Abstract
Purpose
To identify the vascular patterns found in superficial lymph nodes with histologically confirmed lymphomatous involvement and to determine their value in the sonographic diagnosis of lymphadenopathy.
Methods and materials
The study involved the prospective classification of vascular patterns observed during power Doppler and/or color Doppler studies of superficial lymph nodes scheduled for resection. Forty patients (27 men and 13 women, aged 22–84 years; mean age: 58 years) with pathologically proven lymphoma were selected for this study (26 cervical, 13 axillary and 1 inguinal).
Results
A longitudinal vessel with or without branches (pattern I) was found in 14 lymphomatous nodes. Six contained short vessel segments distributed in the hilum area or centrally (pattern II), five had multiple vessels, partially branching, entering the node in a few rows from its longitudinal side (pattern III), seven presented multiple vessels that branched irregularly or chaotically with avascular areas (pattern IV), and eight had a peripheral vessel distribution (pattern V). Therefore, 50% of the lymphomatous nodes had vascular patterns regarded as characteristic of reactive lymph nodes (patterns I and II), and 37.5% had patterns normally described in lymph nodes with metastatic involvement (patterns IV and V); other lymphomatous lymph nodes had ambiguous vascular patterns that have not been previously classified (pattern III).
Conclusion
The angioarchitecture of superficial lymphomatous lymph nodes varies widely and is difficult to classify. It may resemble that reported in normal or reactive lymph nodes or patterns that are associated with metastases. The finding of a normal or benign vascular pattern in a lymph node with suspected lymphomatous involvement does not eliminate the need for a diagnostic biopsy.
Keywords: Lymph nodes, Lymphoma, Ultrasound, Power Doppler, Vessel pattern
Sommario
Scopo
Valutazione dei possibili pattern vascolari dei linfonodi superficiali interessati da linfoma confermato da esame istologico allo scopo di ottenere un elemento aggiuntivo nella diagnosi differenziale ecografica tra patologie interessanti i linfonodi.
Materiali e metodi
È stata effettuata una classificazione ecografica prospettica di pattern vascolari color e power Doppler di 55 linfonodi superficiali programmati per resezione. Sono stati selezionati per questo studio 40 pazienti con diagnosi istologica di linfoma (26 linfonodi cervicali, 13 ascellari e 1 inguinale). Il gruppo di pazienti studiato era comprensivo di 27 uomini e 13 donne, età 22–84 anni (età media: 58 anni).
Risultati
È stato trovato un vaso lungitudinale con o senza ramificazioni (pattern I) in 14 linfoadenopatie; piccoli segmenti vascolari distribuiti nell'area dell'ilo o nella zona centrale del linfonodo (pattern II) in 6 linfoadenopatie. Vasi multipli, parzialmente ramificati, che entrano nel linfonodo in alcune file dal lato longitudinale (pattern III) sono stati riscontrati in 5 linfonodi mentre vasi multipli che si ramificano irregolarmente o in modo caotico con aree avascolari (pattern IV) sono stati visualizzati in 7 linfonodi. È stata trovata una distribuzione vascolare periferica (pattern V) in 8 linfoadenomegalie. Quindi, la angioarchitettura considerata caratteristica per linfonodi reattivi (patterns I and II) è stata trovata nel 50% delle linfoadenopatie di questo studio; il 37,5% dei linfomi ha mostrato una distribuzione dei vasi descritta nelle metastasi (patterns IV e V); altri linfonodi interessati da linfoma avevano una angioarchitettura non precedentemente classificata o equivoca (pattern III).
Conclusioni
La angioarchitettura delle linfoadenopatie superficiali è molto varia e difficile da classificare. Essa può variare da pattern vascolari incontrati in linfonodi normali o reattivi a configurazioni trovate nelle metastasi. Pattern vascolari normali o benigni di un linfonodo in un paziente con sospetto clinico di linfoma non dovrebbe essere un criterio di esclusione quando è contemplata una biopsia diagnostica.
Introduction
Differential diagnosis of lymph node disease is an important clinical issue. Physical examination is limited to superficial nodes. It also lacks sensitivity and is usually not sufficient to allow a diagnosis [1–4]. In contrast, the sonographic examination provides information on the internal structure of lymph nodes, and it can reveal abnormalities even in lymph nodes that are not enlarged. Sonographic assessment of the lymph nodes includes gray-scale studies as well as those based on power or color Doppler. Evaluation of lymph node vascularization is an important part of the sonographic assessment of a lymph node. Several papers have described the vascular patterns associated with reactive and metastatic lymph nodes [5–8]. In the majority of previous publications, the patterns were classified in general terms, e.g., peripheral pattern, hilar pattern, or mixed pattern [4,9]. More detailed classifications were introduced by Na et al. [10] and Tschammler et al. [11].
The aim of this study was to identify the vascular patterns found in superficial lymph nodes with histologically confirmed lymphomatous involvement and to determine their value in the sonographic diagnosis of lymphadenopathy.
Material and methods
The study involved the prospective classification of vascular patterns observed during power Doppler and/or color Doppler studies of 55 superficial lymph nodes scheduled for resection. The patients included 32 men and 23 women aged 17–84 years (mean age: 52 years). All the lymph nodes examined in the study were later resected and subjected to histopathological analysis.
All ultrasound examinations were performed with a Siemens Elegra (Erlangen, Germany) scanner and a 7.5-MHz linear transducer.
Detailed analysis of the vessel pattern in each lymph node was performed during power and/or color Doppler studies. The sample volume was adjusted to the dimensions of the lymph node, and occasionally diminished for better visualization of flow when there were pulsation artifacts from large blood vessels or in large lymph nodes. The velocity and power/color gain were adjusted individually for each lymph node (just above the level of signal noise) to optimize visualization of the vessels' courses. Default settings were used for other study parameters. The vascular architecture was classified on the basis of Na and Tschammler classifications [10,11] with certain modifications. We assessed the following aspects in each node:
-
-
presence of a longitudinal vessel within the node (with or without branches) (Fig. 1);
-
-
presence of only short vessel segments distributed within the hilum or central part of the node (Fig. 2);
-
-
regularity of the courses of vessel branches;
-
-
presence of avascular areas;
-
-
distribution of peripheral vessels (short or branching vessels) (Fig. 3).
Fig. 1.
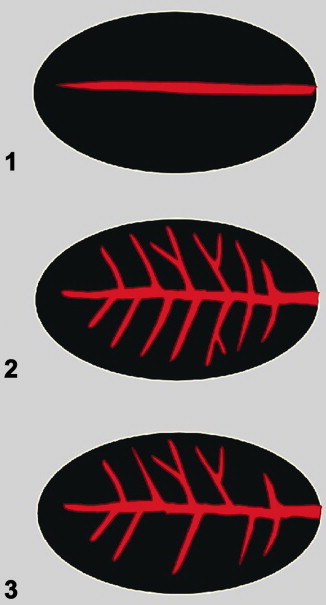
Schematic presentation of central/hilar vessel patterns in lymph nodes: A longitudinal vessel: without branches (1); with symmetric branches (2); with asymmetric branches (3).
Fig. 2.
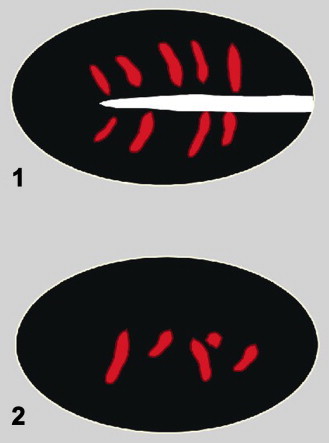
Schematic presentation of central/hilar vessel patterns in lymph nodes: short vessel segments distributed in the hilum area (1) or centrally (2).
Fig. 3.
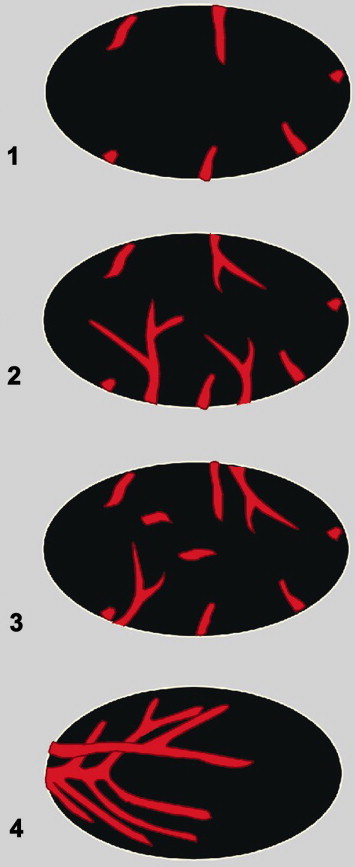
Schematic presentation of peripheral vessel patterns in lymph nodes: short vessel segments (1) or branching vessels (2) and (3) associated with short central signals; multiple vessels branching irregularly or chaotically, with avascular areas (4).
A separate “indeterminate” vascularization pattern was identified in some nodes. It consisted in the presence of multiple vessels, partially branching, entering the node in a few rows from its longitudinal side (Fig. 4).
Fig. 4.

Schematic presentation of the indeterminate vessel pattern in lymph nodes: multiple vessels, partially branching, entering the node in several rows from its longitudinal side (a – longitudinal section; b – transverse section).
Histopathological examination of the resected lymph nodes revealed lymphomatous involvement in 40 nodes (26 cervical, 13 axillary, and 1 inguinal) (Table 1).
Table 1.
Histopathological features of the 40 lymphomatous lymph nodes analyzed in our study
| Three Hodgkin lymphomas | ||
| Thirty-seven non-Hodgkin lymphomas | Low-grade 30 | Small lymphocytic lymphoma (SLL) 22 |
| Nodal marginal zone lymphoma (MZL) 2 | ||
| Plasma-cell myeloma infiltration 2 | ||
| Follicular lymphoma (FL) 2 | ||
| Mantle-cell lymphoma (MCL) 2 | ||
| High-grade 7 | Diffuse large B-cell lymphoma (DLBCL) 7 |
These 40 nodes, which came from 27 men and 13 women aged 22–84 years (mean age: 58 years), are the subject of the present report. Their maximal longitudinal diameters ranged from 8 to 60 mm (mean: 21 mm); minimal transverse diameters ranged from 4 to 40 mm (mean: 10 mm).
Results
The vessel patterns seen in the different histological types of lymphomatous lymph nodes are summarized in Table 2.
Table 2.
Correlation between vessel patterns observed in the power Doppler ultrasound examination and lymphoma types
| Vascular pattern | Detailed description | Hodgkin lymphomas, n = 3 | non-Hodgkin lymphomas |
|
|---|---|---|---|---|
| Low-grade, n = 30 | High-grade, n = 7 | |||
| I | Longitudinal vessel (with or without branches) | 0 | 13 | 1 |
| II | Short vessel segments distributed in the hilum area or centrally | 1 | 5 | 0 |
| III | Multiple vessels, partially branching, entering the node in a few rows from its longitudinal side | 0 | 4 | 1 |
| IV | Peripheral vessel distribution (short vessel segments or branching vessels) | 1 | 5 | 2 |
| V | Vessels branching irregularly or chaotically in avascular areas | 1 | 3 | 3 |
Vascular patterns I and II, which are regarded as characteristic of reactive lymph nodes, were found in 20 of the 40 (50%) lymphomatous nodes we examined. A longitudinal vessel, with or without branches (pattern I), was found in 14 lymphomatous nodes (Figs. 1, 5 and 6). Six nodes contained short vessel segments with a hilar or central distribution (pattern II) (Figs. 2 and 7).
Fig. 5.
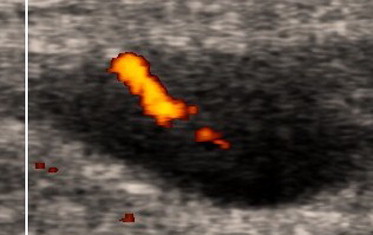
A cervical lymph node in a 58-year-old man with small lymphocytic lymphoma. Power Doppler ultrasound examination shows a longitudinal vessel without branches (Fig. 1, scheme 1).
Fig. 6.
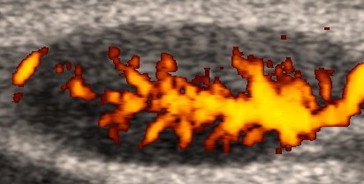
A cervical lymph node in a 64-year-old woman with marginal zone lymphoma. The power Doppler ultrasound examination shows a longitudinal vessel with symmetric branches (Fig. 1, scheme 2).
Fig. 7.
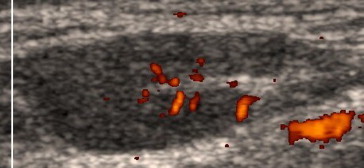
A cervical lymph node in a 46-year-old man with small lymphocytic lymphoma. The power Doppler ultrasound examination shows short vessel segments distributed in the hilum area (Fig. 2, scheme 1).
A previously unclassified or equivocal vessel distribution pattern (pattern III) was observed in five of the 40 lymphomas (12.5%). It consisted in the presence of multiple vessels, partially branching, that entered the node in a few rows from its longitudinal side) (Figs. 4 and 8).
Fig. 8.
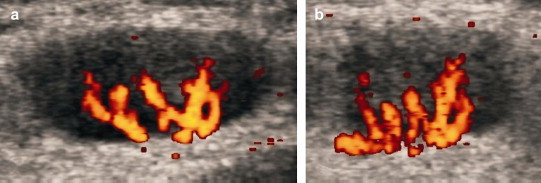
A cervical lymph node in a 70-year-old woman with diffuse large B-cell lymphoma. The power Doppler ultrasound examination shows multiple, partially branching vessels, entering the node in several rows from its longitudinal side (a – longitudinal section; b – transverse section) (Fig. 4).
Vessel distribution patterns typical of metastatic lymph node involvement (patterns IV and V) were found in 15 of the 40 (37.5%) lymphomas. Pattern IV (multiple vessels with irregular or chaotic branching together with avascular areas) was seen in seven lymph nodes (Figs. 3 and 10), and eight lymphomas presented pattern V (presence in the periphery of short vessel segments or branching vessels) (Figs. 3 and 9).
Fig. 10.
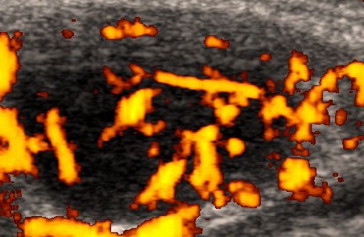
A cervical lymph node in a 46-year-old man with follicular lymphoma. The power Doppler sonographic examination shows multiple vessels branching irregularly or chaotically, with avascular areas (Fig. 3, scheme 4).
Fig. 9.

A cervical lymph node in a 61-year-old man with small lymphocytic lymphoma. The power Doppler ultrasound examination shows peripheral branching vessels (Fig. 3, scheme 2).
Discussion
The vascular patterns observed in lymph nodes may be divided in two basic groups:
-
1.
patterns characteristic of normal or reactive lymph nodes;
-
2.
patterns characteristic of metastatic involvement.
The pattern regarded as typical of benign conditions has been described as central vascularity, central perihilar vascularity, or hilar vascularity [4,9,10,12–14]. More detailed classification patterns were described by Na et al. [10] and Tschammler et al. [11]. Tschammler et al. added descriptions of features that are typical of reactive nodes: the presence of a longitudinal vessel, peripheral branches originating from the longitudinal vessels, small intranodal color dots representing short vessel segments [11].
In the present study, the following vessel patterns were regarded as indicative of “normal or reactive” lymph nodes, based on previously reported data: the presence of a longitudinal vessel (with or without branches), short vessel segments distributed in the hilum area or centrally. Benign patterns were found in 50% (20 of 40) of the lymphomatous nodes we examined (Figs. 5–7). Using less detailed classifications of vascularization, other authors have also reported lymphomas displaying the benign pattern described as “hilar vascularity.” However, the frequency of this pattern varied widely in these reports, ranging from 28.5% in the series examined by Ying et al. [13] to over 90% in those analyzed by Giovagnorio et al. (97% of the non-Hodgkin lymphomas, 94% of the Hodgkin lymphomas) [15].
The presence of central vascularity in a lymphoma lead to diagnostic problems in distinguishing reactive from lymphomatous nodes, thus reducing the sensitivity of Doppler US in the detection of lymphomas.
On the other hand, in many previous studies, metastatic involvement of lymph nodes has frequently been associated with capsular or peripheral vascularity [5,6,9]. Na et al. added more detailed descriptions: deformed radial and aberrant multifocal patterns [10]. Apart from the presence of subcapsular vessels, Tschammler et al. reported the following findings as typical of malignant lymph nodes: displaced (curved) intranodal vessels, focal absence of perfusion, and aberrant vessels (one or more central vessels, excluding longitudinal vessel branches, forming an angle of 30° with the skin surface or with the longitudinal axis of the lymph node) [11].
In our study, the following vessel patterns were regarded as suggestive of metastatic involvement: peripheral vessels (short vessel segments or branching vessels) or vessels branching irregularly or chaotically with avascular areas. Malignant vascularization was found in 37.5% (15 of 40) of the lymphomatous lymph nodes included in our study (Figs. 9 and 10). This finding may cause difficulties in differentiating lymphomatous lymph nodes from those with metastatic involvement. In either case, however, the lymph nodes presenting this pattern would be flagged as abnormal. From a clinical point of view, the risk here is relatively unimportant. Misdiagnosis of lymphomatous lymph nodes as reactive due to the presence of central or hilar blood flow can have much more serious repercussions.
Lymphomatous lymph nodes in studies based on the classification of node vascularity as central, peripheral, or mixed, lymphomatous and metastatic nodes often fall into the “mixed” category (71% and 85%, respectively, according to Na et al.) [10]. Tschammler et al. reported the presence of one or more vascular sonographic signs of malignancy in 92% of the lymphomas they analyzed and in a similar percentage of the lymph node metastases (96%) [11]. Other authors include lymphomatous lymph nodes and metastatic lymph nodes in a single category reported as “malignant lymph nodes,” which precludes comparison of their data [16–18].
One of the vessel patterns observed in our study was difficult to classify as benign or metastatic. This “indeterminate” pattern was characterized by the presence of multiple vessels, partially branching, that entered the node in a few rows from its longitudinal side. This pattern seems to be similar to the aberrant vessels described by Tschammler et al., but it also resembles a benign pattern of branches of a longitudinal vessel in a lymph node with an eccentric hilum. The indeterminate vessel pattern was observed in 12.5% of the lymphomatous lymph nodes we examined (Fig. 8).
Vessel patterns seen in superficial lymphomatous lymph nodes are highly variable. Lymphomas can present patterns suggestive of metastatic involvement or patterns typical of benign nodal disease. There does not appear to be any vascular pattern that is specific for lymphomatous lymph nodes. Multiplicity of lymph nodes with a “fence-like” distribution may be suggestive of non-Hodgkin lymphoma independently of the vascular pattern [19]. Abnormal vessel pattern always should raise the suspicion of malignancy, and further investigation is mandatory to differentiate metastatic lymph nodes from lymphomatous ones. However, findings of a normal, benign vascular pattern on Doppler studies and a normal lymph node appearance in the gray-scale examination are not sufficient to exclude involvement of a lymphoma.
New perspectives for the assessment of parenchymal microperfusion, not only for the evaluation of large vessel branches, give ultrasound the use of second-generation ultrasound contrast agents in scans with a low mechanical index offer new possibilities for the assessment of lymph node vascularity, including parenchymal microperfusion as well as large vessel branches [14].
Conclusions
The angioarchitecture of lymphomatous superficial lymph nodes is not specific and may be difficult to classify. The patterns range from those encountered in normal or reactive lymph nodes to those associated with metastatic involvement. The finding of a normal or benign vascular pattern in a lymph node with suspected lymphomatous involvement does not eliminate the need for a diagnostic biopsy.
Acnowledgements
This work was supported by a grant from the State Committee for Scientific Research, Warsaw, Poland (2003–2006).
Footnotes
EUROSON-SIUMB 2006 – SIUMB Award for the Communication presented at the 18th European Congress of Ultrasound in conjunction with XVII Congresso Nazionale SIUMB.
References
- 1.Esen G., Gurses B., Yilmaz M.H. Gray scale and power Doppler US in the preoperative evaluation of axillary metastases in breast cancer patients with no palpable lymph nodes. Eur J Radiol. 2005;15:1215–1223. doi: 10.1007/s00330-004-2605-9. [DOI] [PubMed] [Google Scholar]
- 2.Gritzmann N., Czembirek H., Hajek P., Karnel F., Türk R., Frühwald F. Sonographie bei zervikalen Lymphknotenmetastasen. Radiologe. 1987;27:118–122. [PubMed] [Google Scholar]
- 3.Prayer L., Winkelbauer H., Gritzmann N., Winkelbauer F., Helmer M., Pehamberger H. Sonography versus palpation in the detection of regional lymph-node metastases in patients with malignant melanoma. Eur J Cancer. 1990;26:827–830. doi: 10.1016/0277-5379(90)90163-n. [DOI] [PubMed] [Google Scholar]
- 4.Saiag P., Bernard M., Beauchet A., Bafounta M.L., Bourgault-Villada I., Chagnon S. Ultrasonography using simple diagnostic criteria vs palpation for the detection of regional lymph node metastases of melanoma. Arch Dermatol. 2005;141:183–189. doi: 10.1001/archderm.141.2.183. [DOI] [PubMed] [Google Scholar]
- 5.Ahuja A.T., Ying M., Ho S.S.Y., Metreweli C. Distribution of intranodal vessels in differentiating benign from metastatic neck nodes. Clin Radiol. 2001;56:197–201. doi: 10.1053/crad.2000.0574. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q., Takashima S., Takayama F. Detection of occult metastatic lymph nodes in the neck with gray-scale and power Doppler US. Acta Radiol. 2001;42:312–319. doi: 10.1080/028418501127346701. [DOI] [PubMed] [Google Scholar]
- 7.Wu C.H., Chang Y.L., Hsu W.C., Ko J.Y., Sheen T.S., Hsieh F.J. Usefulness of Doppler spectral analysis and power Doppler sonography in the differentiation of cervical lymphadenopathies. AJR Am J Roentgenol. 1998;171:503–509. doi: 10.2214/ajr.171.2.9694484. [DOI] [PubMed] [Google Scholar]
- 8.Ying M., Ahuja A. Sonography of neck lymph nodes. Part I: normal lymph nodes. Clin Radiol. 2003;58:351–358. doi: 10.1016/s0009-9260(02)00584-6. [DOI] [PubMed] [Google Scholar]
- 9.Wu C.H., Shih J.C., Chang Y.L., Lee S.Y., Hsieh F.J. Two-dimensional and three-dimensional power Doppler sonographic classification of vascular patterns in cervical lymphadenopathies. J Ultrasound Med. 1998;17:459–464. doi: 10.7863/jum.1998.17.7.459. [DOI] [PubMed] [Google Scholar]
- 10.Na D.G., Lim H.K., Byun H.S., Kim H.D., Ko Y.H., Baek J.H. Differential diagnosis of cervical lymphadenopathy: usefulness of color Doppler sonography. AJR Am J Roentgenol. 1997;168:1311–1316. doi: 10.2214/ajr.168.5.9129432. [DOI] [PubMed] [Google Scholar]
- 11.Tschammler A., Ott G., Schang T., Seelbach-Goebel B., Schwager K., Hahn D. Lymphadenopathy: differentiation of benign from malignant disease-Color Doppler US assessment of intranodal angioarchitecture. Radiology. 1998;208:117–123. doi: 10.1148/radiology.208.1.9646801. [DOI] [PubMed] [Google Scholar]
- 12.Yang W.T., Chang J., Metreweli C. Patients with breast cancer: differences in color Doppler flow and gray-scale US features of benign and malignant axillary lymph nodes. Radiology. 2000;215:568–573. doi: 10.1148/radiology.215.2.r00ap20568. [DOI] [PubMed] [Google Scholar]
- 13.Ying M., Ahuja A., Brook F. Accuracy of sonographic vascular features in differentiating different causes of cervical lymphadenopathy. Ultrasound Med Biol. 2004;30:441–447. doi: 10.1016/j.ultrasmedbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Rubaltelli L., Khadivi Y., Tregnaghi A. Evaluation of lymph node perfusion using continuous mode harmonic ultrasonography with a second-generation contrast agent. J Ultrasound Med. 2004;23:829–836. doi: 10.7863/jum.2004.23.6.829. [DOI] [PubMed] [Google Scholar]
- 15.Giovagnorio F., Galluzzo M., Andreoli Ch, De Cicco M.L., David V. Color Doppler sonography in the evaluation of superficial lymphomatous lymph nodes. J Ultrasound Med. 2002;21:403–408. doi: 10.7863/jum.2002.21.4.403. [DOI] [PubMed] [Google Scholar]
- 16.Chang D.B., Yuan A., Yu C.J., Luh K.T., Kuo S.H., Yang P.C. Differentiation of benign and malignant cervical lymphnodes with color Doppler sonography. Am J Roentgenol. 1994;162:965–968. doi: 10.2214/ajr.162.4.8141027. [DOI] [PubMed] [Google Scholar]
- 17.Giovagnorio F., Caiazzo R., Avitto A. Evaluation of vascular patterns of cervical lymph nodes with power Doppler sonography. J Clin Ultrasound. 1997;25:71–76. doi: 10.1002/(sici)1097-0096(199702)25:2<71::aid-jcu4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Wu C.H., Hsu M.M., Chang Y.L., Hsieh F.J. Vascular pathology of malignant cervical lymphadenopathy. Qualitative and quantitative assessment with power Doppler ultrasound. Cancer. 1998;83:1189–1196. [PubMed] [Google Scholar]
- 19.Koischwitz D., Gritzmann N. Ultrasound of the neck. Radiol Clin N Am. 2000;38:1029–1104. doi: 10.1016/s0033-8389(05)70219-0. [DOI] [PubMed] [Google Scholar]


