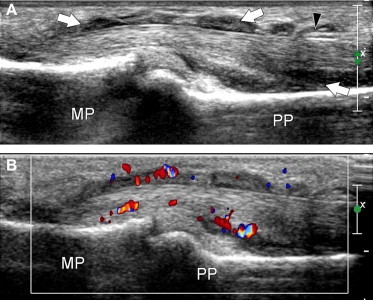
(A, B) Longitudinal color Doppler US images in a patient with hypertrophic tenosynovitis. The sheath of the flexor tendons is substantially thickened (white arrows) due to hypertrophy of the synovium. Color Doppler confirms the presence of increased vascularity due to active local inflammation. MP = middle phalanx, PP = proximal phalanx, black arrowhead = A2 pulley.
Serous tenosynovitis can result from chronic microtrauma, such as that associated with the triggering, or it may be a reflection of early-stage arthritic involvement. In psoriatic arthritis, the finger may be diffusely swollen (the so-called “sausage finger”) as a result of flexor tendon tenosynovitis or involvement of the finger joints. US can demonstrate the sheath involvement and differentiate it from polyarthritis. Differential diagnosis is important because in the latter condition multiple intraarticular injections are required rather then a single injection inside the tendon sheath.
Hypertrophic forms of tenosynovitis are more commonly expressions of systemic arthritis (e.g., rheumatoid or psoriatic), but they can also be the end result of chronic mechanical tendonitis or infectious disease (Fig. 10). Reliable differentiation of these conditions is not possible based on US findings alone: correlations with clinical, laboratory, and radiological findings are essential. In severe cases, the synovium can completely fill the tendon sheath [2,3,27,28]. In the acute phases of inflammation, color and power Doppler imaging demonstrate hyperemic flow within the synovial folds and within the tendon substance (Fig. 10). In these settings, it may help to differentiate synovial pannus from effusion [29]. Substantial reduction of synovial hyperemia can be also observed as a response to medical treatment, and this parameter is a useful index for monitoring the effects of therapy [30].
The presence of an echogenic effusion may be an early sign of infectious tenosynovitis [31,32]. In these cases, however, US-guided aspiration of the sheath fluid is essential to confirm the US imaging finding and test the responsiveness of the infectious agent to antibiotics. In hand wounds, foreign bodies are a common cause of infection of the flexor tendon sheath. The joints should always be carefully examined to rule out the presence of hyperechoic intrasynovial foreign bodies, which require mandatory surgical removal.
Peritendinous disorders
Ganglion cysts
Digital ganglia are fibrous-walled cystic lesions filled with mucoid fluid that develop on tendons or peritendinous structures. They are generally found at the volar aspect of the base of the finger, close to the annular pulleys [33]. They are less common and smaller than wrist ganglia [34,35]. They are caused by microtrauma to the digital annular pulley, which is followed by mucoid degeneration. Most of them are located anterior to the proximal edge of the A2-pulley. The third and fourth fingers are the most commonly involved. Ganglia affecting the dorsal aspect of the fingers are rare (Fig. 11).
Fig. 11.
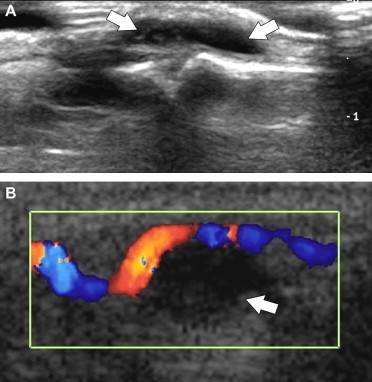
(A) Longitudinal US images of a patient with a mucoid cyst located at the extensor aspect of the middle finger. The cyst appears as an anechoic mass. (B) Longitudinal color Doppler US image in a patient with a mucoid cyst of the flexor tendon. The cyst presents no internal flow signals. Note dislocation of the adjacent normal digital artery.
US depicts these cysts as small anechoic lesions with no discernible wall and posterior acoustic shadowing. They extend along the lateral and medial aspects of the flexor tendons. Dynamic examination demonstrates smooth gliding movement of the flexor tendons that does not affect the size or position of digital ganglia. Occasionally, the A2-pulley can be visualized as a thin hyperechoic band lying between the tendons and the ganglion. The size of digital ganglia can vary over time due to alternating increases and decreases in the mucoid content. In general, size changes correlate well with the patient's symptoms. Color Doppler shows an absence of internal flow signals. It can be used to determine the relationship of the cystic lesion with the adjacent vessels (Fig. 11).
Giant-cell tumor of the tendon sheath
Giant-cell tumors of the tendon sheath are considered a localized form of pigmented villonodular synovitis. They represent the second most common space-occupying lesion of the hand that involves peritendinous tissues. It appears as a painless, slow–growing, solid extra-articular mass on the volar aspect of the fingers; lateral and circumferential extension is usually present. Typically, the giant-cell tumor lies adjacent to normally appearing flexor tendons [34].
On US, the tumor is seen as a hypoechoic mass with sharp margins. Unlike digital ganglia, giant-cell tumors of the tendon sheath have internal echoes and no posterior acoustic enhancement (Fig. 12). Some exhibit internal vasculature at color and power Doppler imaging. US may reveal displacement of the digital arteries and cortical erosions of the phalanges secondary to pressure from the overlying lesion. After surgery, US can be useful in screening for local recurrences.
Fig. 12.
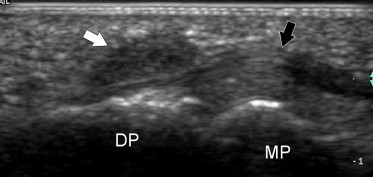
Longitudinal US image of a small giant-cell tumor of the tendon sheath (white arrow) located at the palmar aspect of the distal insertion of the flexor digitorum profundus tendon (black arrow) of the index finger. The nodule appears at US as a solid hypoechoic mass without posterior enhancement. DP = distal phalanx, MP = middle phalanx.
Footnotes
EUROSON-SIUMB 2006 – SIUMB Award for the poster presented at 18th European Congress of Ultrasound in conjunction with XVIII Congresso Nazionale SIUMB.
References
- 1.Bianchi S. Editorial. J Radiol. 2005;86:1829. [Editorial] [Google Scholar]
- 2.Bianchi S., Martinoli C., Abdelwahab I.F. High-frequency ultrasound examination of the wrist and hand. Skeletal Radiol. 1999;28:121–129. doi: 10.1007/s002560050488. [DOI] [PubMed] [Google Scholar]
- 3.Teefey S.A., Middleton W.D., Boyer M.I. Sonography of the hand and wrist. Semin Ultrasound CT MR. 2000;21:192–204. doi: 10.1016/s0887-2171(00)90042-8. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi S., Martinoli C., Sureda D., Rizzato G. Ultrasound of the hand. Eur J Ultrasound. 2001;14:29–34. doi: 10.1016/s0929-8266(01)00143-4. [DOI] [PubMed] [Google Scholar]
- 5.Moschilla G., Breidahl W. Sonography of the finger. AJR Am J Roentgenol. 2002;178:1451–1457. doi: 10.2214/ajr.178.6.1781451. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi S., Martinoli C., Montet X., Fasel J. Wrist and hand ultrasound. Radiologe. 2003;43:831–840. doi: 10.1007/s00117-003-0961-0. [DOI] [PubMed] [Google Scholar]
- 7.Rispler D., Greenwald D., Shumway S., Allan C., Mass D. Efficiency of the flexor tendon pulley system in human cadaver hands. J Hand Surg [Am] 1996;21:444–450. doi: 10.1016/S0363-5023(96)80361-3. [DOI] [PubMed] [Google Scholar]
- 8.Lin G.T., Amadio P.C., An K.N., Cooney V.P. Functional anatomy of the human digital flexor pulley system. J Hand Surg [Am] 1989;14:949–956. doi: 10.1016/s0363-5023(89)80043-7. [DOI] [PubMed] [Google Scholar]
- 9.Lin G.T., Cooney W.P., Amadio P.C., An K.N. Mechanical properties of human pulleys. J Hand Surg [Br] 1990;15:429–434. doi: 10.1016/0266-7681(90)90085-i. [DOI] [PubMed] [Google Scholar]
- 10.Phillips C., Mass D. Mechanical analysis of the palmar aponeurosis pulley in human cadavers. J Hand Surg [Am] 1996;21:240–244. doi: 10.1016/S0363-5023(96)80107-9. [DOI] [PubMed] [Google Scholar]
- 11.Hauger O., Chung C.B., Lektrakul N. Pulley system in the fingers: normal anatomy and simulated lesions in cadavers at MR imaging, CT and US with and without contrast material distension of the tendon sheath. Radiology. 2000;217:201–212. doi: 10.1148/radiology.217.1.r00oc40201. [DOI] [PubMed] [Google Scholar]
- 12.Boutry N., Titecat M., Demondion X., Glaude E., Fontaine C., Cotten A. High-frequency ultrasonographic examination of the finger pulley system. J Ultrasound Med. 2005;24:1333–1339. doi: 10.7863/jum.2005.24.10.1333. [DOI] [PubMed] [Google Scholar]
- 13.Martinoli C., Bianchi S., Nebiolo M., Derchi L.E., Garcia J. Sonographic evaluation of digital annular pulleys tears. Skeletal Radiol. 2000;29:387–391. doi: 10.1007/s002560000226. [DOI] [PubMed] [Google Scholar]
- 14.Bollen S.R. Injury to the A2 pulley in rock climbers. J Hand Surg [Br] 1990;15:268–270. doi: 10.1016/0266-7681_90_90135-q. [DOI] [PubMed] [Google Scholar]
- 15.Bodner G., Rudisch A., Gabl M., Judmaier W., Springer P., Klauser A. Diagnosis of digital flexor tendon annular pulley disruption: comparison of high frequency ultrasound and MRI. Ultraschall in Med. 1999;20:131–136. doi: 10.1055/s-1999-8904. [DOI] [PubMed] [Google Scholar]
- 16.Klauser A., Bodner G., Frauscher F., Gabl M., Zur Nedden D. Finger injuries in extreme rock climbers. Assessment of high resolution ultrasonography. Am J Sports Med. 1999;27:733–737. doi: 10.1177/03635465990270060801. [DOI] [PubMed] [Google Scholar]
- 17.Klauser A., Frauscher F., Bodner G. Finger pulley injuries in extreme rock climbers: depiction with dynamic US. Radiology. 2002;222:755–761. doi: 10.1148/radiol.2223010752. [DOI] [PubMed] [Google Scholar]
- 18.Martinoli C., Bianchi S., Cotten A. Imaging of rock climbing injuries. Semin Musculoskelet Radiol. 2005;9:334–345. doi: 10.1055/s-2005-923378. [DOI] [PubMed] [Google Scholar]
- 19.Le Viet D., Rousselin B., Roulot E., Lantieri L., Godefroy D. Diagnosis of digital pulley rupture by computed tomography. J Hand Surg [Am] 1996;21:245–248. doi: 10.1016/S0363-5023(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 20.Parellada J.A., Balkissoon A.R., Hayes C.W., Conway W.F. Bowstring injury of the flexor tendon pulley system: MR imaging. AJR Am J Roentgenol. 1996;67:347–349. doi: 10.2214/ajr.167.2.8686601. [DOI] [PubMed] [Google Scholar]
- 21.Serafini G., Derchi L.E., Quadri P. High resolution sonography of the flexor tendons in trigger fingers. J Ultrasound Med. 1996;15:213–219. doi: 10.7863/jum.1996.15.3.213. [DOI] [PubMed] [Google Scholar]
- 22.Guerini H., Drapé J.-L., Le Quintrec J.-S. Aspect echographique des doigts en ressalt. In: Brasseur J.-L., Zeitoun-Eiss D., Grenier P., editors. Actualités en echographie de l'appareil locomoteur. Sauramps médical; Montpellier: 2005. pp. 275–282. [Google Scholar]
- 23.Soussi M., Ebelin M., Rigot J. III. Pathologie traumatique et inflammatoiore des tendons flechisseurs des doigts de la main. J Radiol. 1989;70:352–355. [PubMed] [Google Scholar]
- 24.Corduff N., Jone R., Ball J. The role of ultrasound in the management of zone I flexor tendon injuries. J Hand Surg. 1984;19B:76–80. doi: 10.1016/0266-7681(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 25.Gooding G.A., Hardiman T., Sumers M., Stess R., Graf P., Grunfeld C. Sonography of the hand and foot in foreign body detection. J Ultrasound Med. 1987;6:441–447. doi: 10.7863/jum.1987.6.8.441. [DOI] [PubMed] [Google Scholar]
- 26.Souissi M., Giwerc M., Ebelin M. Echography of the flexor tendons of the fingers. Presse Med. 1989;18:463–466. [PubMed] [Google Scholar]
- 27.Grassi W., Tittarelli E., Blasetti P., Pirani O., Cervini C. Finger tendon involvement in rheumatoid arthritis. Evaluation with high-frequency sonography. Arthritis Rheum. 1995;38:786–794. doi: 10.1002/art.1780380611. [DOI] [PubMed] [Google Scholar]
- 28.Fornage B.D. Soft tissue changes in the hand in rheumatoid arthritis: evaluation with US. Radiology. 1989;173:735–737. doi: 10.1148/radiology.173.3.2682774. [DOI] [PubMed] [Google Scholar]
- 29.Newman J.S., Laing T.J., McCarthy C.J., Adler R.S. Power Doppler sonography of synovitis: assessment of therapeutic response: preliminary observations. Radiology. 1996;198:582–584. doi: 10.1148/radiology.198.2.8596870. [DOI] [PubMed] [Google Scholar]
- 30.Breidahl W.H., Stafford Johnson D.B., Newman J.S., Adler R.S. Power Doppler sonography in tenosynovitis: significance of the peritendineous hypoechoic rim. J Ultrasound Med. 1998;17:103–107. doi: 10.7863/jum.1998.17.2.103. [DOI] [PubMed] [Google Scholar]
- 31.Schecter W.P., Markison R.E., Jeffrey R.B., Barton R.M., Laing F. Use of sonography in the early detection of suppurative flexor tenosynovitis. J Hand Surg. 1989;14A:307–310. doi: 10.1016/0363-5023(89)90027-0. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey R.B., Jr., Laing F.C., Schechter W.P., Markison R.E., Barton R.M. Acute suppurative tenosynovitis of the hand: diagnosis with US. Radiology. 1987;162:741–742. doi: 10.1148/radiology.162.3.3544036. [DOI] [PubMed] [Google Scholar]
- 33.Garcia J., Bianchi S. Diagnostic imaging of tumors of the hand and wrist. Eur Radiol. 2001;11:1470–1482. doi: 10.1007/s003300000751. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi S., Abdelwahab I.F., Zwass A. Sonographic findings in examination of digital ganglia: retrospective study. Clin Radiol. 1993;48:45–47. doi: 10.1016/s0009-9260(05)80107-2. [DOI] [PubMed] [Google Scholar]
- 35.Hoglund M., Tordai P., Muren C. Diagnosis of ganglions in the hand and wrist by sonography. Acta Radiol. 1994;35:3539. [PubMed] [Google Scholar]


