Abstract
Transplantation is considered definitive therapy for acute or chronic irreversible pathologies of the liver, and the increased survival rates are mainly due to improved immunosuppressive therapies and surgical techniques. However, early diagnosis of possible graft dysfunction is crucial to liver graft survival. Diagnostic imaging plays an important role in the evaluation of the liver before and after transplant and in the detection of complications such as vascular and biliary diseases, acute and chronic rejection and neoplastic recurrence. Integrated imaging using color-Doppler, CT, MRI and traditional x-ray reach a high level of sensitivity and specificity in the management of transplanted patients.
Keywords: Hepatic-transplant, Diagnostic imaging, Color-Doppler US, x-ray, CT, MRI
Sommario
Il trapianto epatico è il migliore trattamento per l'insufficenza epatica terminale.
Il miglioramento della sopravvivenza postoperatoria è dovuta soprattutto al perfezionamento della terapia immunosoppressiva e delle tecniche chirurgiche.
L'imaging radiologico riveste un ruolo importante sia per la valutazione preoperatoria dei pazienti, identificando eventuali varianti anatomiche che richiedono una modifica della tecnica chirurgica, sia per la diagnosi e la terapia precoce delle complicanze post trapianto, contribuendo alla riduzione della mortalità e della morbilità.
Introduction
Liver transplant is currently considered definitive therapy for acute or chronic irreversible pathologies of the liver and the increased survival rates are mainly due to better immunosuppressive therapies and improved surgical techniques.
Diagnostic imaging plays an important role in the preoperative evaluation of the recipient and in the selection of the donors and also in the follow-up after surgery, thus contributing to the reduction of mortality and morbidity rates.
Color-Doppler Ultrasound (US), multislice-CT and MRI are non-invasive methods used in the preoperative study to evaluate:
-
–
volume and morphology of the liver;
-
–
arterial and venous vascularization in order to identify possible anatomic variations and to evaluate patency;
-
–
the presence of focal and diffuse parenchymal diseases; and
-
–
the presence of portosystemic ascites or shunts.
Hepatic volume is measured to compare the volume of the recipient's liver with that of the donor's to avoid excessive discrepancy between the donor and recipient vessels. The donor liver volume should not exceed that of the recipient's with more than 20%. Small donor livers are better tolerated: in this case a discrepancy of up to 50% is acceptable [5–18].
Evaluation of the portal vein is important as the presence of portal vein thrombosis is a contraindication to transplant, particularly in case of neoplastic thrombosis. The detection of arterial blood flow within the thrombosis at color-Doppler US, or an opaque image at contrast-enhanced CT suggests that the thrombosis is neoplastic. Identification of arterial variations is important to facilitate surgical planning and decide on the type of anastomosis [1–16].
Generally the hepatic arterial vascularity of the donor arises entirely from the celiac trunk. In that case the celiac trunk and a portion of the aortic wall are excised from the donor and subsequently anastomosed to the hepatic artery of the recipient.
In some donors the liver presents a double vascularization: a right hepatic artery arising from the upper mesenteric artery and a left hepatic artery originating from the celiac trunk. In that case a single arterial donor trunk can be created and anastomosed to the hepatic artery in the recipient.
Due to the surgical complexity of a liver transplant and the debilitated condition of the patient, post-transplant complications are frequent. Early diagnosis and therapy are therefore crucial to graft survival.
Vascular complications
Vascular complications occur in 9% of cases mainly related to the artery: thrombosis and stenosis of the hepatic artery, pseudoaneurysm and arteriovenous fistula [4,5]. Venous complications are less frequent and comprise thrombosis or stenosis of the inferior vena cava or the portal vein.
Complications of the hepatic artery
The most frequent post-transplant complication is early or late thrombosis of the hepatic artery which occurs in about 7% of cases, usually at the level of the anastomosis [4]. The main risk factors are ABO blood incompatibility, small vessel dimensions and poor blood flow, damage to the graft associated with prolonged cold ischemia and rejection.
Clinical evolution of artery thrombosis of the liver graft varies considerably, from acute fulminating hepatic necrosis requiring retransplant or bile leak due to biliary necrosis and sepsis. The first clinical–biochemical signs of thrombosis occur when liver parenchyma and gall bladder are already compromised. A careful color-Doppler US monitoring of the patient is therefore necessary in the first weeks after transplant. Color-Doppler US is a reliable technique for evaluating hepatic artery patency with a sensitivity of 60%–80%. Most cases of thrombosis are characterized by absence of hepatic arterial blood flow [5–17], and sometimes intrahepatic arterial flow and collateral formation of vessels can be observed.
However, absence of arterial flow at the level of the hepatic hilus with parvus-tardus waveform in the intrahepatic arterial blood flow suggests thrombosis in the hepatic artery.
The outcome can be false-positive in patients with substantially reduced arterial blood flow due to systemic hypertension. Arteriography therefore remains gold standard to confirm a US diagnosis suggesting thrombosis.
However, thanks to the technological evolution in the recent years, there is now a valid alternative to the invasive techniques for identifying thrombosis of the hepatic artery represented by multislice-CT and the development of 3D reconstruction algorithms which present a sensitivity of 100% and specificity of 89%. Angio-MR reaches a sensitivity of 86%, as false-positive outcomes are often caused by artefacts due to the presence of metal clips proximal to the hepatic artery anastomosis [5].
Hepatic artery stenosis occurs in 3%–10% of cases within the first three months from transplant, generally situated proximal to the anastomosis [7]. Color-Doppler US can evidence a hemodynamically significant hepatic artery stenosis when peak systolic velocity (Vps) exceeds 2–3 m/s and there is turbulence flow at the level of the stenosis.
Also a tardus-parvus waveform distal to the anastomosis in the intrahepatic artery suggests a hemodynamically significant stenosis in the hepatic artery with a sensitivity and specificity of 73% [5]. If there is suspicion of stenosis, a further diagnostic study using angio-CT and/or angiography is required.
Early diagnosis of hepatic artery stenosis is crucial as immediate balloon angioplasty is required to avoid progress of the thrombosis [12] and consequent hepatic hypoperfusion, biliary ischemia and graft failure.
Post-transplant pseudoaneurysm in the hepatic artery is often situated proximal to the anastomosis. It is a rare complication, which can become lethal if it is not embolized [6]. Pseudoaneurysms are usually asymptomatic, and if they are not detected they can burst and cause biliary tract hemorrhage and peritoneal or gastrointestinal hemorrhage. Diagnosis can be made using contrast enhanced CT or color-Doppler US. At ultrasound examination a pseudoaneurysm appears as a hypoechogenic area mimicking a fluid collection. A correct diagnosis can be obtained using color-Doppler US which can detect arterial blood flow within the lesion. Angio-CT can confirm the diagnosis showing vascular enhancement after injection of contrast agent [4–15], and arteriography can further confirm the diagnosis and is used also in embolization treatment.
Artery–portal vein fistula is a frequent complication in transplanted patients who undergo repeated liver biopsies to exclude rejection. It usually occurs within one week from biopsy [4–13]. A fistula can be diagnosed using angio-CT or angiography [20]. In the arterial phase it appears as an early enhancement of a peripheric portal branch or as a limited, triangular area of parenchymal hyperdensity.
Vein complications
Vein complications are less frequent and include thrombosis or stenosis of the portal vein or the inferior vena cava [5–14].
Portal vein complications are often asymptomatic and occur in 2%–3% of transplants due to significant discrepancies between the dimensions of the recipient and donor, excessive vessel length, or hypercoagulability [4,5,9]. Possible symptoms are hypertension with gastric varices, ascitis or enlarged spleen.
Color-Doppler US can detect thrombosis (Fig. 1), evidencing absence of intraportal venous flow, or stenosis showing aliasing effects with increased peak systolic velocity as compared to the vein before the stenosis (Figs. 2 and 3).
Fig. 1.

Color-Doppler US shows portal vein thrombosis.
Fig. 1 L'esame eco-color-Doppler dimostra trombosi della vena porta.
Fig. 2.
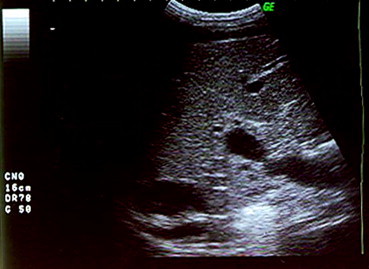
Baseline US shows portal vein focal stenosis.
Fig. 2 L'esame ecografico basale dimostra la presenza di una stenosi focale della vena porta.
Fig. 3.
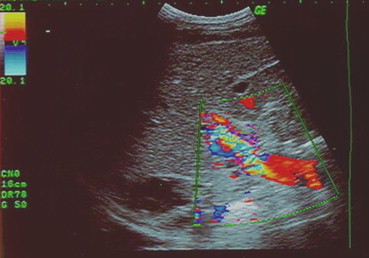
Color-Doppler US shows significant aliasing artefacts within the portal vein suggesting portal vein stenosis.
Fig. 3 L'esame eco-color-Doppler dimostra significative turbolenze di flusso (aliasing) all'interno della vena porta come per stenosi.
Angio-CT provides a good visualization of the thrombosis as an endoluminal filling defect, and the stenosis showing a reduced blood vessel calibre at the level of the anastomosis [9,22–25].
Inferior vena cava complications are very rare, occurring in only 0.8%–2% of transplants. It is an acute lesion in the anastomotic site resulting from technical problems during surgery or due to ab-estrinseco compression caused by hematomas or adjacent fluid collections [5]. Retransplanted patients are at higher risk of late stenosis in the vena cava due to fibrosis in the previous anastomosis. Stenosis at the level of the suprahepatic anastomosis can cause obstruction of the hepatic veins and lead to Budd–Chiari syndrome, abnormal renal function and edema of the legs. It can also lead to thrombosis of the vein distal to the caval stenosis. Color-Doppler US and particularly multislice-CT reconstruction can provide an early diagnosis of stenosis of the vena cava and possible thrombosis before acute hepatic vein obstruction occurs.
At color-Doppler US, the vena cava usually presents biphasic waveforms due to the pulsating movements of the heart. In presence of stenosis the flow will decrease or even reverse at the level of the inferior and suprahepatic vena cava. In order to avoid further pathologies, angioplasty and positioning of an intravascular stent are useful in this case [23–26].
Biliary tract complications
After rejection, biliary tract complications are the second most frequent cause of hepatic graft dysfunction (Figs. 4 and 5). They occur within the first months after transplant in 10%–15% of cases and they result in bile leak or biliary stenosis [4,5]. In order to understand the reason why biliary tract complications occur it should be kept in mind that the biliary tree of the graft is critically dependent on the hepatic artery. Biliary tract complications are thus associated with 90% of cases of arterial thrombosis and more than 70% of cases of hepatic artery stenosis [4].
Fig. 4.

Baseline US shows main biliary duct and right intrahepatic biliary duct dilation.
Fig. 4 L'ecografia basale dimostra la presenza di dilatazione della via biliare principale e delle vie biliari intraepatiche del lobo destro.
Fig. 5.
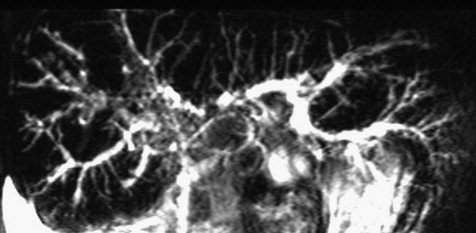
CPRM shows irregular aspect and dilation of the intrahepatic biliary ducts suggesting post-transplant cholangitis.
Fig. 5 Colangio pancreato RM: irregolarità del calibro delle vie biliari intraepatiche come per colangite post trapianto.
Bile leak
Early complications are mainly bile leaks, generally caused by technical problems at the level of the anastomosis or at the Kehr tube.
Management of biliary fistulas depends on the volume. A small leak usually closes spontaneously while a substantial leak can form a biliary collection, and it therefore requires percutaneous drain insertion.
Cholangiography can show a “double duct” sign of contrast enhancement along the T-tube and sometimes a bile collection in the subhepatic space, which can be evidenced also at CT or US examination [5].
In addition to anastomotic bile leaks, also non-anastomotic leaks can be observed, although less frequently. A non-anastomotic leak is a sign of serious complication caused by diffuse necrosis of the bile ducts as a result of thrombosis or hepatic artery stenosis or vasculitis in connection with acute rejection (Fig. 6).
Fig. 6.
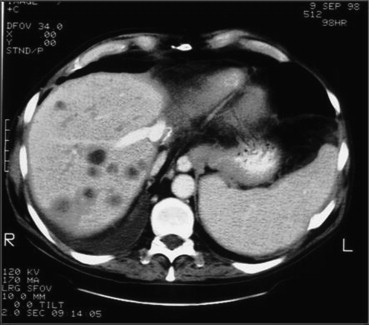
Contrast-enhanced CT during the portal phase shows biliary duct dilation within the right hepatic lobe.
Fig. 6 TC dopo mezzo di contrasto durante la fase portale mostra dilatazione delle vie biliari nel contesto del lobo destro.
Cholangiography can evidence substantial amounts of contrast agent leaking from necrotic and distended biliary ducts. Non-anastomotic leaks associated with hepatic artery thrombosis generally require retransplant.
Obstruction of the biliary ducts
Biliary duct stenosis is one of the most frequent complications, and it can occur both proximal to the anastomosis and elsewhere. Stenosis of the extrahepatic bile ducts at the level of the anastomosis is in most cases caused by surgical problems, and it occurs more often if the bile duct anastomosis is constructed to the recipient's own bile duct rather than to the small intestine [2,4]. It is a result of exaggerated scarring of the perianastomotic tissues or of insufficient vascularization in the distal part.
A stenosis can be detected using cholangiography or, nowadays, cholangio-MR which has shown a sensitivity of 87% and a specificity of 92% [21]. Significant anastomotic stenosis appears as a focal narrowing at the level of the anastomosis and a dilation of the proximal biliary tract, where sometimes calculi or biliary mud is formed. This type of stenosis is successfully treated using percutaneous balloon dilation.
Non-anastomotic biliary stenosis is more complicated, and it occurs in 8% of transplants after ischemia of the biliary ducts and consequent formation of fibrosis and stenosis of the ducts. The main causes of ischemia can be thrombosis or hepatic artery stenosis, ABO blood incompatibility between donor and recipient and chronic rejection. Non-anastomotic biliary stenosis can occur anywhere on the biliary tree, but usually in the biliary hilus. Temporary therapy, while waiting for a suitable donor for retransplant, consists of positioning of one or several biliary stents.
If early or late biliary complications are suspected, first line examinations are US and color-Doppler US to evaluate hepatic artery perfusion. A positive outcome should be confirmed using angio-CT or hepatic arteriography [3,24,26].
Mucocele of the cystic duct
Mucocele of the cystic duct is a rare complication (3%–4% of cases). It occurs when the cystic duct remnant of the graft is overstretched due to an accumulation of mucus inside the duct. The accumulation can become so voluminous that the duct is compressed and obstructed [4].
Rejection
Rejection of the graft is an inflammation caused by genetic incompatibility between the donor and the recipient. The first symptoms usually show at the level of the biliary duct and the portal and suprahepatic vascular endothelium, and in some cases also the arterial branches are involved.
Rejection can be classified as acute cellular rejection and chronic rejection. Acute rejection is more frequent (50%–74% of patients). It generally occurs within the first three weeks from transplant and is by far the main reason for liver graft failure [4,5].
Generally good results are obtained with increased doses of immunosuppressive drugs.
Chronic rejection usually occurs from 5 weeks to 1 year from transplant, fortunately at a lower incidence rate than acute rejection: 2%–17% of patients [4,5]. The symptoms of chronic rejection are varied, ranging from slight bile duct loss and light cholestasis, a situation which is potentially reversible, to a serious rejection leading to loss of the interlobular and septal ducts and serious cholestasis which does not respond to therapy. Histologically, chronic rejection is characterized by a progressive destruction of the intrahepatic bile ducts due to ischemic damage caused by intimal and subintimal infiltration of the arterial branches by macrophage foam cells. Sensitivity and specificity of clinical symptoms and imaging are low.
In acute rejection, color-Doppler US shows only an inhomogeneous parenchyma associated with a periportal hypoechogenic area, and CT scan evidences a hypodensity proximal to the first portal branches.
However, these findings are not specific as they are seen also immediately after transplant due to hepatic perivascular lymphedema, but with no rejection in progress.
Diagnostic imaging is mainly useful to rule out vascular, biliary and infective complications, which can clinically mimic rejection, but the only way to obtain a definitive diagnosis is biopsy.
Infective complications
The principal cause of death after transplant is infection, mainly lung infections and hepatic/biliary infections [4]. During the first month after transplant, bacterial infections are the most frequent, generally caused by mycotic Gram-e, particularly Candida and Aspergillus. As the effect of immunosuppressive drug increases, secondary infections can arise in the third–fourth month due to Mycobacterium tuberculosis and Pneumocystis carinii. As lung infections are frequent, vulnerable patients should have regular chest x-ray examinations [19].
Radiological images of bacterial lung infections are characterized by areas of single or multiple parenchymal consolidations with distribution at the lobar and segmental levels, often associated with small pleural fluid collections.
Viral pneumonia caused by P. carinii is characterized by diffuse interstitial infiltrations with a predominantly reticular pattern, but not associated with pleural fluid collections.
In patients with a clinical or radiological suspicion of lung infection, high resolution CT can provide a definitive diagnosis and efficiently monitor the response to therapy.
Post-transplant neoplastic lesions
Transplanted patients are at high risk of neoplastic pathologies due to the immunosuppressive therapy required to reduce the risk of rejection. The risk of developing neoplastic lesions is 100 times higher compared with control subjects of the same age [5], particularly Hodgkin's lymphoma, B-cell or squamous cell neoplastic cutaneous lesions and Kaposi's sarcoma.
Recurrence of the original disease has also been an important reason for liver graft failure, and this fact has led to a revision of the criteria for including transplant candidates on the national list. Liver transplant is now permitted in candidates with Hepatocellular Carcinoma (HCC) only if the lesion is monofocal <5 cm, or in case of multiple lesions with a maximum of three lesions of <3 cm diameter. With these new criteria the recurrence rate has been reduced to less than 10% and survival rate has reached 70%–75% [4–10].
Fluid collections
Post-transplant fluid collections, hematomas, seromas and bilomas, are frequent. A hematoma can occur in or proximal to the graft or in the adrenal gland. Adrenal hematomas are always right-sided and they are due to accidental ligation of the adrenal vein during anastomosis of the inferior vena cava causing venous infarction of the gland [8].
Fluid collections are easily identified on a CT scan or US image. It should be borne in mind that they can develop infection in immunodepressed patients, and percutaneous needle aspiration is therefore required particularly in extensive lesions.
Conclusions
Invasive and non-invasive diagnostic imaging plays a crucial role in the follow-up after transplant in monitoring the vascular and biliary anastomosis, detection of pathologies which can arise as a consequence of immunosuppressive drugs, and identification of new or recurrent neoplastic lesions. Post-transplant monitoring should include daily ultrasound and color-Doppler US examinations combined with daily chest x-rays during the first week in order to rule out infective pathologies.
In patients with clinical suspicion of post-transplant complications, angio-CT and angio-MR should be carried out, and in particular cases also invasive angiography, which is also required in the preoperative treatment.
Thanks to improved surgical techniques, a more adequate selection of candidates and more accurate monitoring of the grafts, the 1-year mean survival rate is now over 85% both in adults and in pediatric patients, whereas the 1-year death rate is under 1%.
References
- 1.Romano S., Tortora G., Scaglione M. MDCT imaging of post intervention liver: a pictorial essay. Eur J Radiol. 2005 Mar;53(3):425–432. doi: 10.1016/j.ejrad.2004.12.019. [review] [DOI] [PubMed] [Google Scholar]
- 2.Gualdi G., Di Blasi C., Cascinai E. Universo; 2002. Imaging clinico. p. 345–7. [Google Scholar]
- 3.Almusa O., Federle M.P. Abdominal imaging and intervention in liver transplantation. Liver Transpl. 2006 Feb;12(2):184–193. doi: 10.1002/lt.20697. [review] [DOI] [PubMed] [Google Scholar]
- 4.Golfieri R., Giampalma E., Fusco F. Il trapianto ortotopico di fegato (OLT): il contributo della diagnostica per immagini e della radiologia interventistica nella preparazione al trapianto e nella gestione delle complicanze parte 1–2. Radiol Med (Torino) 2005 Nov–Dec;110(5–6):391–481. [Google Scholar]
- 5.Gore R.M., Levine M.S. vol. 3. Verduci; 2002. Imaging del fegato trapiantato. (Trattato di radiologia gastro-intestinale). [sezione XI, fegato] [Google Scholar]
- 6.Leelaudomlipi S., Bramhall S.R., Gunson B.K. Hepatic artery aneurysm in adult liver transplantation. Transpl Int. 2003 Apr;16(4):257–261. doi: 10.1007/s00147-003-0551-0. [DOI] [PubMed] [Google Scholar]
- 7.Abbasoglu O., Levy M.F., Vodapally M.S. Hepatic artery stenosis after liver transplantation: incidence, presentation, treatment and long term outcome. Transplantation. 1997;63:250–255. doi: 10.1097/00007890-199701270-00013. [DOI] [PubMed] [Google Scholar]
- 8.Pandharipande P.V., Lee V.S., Morgan G.R. Vascular and extravascular complications of liver transplantation. AJR Am J Roentgenol. 2001;177:1101–1107. doi: 10.2214/ajr.177.5.1771101. [DOI] [PubMed] [Google Scholar]
- 9.Katyal S., Oliver J.H., III, Buck D.G. Detection of vascular complications after liver transplantation: early experience in multislice CT angiography with volume rendering. AJR Am J Roentgenol. 2000;175:1735–1739. doi: 10.2214/ajr.175.6.1751735. [DOI] [PubMed] [Google Scholar]
- 10.Vivarelli M., Bellusci R., Cucchetti A. Low recurrence rate of hepatocellular carcinoma after liver transplantation: better patient selection or lower immunosuppression? Transplantation. 2002;27:1664–1665. doi: 10.1097/00007890-200212270-00017. [DOI] [PubMed] [Google Scholar]
- 11.Shaw A.S., Ryan M., Beese R.C. Liver transplantation. Imaging. 2002;14:314–328. [Google Scholar]
- 12.Nolten A., Sproat I.A. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553–559. doi: 10.1148/radiology.198.2.8596865. [DOI] [PubMed] [Google Scholar]
- 13.Nghiem H.V., Tran K., Winter T.C. Imaging of complication in liver transplantation. Radiographics. 1996;16:825–840. doi: 10.1148/radiographics.16.4.8835974. [DOI] [PubMed] [Google Scholar]
- 14.Ito K., Siegelman E.S., Stolpen A.H. MR imaging of complications after liver transplantation. AJR Am J Roentgenol. 2000;175(4):1145–1149. doi: 10.2214/ajr.175.4.1751145. [DOI] [PubMed] [Google Scholar]
- 15.Quiroga S., Sebastia M.C., Margarit C. Complication of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085–1102. doi: 10.1148/radiographics.21.5.g01se061085. [DOI] [PubMed] [Google Scholar]
- 16.Pirat A., Ozgur S., Torgay A. Risk factors for postoperative respiratory complications in adult liver transplant recipients. Transplant Proc. 2004;36:218–220. doi: 10.1016/j.transproceed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 17.De Gaetano A.M., Cotroneo A.R., Maresca G. Color Doppler sonography in the diagnosis and monitoring of arterial complications after liver transplantation. J Clin Ultrasound. 2000;28:373–380. doi: 10.1002/1097-0096(200010)28:8<373::aid-jcu1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Zetterman R.K., McCashland T.M. Long term follow-up of the orthotopic liver transplantation patient. Semin Liver Dis. 1995;15:173–180. doi: 10.1055/s-2007-1007274. [DOI] [PubMed] [Google Scholar]
- 19.Robiati S., Veltri A., Martina A.C. La TC nello studio delle complicanze toraco-polmonari dopo trapianto del fegato. Radiol Med. 2000;100:444–452. [PubMed] [Google Scholar]
- 20.Vignali C., Cioni R., Petruzzi P. Role of interventional radiology in the management of vascular complications after liver transplantations. Transplant Proc. 2004;36:552–554. doi: 10.1016/j.transproceed.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Beltran M.M., Marugan R.B., Oton E., Blesa C., Nuno J. Accuracy of magnetic resonance cholangiography in the evaluation of late biliary complications after orthotopic liver transplantation. Transplant Proc. 2005 Nov;37(9):3924–3925. doi: 10.1016/j.transproceed.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Shah T.U., Semelka R.C., Voultsinos V. Accuracy of magnetic resonance imaging for preoperative detection of portal vein thrombosis in liver transplant candidates. Liver Transpl. 2006 Nov;12(11):1682–1688. doi: 10.1002/lt.20873. [DOI] [PubMed] [Google Scholar]
- 23.Alfidja A., Abergel A., Chabrot P. Portal vein stenosis and occlusion stenting after liver transplantation in two adults. Acta Radiol. 2006 Mar;47(2):130–134. doi: 10.1080/02841850500444705. [DOI] [PubMed] [Google Scholar]
- 24.Alsharabi A., Zieniewicz K., Patkowski W. Assessment of early biliary complications after orthotopic liver transplantation and their relationship to the technique of biliary reconstruction. Transplant Proc. 2006 Jan–Feb;38(1):244–246. doi: 10.1016/j.transproceed.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Perkins J. Hepatic venous outflow obstruction after piggyback orthotopic liver transplantation. Liver Transpl. 2006 Jan;12(1):159–160. [PubMed] [Google Scholar]
- 26.Amesur N.B., Zajko A.B. Interventional radiology in liver transplantation. Liver Transpl. 2006 Mar;12(3):330–351. doi: 10.1002/lt.20731. [review] [DOI] [PubMed] [Google Scholar]


