Abstract
Orthotopic liver transplantation (OLT) involves the substitution of a diseased native liver with a normal liver (or part of one) taken from a deceased or living donor. Considered an experimental procedure through the 1980s, OLT is now regarded as the treatment of choice for a number of otherwise irreversible forms of acute and chronic liver disease.
The first human liver transplantation was performed in the United States in 1963 by Prof. T.E. Starzl of the University of Colorado. The first OLT to be performed in Italy was done in 1982 by Prof. R. Cortesini. The procedure was successfully performed at the Policlinico Umberto I of the University of Rome (La Sapienza).
The paper reports the indications for liver transplantation, donor selection and organ allocation in our experience, surgical technique, immunosuppression, complications and results of liver transplantation in our center.
Keywords: Liver transplantation, Piggyback, Living donor, Split-liver, Recipient outcome, Immunosuppression, Hepatocellular carcinoma
Sommario
Il trapianto di fegato (OLT), cioè la sostituzione del fegato nativo, malato, con un fegato normale, intero o con una parte di esso ottenuto da un donatore cadavere (deceased donor) o vivente (living donor), si è trasformato da una procedura considerata, fin negli anni '80, sperimentale, a una indicazione terapeutica elettiva per la cura di molte patologie epatiche acute o croniche altrimenti incurabili.
Il primo trapianto di fegato nell'uomo è stato effettuato nel 1963 dal Prof. T.E. Starzl presso l'Università del Colorado (USA). Nel 1982 il Prof. R. Cortesini portò a termine con successo, presso il Policlinico Umberto I, Università “La Sapienza” di Roma, il primo OLT effettuato in Italia.
In questo lavoro riportiamo le indicazioni al trapianto, la selezione dei donatori e allocazione dei graft secondo le nostre procedure, la tecnica chirurgica, la terapia immunosoppressiva, le complicanze e i risultati di questa tecnica presso il nostro centro.
Introduction
Orthotopic liver transplantation (OLT) involves the substitution of a diseased native liver with a normal liver (or part of one) taken from a deceased or living donor. Considered an experimental procedure through the 1980s, OLT is now regarded as the treatment of choice for a number of otherwise irreversible forms of acute and chronic liver disease.
The first human liver transplantation was performed in the United States in 1963 by Prof. T.E. Starzl of the University of Colorado [1]. By 1967 nine other liver transplantations had been carried out in various parts of the world, but the results were unsatisfactory. That same year, Starzl himself performed the first transplantation associated with recipient survival of more than one year [2]. Between 1968 and 1983, a total of 170 OLTs were carried out at the University of Colorado, and the one-year survival rate was 30%. The immunosuppressant regimen used in those days was based on azathioprine, corticosteroids, and anti-thymocyte globulins.
During the same period, 138 OLTs were done in Cambridge (UK), and the results were just as disappointing [3]. However, in the early 1980s, introduction of the new immunosuppressant, cyclosporine, produced a rapid and significant increase in survival rates [4].
The first OLT to be performed in Italy was done in 1982 by Prof. R. Cortesini. The procedure was successfully performed at the Policlinico Umberto I of the University of Rome (La Sapienza).
In 1988, the first living-donor liver transplantation (LDLT) was carried out by Raia in Brazil [5], and the second was performed in 1989 by Strong in Australia [6]. In 1990, LDLT programs were set up in Japan by Nagasue and by Broelsch in the United States [7]. LDLT was first performed in Italy in 1997 by Prof. D.F. D'Amico of Padua.
Over 40 years have passed since the first ground-breaking steps were taken, and the results have been so remarkable that OLT is now considered the treatment of choice for many patients suffering from acute or chronic liver diseases.
Data from the European Liver Transplant Registry (ELTR) reveal that there are 137 active Transplant Centers in 23 countries. In Europe, a total of 68,776 OLTs were performed in 61,718 recipients from 1968 through December 2005, and 4736 procedures were performed in 2005 alone.
Indications for liver transplantation
Liver transplantation is indicated for adults and children with acute or chronic liver disease that cannot be treated medically or with alternative forms of surgery. The most common indications are cirrhosis (viral, alcohol-related, cryptogenic), cholestatic liver disease, metabolic liver disease, acute liver failure, liver tumors, and other forms of liver disease.
Causes of chronic liver failure
Hepatic cirrhosis
Hepatic cirrhosis can lead to end-stage liver disease. It has various causes, the most common being chronic infection with the hepatitis C (HCV) or B (HBV) virus or autoimmune forms of hepatitis.
-
•
HCV: Today, HCV-related cirrhosis is the most common indication for OLT [8]. Approximately 3.9 million Americans suffer from chronic HCV infections [9], and it has been estimated that around 20% of these individuals will develop cirrhosis within 20 years of the onset of chronic infection [10]. The process seems to be accelerated by alcohol abuse [11]. Although 10-year survival rates of around 80% have been documented among patients with well-compensated cirrhosis, this figure drops dramatically when complications occur (<50% at five years) [12]. Patients with cirrhosis are also at increased risk for hepatocellular carcinoma (HCC), approximately 1–4% per year [13]. Antiviral therapy is not recommended in patients with cirrhosis and signs of liver failure [15]. Liver transplantation represents the only treatment option in these cases although virtually all recipients have persistent viremia after transplantation [16]. The risk that cirrhosis will also develop in the graft is increased by several factors including high-level viremia, early recurrence (<1 year after OLT), donor age >50 years, poor organ quality, and re-transplantation; there is currently a good deal of controversy over the role of donor status (live versus deceased). Over the past decades, mortality rates among HCV-positive patients undergoing OLT have dropped progressively, but the cause of this decline is unknown [17]. There is a clear need for longer-term studies on the natural history of the disease after liver transplantation and the development of new antiviral drug protocols for the post-transplant management of persistent hepatitis C.
-
•
HBV: The natural history of chronic hepatitis B is variable. Patients may develop cirrhosis, liver failure, or HCC. The prognosis is closely related to the severity of the histological damage and the presence of active viral replication. Thirty to forty percent of all patients with chronic active HBV-related infections respond positively to interferon therapy [18], but this approach is not recommended in patients with cirrhosis due to the high risk of adverse effects [19]. Interferon has also proved to be of little value in the treatment of recurrent infection after the transplantation [20]. The prospect for HBV-positive patients was quite bleak due to the high frequency of recurrent infections that led to rapidly progressive forms of chronic hepatitis. The picture changed dramatically with the demonstration that the administration of immunoglobulins raised against the HBV (HBIG) reduced both the incidence and severity of recurrent HBV infections. Today, mid- and long-term survival rates among these patients are excellent: 76% at five years and 65% at 10 years according to the ELTR. The high cost of immunoglobulin therapy has led to the development of new drugs like lamivudine and adefovir, which offer new possibilities for the pre-transplant treatment of viremia before [14] (viremia <105 copies/mL at the time of surgery) and after transplantation [21].
-
•
HDV: The “defective” delta virus causes hepatitis only when the patient is or has been infected with HBV. In Europe from 1968 through 2004, no more than 8% of all cases of viral cirrhosis were caused by this virus.
-
•
Autoimmune hepatitis: The cause of this disease is unknown. It shows a clear predilection for women and involves progressive inflammatory and fibrotic changes in the liver that can result in cirrhosis. Corticosteroid therapy produces clinical remission in up to 80% of all cases and has improved both short- and long-term survival rates. Nonetheless, some patients develop portal hypertension and liver failure, and for these individuals, the only hope is liver transplantation. The results are generally excellent [22].
Alcohol-related cirrhosis
Cirrhosis caused by alcohol abuse is the second most frequent cause of OLT in both Europe and the United States [17]. In the latter country, an estimated 12,000 people die each year from alcohol-related liver disease [23]. Abstinence is the only solution for many patients, and it significantly increases survival rates even among those who have already developed cirrhosis. The use of liver transplants in patients with alcoholic cirrhosis is controversial: the main issues are how long the patent should be required to abstain from drinking before transplantation and the risk that alcohol abuse will resume after the transplantation. Many transplant centers insist upon a period of abstinence of at least 6 months before a patient can even be inserted in the transplant waiting list, and prevention of relapses can only be accomplished with a multi-disciplinary approach that includes psychiatric and psychological support.
Cholestatic liver disease
A number of conditions in this category can give rise to chronic liver failure, including primary and secondary biliary cirrhosis (PBC and SBC) and the cholestatic disorders of childhood and infancy.
-
•
PBC: This is a rare disease that affects mainly women between the fourth and seventh decades of life. The causes are currently unknown. Treatment with ursodeoxycholic acid (UDCA) can attenuate symptoms and delay the need for liver transplantation [24], but organ replacement is the only approach that is potentially curative [25]. The use of liver transplants in these patients has dramatically increased survival rates over those estimated by means of the Mayo Clinic model [26].
-
•
SBC: Secondary biliary cirrhosis is a multifactorial disorder that involves progressive inflammatory stenosis of the intra- and extrahepatic bile ducts. A number of causes have been identified including postoperative stenosis, gall stones, biliary atresia, and cystic fibrosis.
-
•
PSC: Primary sclerosing cholangitis is generally diagnosed in young males, and 70–75% are suffering from inflammatory bowel disease (usually ulcerative colitis) [27]. Use of UDCA can lead to clinical improvement but it has had little impact on survival rates. Symptomatic forms of SBC generally result in liver failure within 10 years of diagnosis [28]. OLT is the only hope in these cases, and three-year survival rates of over 70% have been achieved [29,30].
-
•
Cholestatic liver disease in pediatric patients: This category comprises various conditions, including extrahepatic biliary atresia [31], Alagille disease [32], Caroli disease [33], congenital biliary fibrosis, choledochal cysts, Byler disease (familial intrahepatic cholestasis), and alpha-1 antitrypsin deficiency [34]. The one responsible for most liver transplantations is congenital biliary atresia, a disorder that causes fibrosis of the intra- and extrahepatic bile ducts. Its cause is unknown. The prognosis is poor, and there is currently no medical treatment for this disease. Death generally occurs within the first or second year of life [35]. If the condition is diagnosed early, the Kasai portoenterostomy can produce excellent results [36]. Survival rates are significantly better in infants who undergo this procedure within the first 3 months of life. If this surgical approach fails, however, or when the disease is diagnosed after the age of 3 months, OLT is the only option [37]. Many children treated with the Kasai procedure go on to develop cirrhosis that requires OLT, but delaying transplantation until the child is 5–10 years of age (as opposed to performing an OLT in a newborn) has obvious advantages [38].
Metabolic disorders
With the passage of time, many metabolic diseases can lead to hepatic cirrhosis. The most common examples are hereditary hemochromatosis, Wilson disease, and alpha-1 antitrypsin deficiency.
-
•
Hereditary hemochromatosis: This autosomal recessive disorder has an incidence in Caucasians of 1:200–1:300. It is caused by the progressive accumulation of iron in the tissues due to a defect in intestinal absorption [39]. The long-term effects include hepatic cirrhosis, HCC, dilatative cardiomyopathy, diabetes mellitus, arthritis, and hypogonadism [40]. Medical treatment consists in repeated phlebotomies or the use of alkylating agents. Liver transplantation is the treatment of choice for hemochromatosis patients with end-stage liver disease [41], but the results in these cases are significantly worse than they are in patients with other diseases [42]. Cardiomyopathy, the high incidence of postoperative infections, and the high levels of iron seem to contribute to this [43].
-
•
Wilson disease: This disease, which is also autosomal recessive, is caused by abnormal metabolism of copper [44]. It can produce acute and chronic forms of liver disease, as well as neurological disturbances, hemolytic anemia, and renal involvement. Chelating therapy based on the use of penicillamine or trientene produces excellent results in many patients with chronic disease [45]. In some cases, especially those characterized by acute liver failure, transplantation is the only option [46]. In general, liver transplantation cures the genetic abnormality that causes copper to accumulate in the body, putting an end to all of the metabolic disturbances. Chelating therapy can thus be discontinued [47].
-
•
Alpha-1 antitrypsin deficiency: This is the most common hereditary indication for OLTs in pediatric patients [34]. Twenty-five percent of children with this autosomal co-dominant disorder develop jaundice and cirrhosis by age 10; in another 25% of cases, they develop during the second decade of life [48]. Liver transplantation is currently the only potentially curative therapeutic option. In fact, transplantation results in expression of the donor's normal alpha-1 antitrypsin phenotype. The results are excellent [49].
-
•
Other diseases: Liver transplantation may be required in a number of other metabolic disorders, such as cystic fibrosis, type 1 Crigler–Najjar disease, protoporphyria, primary hyperoxaluria, tyrosinemia, glycogenoses, homozygous hypercholesterolemia, and familial amyloid polyneuropathy.
Cryptogenic cirrhosis
Cryptogenic cirrhosis still represents a significant percentage of all cases of hepatic cirrhosis: in up to 8% of all cases of cirrhosis, the cause cannot be identified. Recent studies conducted in Europe and the United States have shown that in a high percentage of these cases the cirrhosis is the result of non-alcoholic steato-hepatitis (NASH) [50,51]. This is a particular variant of non-alcoholic fatty liver disease (NAFLD), which includes also hepatic steatosis, commonly associated with obesity, hypertension, type 2 diabetes mellitus, hyperinsulinemia and dyslipidemia. The main cause of this disease is the Western lifestyle as a whole, and an increase in its incidence seems inevitable.
Acute liver failure
The term acute liver failure (ALF) refers to the onset of hepatic encephalopathy, seriously altered coagulation, and jaundice in the absence of previous signs of chronic liver disease [52]. This condition must be distinguished from acute liver failure that develops as the result of decompensation in a patient with chronic liver disease, which is referred to as acute-on-chronic liver failure (ACLF).
The various causes of ALF include hepatitis caused by HAV, HBV, HDV, or HEV; hepatotoxicity provoked by paracetamol or other drugs (Reye syndrome in children treated with acetylsalicylic acid); poisoning caused by the ingestion of hepatotoxins (Amanita phalloides); the acute form of Wilson disease; trauma; and surgery (damage to or ligation of the vessels of the hepatic hilum) [53,54]. Often the cause of ALF cannot be identified (cryptogenic forms) [55].
Patients with ALF experience progressive deterioration of liver function that leads to complete decompensation within 2 to 6 months of symptom onset [56].
In some cases, the disease resolves spontaneously without provoking any long-term damage, but this outcome is rare in children under 10 or adults over the age of 40 [57,58].
There is currently no specific treatment for severe cases of ALF. The only solution is an emergency liver transplantation, and until a suitable organ can be found, the patient is generally placed in the Intensive Care Unit. Artificial liver support (e.g., the MARS system) can be useful in these cases [59]. In the past, the results of OLT in ALF patients were not very encouraging for two main reasons [60]. First of all, when the transplantations were performed the patients were already suffering from cerebral edema and multi-system failure (hepato-renal syndrome, MOF) [61]; second, due to the extreme urgency of the procedure, the organs transplanted were of marginal quality and in some cases incompatible with the donor's ABO-blood type.
Liver tumors
-
•
HCC: This is the most common liver tumor and the fifth most common malignant tumor in the world [62,63]. The risk factors that contribute to its development are multiple and include hepatic cirrhosis and all of its causes (alcohol abuse, HCV, HBV, cryptogenic cirrhosis, hemochromatosis, PBC, and SBC) [64]. The role of OLT in the treatment of HCC has changed continually over the past two decades. Initially, the results were decidedly disappointing (high rates of recurrence, one- and five-year survival rates of no more than 25% and 18%, respectively) [65] whereas today transplantation is rightly considered the treatment of choice, at least in a certain well selected subset of patients with HCC [66,67]. In 1996 Mazzaferro et al. published a report that radically modified the indications for OLT in HCC patients [68]. The survival rates they reported were similar to those in patients with non-tumoral liver disease (75% at four years), and the recurrence rate was less than 10%. Today, the Milan Criteria proposed by this group (single HCC ≤5 cm in diameter or two to three lesions, none exceeding 3 cm; absence of macroscopic vascular invasion or distant metastases) are used by transplant centers throughout the world to identify HCC patients eligible for OLT. Nonetheless, there are still a number of unresolved issues. As a result of the adoption of extremely rigid selection criteria, a significant number of HCC patients have been denied potentially curative treatment. Proposals to extend eligibility to patients with large HCCs are currently being examined [69,70]. Five-year survival rates of 50% have been reported with the University of California San Francisco (UCSF) criteria (a single nodule measuring <6.5 cm in diameter or up to three nodules, each measuring ≤4.5 cm with a total tumor diameter of 8 cm or less) [71].
-
•
Cholangiocarcinoma: Intrahepatic cholangiocarcinomas and those involving the extrahepatic bile ducts (e.g., Klatskin tumors) are particularly aggressive tumors, and in many cases distant metastases are already present when the primary tumor is diagnosed. Due to the currently limited availability of donors, the potential role of OLT in the treatment of these tumors has yet to be satisfactorily defined. Very few series have been reported thus far, but those that have are all characterized by high recurrence rates and disappointing results in terms of long-term survival [72–74]. However, good results were reported for a series of 28 rigorously selected cases (2% of all patients) treated at the Mayo Clinic with OLT and adjuvant chemotherapy [75]. Selection criteria must be extremely stringent, however, and as a result, very few patients with this type of liver tumor will be eligible for OLT.
-
•
Metastases: The use of OLT to manage liver metastases from colorectal cancer or other unresectable tumors has produced discouraging results with high rates of recurrence involving the transplanted organ or other sites. At this time, the only indication for OLT in the treatment of metastatic disease is the rare occurrence of liver metastases from neuroendocrine tumors [76].
-
•
Benign tumors: In some cases, a patient with a benign liver tumor (e.g., hepatic adenoma, adenomatosis, hemangioma, focal nodular hyperplasia, regenerative nodular hyperplasia) may require OLT. Transplantation is an option only in cases in which the tumor produces severe symptoms (pain, compression) or is so large that it compromises organ function. Since the underlying pathology is benign, the results are generally excellent.
-
•
Other tumors: In rare cases, patients with hepatoblastomas, angiosarcomas, carcinosarcomas, or epithelioid hemangioendotheliomas have undergone OLT [77]; priority is given to maintaining a five-year survival rate of at least 50%.
Other diseases
OLT may also be indicated for the management of certain rare diseases, such as the Budd–Chiari syndrome, which causes thrombosis of the hepatic veins; polycystic liver disease (Fig. 1), a genetic disease often associated with polycystic renal disease and with chronic renal failure; and parasitic diseases (e.g., schistosomiasis, alveolar echinococcosis, cystic hydatidosis).
Fig. 1.

Polycystic liver disease. Intraoperative image before liver removal. Personal experience.
Fig. 1 Malattia policistica del fegato. Foto intraoperatoria prima dell'epatectomia. Esperienza personale.
In evaluating patients for possible liver transplantations, the most important question to ask is whether the procedure is really needed. The natural history of the liver disease should be compared with the survival rates observed after OLT [78]. A number of prognostic models have been developed for this purpose.
The Mayo Clinic prognostic model for primary biliary cirrhosis is widely used to evaluate candidates with this condition [26], but it cannot be used for patients with other types of liver disease.
The Child–Turcotte–Pugh (CTP) [79] classification was developed to estimate the risk associated with surgery to create portocaval shunts in patients with cirrhosis and bleeding esophageal varices. It has been widely acclaimed as a simple approach for estimating the general prognosis in cases of cirrhosis. Patients are divided into three classes (A, B, and C) based on scores assigned to five variables (ascites, encephalopathy, PT%, serum bilirubin, and serum albumin). Despite its limitations (e.g., subjective evaluation of parameters like ascites or encephalopathy), this model has been widely used in clinical practice. Over a third of all CTP class C patients (10 points or more) on waiting lists for OLT die within one year. Those in class B (scores between 7 and 9) have an 80% probability of surviving for five years without OLT, and this figure increases to 90% for patients in class A (scores between 5 and 6).
The Model for End-stage Liver Disease (MELD) [80] score is currently considered the ideal method for quantifying the risk of mortality of patients with cirrhosis on waiting lists for liver transplantation. It was originally developed as a tool for predicting short-term survival of patients with chronic liver disease who received transjugular intrahepatic portosystemic shunts (TIPS). However, the MELD score system has recently been retrospectively validated as a method for risk-stratification and prediction of three-month mortality rates in this population, independent of the complications related to portal hypertension (bleeding esophageal varices, spontaneous bacterial peritonitis, portosystemic encephalopathy). MELD scores range from 6 to 40, which correspond to estimated probabilities of survival at 3 months of 90% and 7%, respectively.
Since 27 February 2002, the MELD score has been used by the United Network of Organ Sharing (UNOS) in the United States as a criterion for allocating donor organs to patients with cirrhosis on waiting lists for liver transplantations.
The score is calculated as follows:
A similar model known as the PELD has been developed for use in pediatric patients. In addition to serum bilirubin levels and the INR, other parameters such as serum albumin levels, age <1 year, and growth deficiency (<2 SD with respect to the age-specific mean) are considered in calculation of the PELD score. Both scores can be easily calculated on the following Website: http://www.unos.org/resources/meldpeldcalculator.asp.
Complications like ascites, bleeding varices, hepatic encephalopathy, spontaneous bacterial peritonitis, and the hepato-renal syndrome can also have prognostic implications for a patient with cirrhosis.
Five-year survival rates among patients who develop any one of these complications are 20–50% of those observed in patients with compensated cirrhosis. For example, fewer than half of those who develop spontaneous bacterial peritonitis are alive one year after onset of this complication, and in patients who develop the type 1 hepato-renal syndromes (the rapidly progressive form), mean survival is no more than 2 weeks.
The natural history of the underlying disease has to be compared with the estimated chances of survival after transplantation. In the United States, one-, three-, and five-year post-transplant survival rates are currently 88%, 80%, and 75%, respectively (http://www.optn.org/latestdata/step2.asp).
At the present time, only patients with MELD scores of 15 or higher and CTP scores of 7 or more can expect significant survival benefits from liver transplantation (the “transplant benefit” concept) [81–83].
The Transplant Center of the Policlinico Umberto I in Rome uses the following criteria for placing patients on the waiting list for deceased-donor liver grafts:
-
•
Hepatic cirrhosis with liver function impairment corresponding to a MELD score of ≥10; in the presence of complications severe enough to reduce the quality of life and/or life expectancy (based on an assessment of the case in question), a MELD score of <10 may be acceptable.
-
•
Cholestatic cirrhosis that severely diminishes the quality of life (e.g., due to intractable pruritus) or is associated with evidence of rapid disease progression (i.e., a score of 6 or more in the shortened version of the Mayo Clinic Model for predicting the prognosis of PBC).
-
•
HCC in the form of a single nodule measuring ≤5 cm in diameter or up to three nodules, each with a maximum diameter of no more than 3 cm with no evidence of vascular invasion or extrahepatic metastases. “Down-staging” measures, such as chemoembolization, alcohol ablation, thermal ablation, or surgical resection, can be used to help the patient meet these criteria.
-
•
As for patients whose cirrhosis is related to viral infections, those who are HBsAg-positive are placed on the waiting list even if they are also positive for HBV DNA, but they are not included in the active waiting list until their serum HBV DNA levels have dropped to <100,000 copies/mL (PCR) spontaneously or as a result of antiviral therapy. Patients who are HCV-RNA-positive are included in transplant lists without exclusion.
The following are considered indications for urgent transplantation:
-
•
Acute liver failure defined as the onset of hepatic encephalopathy within 26 weeks of onset of the first symptoms of the disease in a patient with no previous signs of liver disease.
-
•
Primary-non-function of a transplanted liver occurring within ≤10 days of the original transplantation.
-
•
Post-traumatic hepatectomy resulting in total loss of liver function.
-
•
Thrombosis of the hepatic artery occurring within ≤15 days of the original liver transplantation.
-
•
Acute liver failure in a patient with Wilson disease.
Contraindications
The contraindications to liver transplantation can be regarded as absolute and relative.
Absolute contraindications
-
•
Frank AIDS
-
•
HIV positivity (for urgent transplants)
-
•
Age >70 years
-
•
BMI >35 kg/m2
-
•
Active non-hepatobiliary infections
-
•
Active drug addiction (abstinence of <6 months)
-
•
Active alcohol abuse (abstinence of <6 months)
-
•
Severe psychiatric disease
-
•
Extrahepatic neoplastic disease
-
•
Metastases from an occult primary tumor
-
•
Documented decerebration
Relative contraindications
-
•
Age between 60 and 70 years
-
•
HIV positivity (only in controlled trials according to ministerial guidelines)
-
•
Portal vein thrombosis
-
•
Cardiological and/or anesthesiological risk
-
•
Probability of poor compliance (reflected by logistic, family-related, psychological, and/or reliability issues)
The criteria related to age, obesity, and HIV status need to be critically reviewed.
-
•
Age: There is no specific age limit for liver transplantation. However, long-term survival in older recipients is lower than that in younger patients [84,85]. Recipient age represents a specific risk factor and a continuous variable in calculations of the risk of post-transplant mortality. Therefore, in our center, patients over 60 are considered eligible for transplantation only in the absence of co-morbidity (e.g., cardiac, pulmonary, renal disease).
-
•
Obesity: This is the most common problem encountered in patients being considered for liver transplantations, especially among women and patients with cryptogenic cirrhosis. Obesity has a negative impact on short- and long-term survival that is particularly significant when the BMI exceeds 35 kg/m2 (pathological obesity) [86].
-
•
HIV positivity: Early experiences with liver transplantation in HIV-positive patients yielded discouraging results that were mainly related to infectious complications [87]. However, after the introduction of highly active antiretroviral therapy (HAART), survival rates have improved significantly. In addition, many patients with well controlled HIV infections succumb to infections with the HCV, and the potential need for transplants in this patient group is thus on the rise [88]. Recent findings indicate that short-term post-transplant survival rates are similar in HIV-positive and HIV-negative patients. In many cases, HIV RNA levels remain below the detection limits after transplantation, but there have also been numerous reports of severe effects caused by interactions between antiretroviral agents and immunosuppressant drugs [89]. In addition, severe recurrent hepatitis C has been reported in many patients. One thing is certain: OLT in a patient who is HIV-positive requires a well coordinated, multi-disciplinary team with experience in managing liver transplantations as well as HIV infections [90].
Donor selection and organ allocation
As a result of the limited availability of donor organs and the increasing length of transplant waiting lists, there is a high probability that patients will die while waiting for a liver transplantation. This risk has led Transplant Centers to relax donor selection criteria. The current use of “extended criteria donors” (ECD) [91–93] and improved organ allocation criteria based on the MELD score have produced excellent results [94].
In our center, organ donors are classified as standard and non-standard. The latter label may be justified by the presence of a variety of factors, each with its own specific weight:
-
•
Age >60 years
-
•
Documented history of alcoholism
-
•
HbcAb (anti-core) positivity with or without the presence of antibodies against other HBV antigens
-
•
Hemodynamic instability and/or severe hypotension (BP <60 mmHg) for at least 2/12 h prior to organ retrieval
-
•
Presence of high levels of vasoactive amines for more than 6/12 h prior to organ retrieval
-
•
Serum sodium levels >160 mEq/L for more than 12 h (continuous)
-
•
Prothrombin activity <40%
-
•
Imaging findings (ETG, CT, etc.) consistent with severe fatty liver changes
-
•
Orotracheal intubation for >10 days
The patient's position on the waiting list for non-urgent transplantations and the allocation of available grafts are determined by the following factors:
-
•
The MELD score, which reflects the severity of the candidate's disease (If a patient with stage T2 HCC has a MELD score of less than 22, the score will be raised to 22 to avoid excessive penalization of patients with tumor disease and chronic compensated liver disease.)
-
•
Donor characteristics: height and weight; standard versus non-standard status (organs from the former are assigned to recipients with the highest MELD score while those from non-standard donors are assigned on a case-by-case basis and never to a patient with a MELD score of ≤15 unless there are exceptional reasons for performing the transplantation at the time when the organ becomes available); serological profile (organs from HCV-positive donors are assigned exclusively to HCV-positive recipients; those from anti-core positive donors are assigned to patients who are positive for HbsAg and/or anti-HBc antibody or to those who are in particularly urgent need of a transplant.)
-
•
ABO-blood group compatibility
-
•
Length of time on the waiting list
Surgical technique
The technique used for liver transplantation was standardized years ago. There are two main variants. The so-called “conventional” approach calls for removal of the infrahepatic portion of the recipient's inferior vena cava and creation of a special by-pass for extracorporeal circulation. In contrast, during the “piggyback” procedure, the native organ is separated from the inferior vena cava, which is then anastomosed to the stumps of the hepatic veins, or a side-to-side anastomosis is created between the donor and recipient vena cavas.
For years, the conventional approach was the standard technique for liver transplantations, and it is still considered the method of reference, especially in centers with more limited transplantation experience. Some authors maintain that the piggyback technique shortens operative times, reduces operative morbidity, and saves money by eliminating the cost of lines for extracorporeal circulation. However, difficulties may arise when it is used to transplant a particularly large liver with a caudate lobe that encircles the inferior vena cava or in some cases in which the recipient has a TIPS.
Preparation of the patient and incision
The patient is placed in a supine position on the operating table with his/her arms spread 90°. The anesthetist inserts a central venous line, an arterial line for continuous blood-gas monitoring, a Swan-Ganz catheter for control of central venous pressure at the atrial level, and a variable number of peripheral venous catheters; the bladder is also catheterized.
The operative field includes the chest, the entire surface of the abdomen, both axillae, and the inguinal regions. If a venous by-pass is being planned, two vascular access points are created for this purpose; in general, vessels on the left are used. A left inguinal incision is made, and the internal saphenous vein is identified and prepared. The vessel is encircled with a loop near its confluence with the femoral vein. A longitudinal incision is made on the volar aspect of the left arm, and the axillary vein is identified and prepared.
The procedure itself begins with bilateral subcostal cutaneous incisions extending along the median line to the xyphoid process. If the clinical and surgical conditions are favorable, less extensive incisions can be used to reduce abdominal wall trauma. Control of bleeding from subcutaneous veins is of fundamental importance, especially in patients with cirrhosis. An electric scalpel is used to incise the muscle layer along with the peritoneum.
Hepatectomy
As soon as the incision has been completed, the round ligament is sectioned and ligated, and the falciform ligament is sectioned. Before initiating the hepatectomy, the abdominal retractor can be positioned.
Elements of the hepatic peduncle are then isolated; the dissection begins laterally with identification of the common bile duct (CBD). Accessing the hepatoduodenal ligament can be very difficult. In some cases, identification and isolation of the various structures is complicated by the presence of lymph-node packets and a rich plexus of venous and lymphatic collaterals, as well as adhesions from previous surgical interventions.
The CBD is prepared and divided between ties. The proper hepatic artery and its branches are then identified and prepared: they should be ligated and sectioned at the most distal point possible. The segment of the hepatic artery proximal to the emergence of the gastroduodenal artery is then isolated and prepared for subsequent anastomosis. Residual connective and lymphatic tissues around the portal vein are sectioned so as to completely skeletonize it.
The triangular and coronary ligaments are sectioned on the right and left, the liver is completely freed, and the infrahepatic vena cava is isolated; this step is executed in different ways depending on whether the conventional or piggyback approach is being used. With the former, the vena cava is cross-clamped, and a veno-venous by-pass is used for extracorporeal circulation; with the piggyback technique, the inferior vena cava is preserved.
Conventional technique
The retroperitoneal segment of the infrahepatic vena cava is isolated and freed, laterally and posteriorly. While the right side is being freed, care should be taken to identify the right adrenal vein, which can be ligated and sectioned.
Once the inferior vena cava has been completely freed, the left saphenous and axillary veins (previously prepared and isolated) can be cannulated. The portal vein is clamped, sectioned, and cannulated with the initiation of extracorporeal circulation. Veno-venous by-pass serves to eliminate episodes of hemodynamic instability caused by complete caval and portal clamping, which alter cardiac output and cause harmful splanchnic venous congestion.
The by-pass circuit consists of three cannulas connected to three tubes; the femoral and portal cannulas are joined with a Y connector to form a single tube that is attached to the front of the “bell” of the pump; the tube connected to the axillary cannula is attached to the side of the bell. Blood from the splanchnic district and from the inferior vena cava enters the circuit from the femoral and portal cannulas and is pumped to the superior caval district through the axillary cannula. The circuit is prepared under sterile conditions while the hepatectomy is being performed. The tubes and the bell are primed with sterile normal saline. Before the pump is activated, care must be taken to ensure that there are no air bubbles within the circuit that could cause embolism. A flow meter attached to the axillary tube allows the pump technician to regulate the pump rate during surgery.
When the pump has been activated and is functioning correctly, the inferior vena cava is clamped above and below the liver that is being removed. The cuff of the native suprahepatic vena cava is prepared and anastomosed to the suprahepatic vena cava of the donor with continuous, nonabsorbable sutures (Fig. 2). An anastomosis is then created between the infrahepatic vena cava of the donor and the suprarenal vena cava of the recipient, again with continuous, nonabsorbable sutures.
Fig. 2.
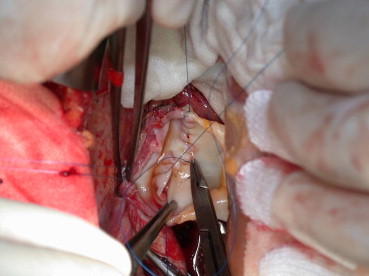
Upper caval anastomosis, back wall is constructed in a running fashion. Personal experience.
Fig. 2 Anastomosi cavale superiore, sutura in continua della parete posteriore. Esperienza personale.
Piggyback technique
The piggyback technique involves separation of the inferior vena cava from the posterior aspect of the liver; the retrohepatic veins are ligated and sectioned, and their ostia are transfixed with 4/0 or 5/0 prolene sutures. The venous ligament is then sectioned so that the liver is attached exclusively to the hepatic veins.
The inferior vena cava is clamped tangentially so that venous return to the heart is not completely interrupted; the clamped segment of the hepatic veins should be as long as possible. Preparation of the cuffs of the hepatic veins involves sectioning the tissue spur separating the common ostia of the left and middle hepatic veins from that of the right hepatic vein so as to create a single orifice. The donor suprahepatic vena cava is then anastomosed to the caval opening of the recipient hepatic veins, and the infrahepatic portion of the donor vena cava is sutured. In some cases, however, the right hepatic vein lies within a plane that is much more caudal than that of the middle and left hepatic veins, and it is thus impossible to include the three veins in the clamp. The anastomosis is thus created with the common trunk of the middle and left hepatic veins, and the right is closed with suture.
Creation of the caval anastomosis involves clamping and sectioning the portal vein, which can lead to temporary splanchnic congestion.
Side-to-side piggyback technique
A variant piggyback technique introduced by Belghiti provides for the creation of a side-to-side anastomosis between the retrohepatic portion of the donor vena cava and the vena cava of the recipient. Like the classic piggyback technique, this variant calls for hepatectomy with preservation of the recipient's retrohepatic vena cava. However, instead of being prepared and clamped for anastomosis, the three hepatic veins are oversewn with polypropylene sutures or a vascular stapler. Tangential longitudinal clamping of the recipient vena cava is carried out so that good caval flow is preserved, and a side-to-side anastomoses is created between the two vena cavas that includes almost the full length of the donor vessel. The upper and lower ends of the donor vena cava are sutured during the back-table procedure.
The piggyback technique offers several advantages over the classic approach:
-
•
Caval flow is maintained, providing greater hemodynamic stability and eliminating the need for venous by-pass.
-
•
It maintains the integrity of the retrocaval and right adrenal regions.
-
•
Creation of a single caval anastomosis reduces the anhepatic phase and the risk of warm ischemia.
The end-to-end portal anastomosis is created with continuous prolene sutures (5/0 or 6/0), and a “growth factor” is left when the suture is tied to reduce the risk of anastomotic stenosis.
Before the liver is revascularized, it is perfused with a 20% solution of albumin through a catheter inserted in the portal vein. The caval and portal clamps are then removed and vascular reperfusion of the transplanted organ begins. At this point, bleeding should be controlled at the level of the anastomosis, from vessels sectioned when the organ was removed, and from the gall bladder bed.
The arterial anastomosis can be created at various levels depending on the preference of the surgeon and the characteristics of the vessel. In general, the donor celiac trunk is anastomosed to the recipient's common hepatic artery where the gastroduodenal artery emerges since the lumen is larger at this point; continuous or non-continuous monofilament (6/0 or 7/0) sutures can be used depending on the caliber of the vessel.
Sometimes the recipient's hepatic artery is not suitable for direct anastomosis. The most common problems are an excessively small caliber, the presence of a large right accessory hepatic artery arising from the superior mesenteric artery, or structural changes in the wall of the vessel caused by arterial embolization performed prior to transplantation for treatment of a hepatocellular carcinoma. In these cases, the recipient's splenic artery can be used for the anastomosis: a segment approximately 2 cm from the celiac tripod is isolated and prepared for direct or lateral anastomosis with the donor's hepatic artery (Fig. 3).
Fig. 3.

Patch of donor aorta anastomosed with the splenic artery. Personal experience.
Fig. 3 Anastomosi arteriosa, patch aortico del donatore anastomizzato con l'arteria splenica. Esperienza personale.
Alternatively, an iliac-artery jump graft taken from the donor can be placed between the donor hepatic artery and the recipient aorta; the iliac artery is anastomosed, end-to-side, with the subrenal aorta of the recipient and its distal head is anastomosed, end-to-end, with the hepatic artery of the graft.
The procedure is completed with the creation of an end-to-end anastomosis between the bile ducts using non-continuous PDS 6/0 sutures. In many centers, a Kehr T-tube is inserted to protect the biliary anastomosis; it emerges from the wall of the bile duct 1–2 cm below the anastomosis. It is generally removed 3 months after the transplantation (Fig. 4).
Fig. 4.

Completed anastomosis is shown. Portal, arterial and biliary. Personal experience.
Fig. 4 Anastomosi portale, arteriosa e biliare completate. Esperienza personale.
If the bile duct stumps are poorly vascularized or when the transplantation is being performed for diseases like sclerosing cholangitis or secondary biliary cirrhosis, a bilioenteric anastomosis is created with the Roux-en-Y loop procedure.
The procedure is completed with thorough control of all bleeders, placement of subdiaphragmatic, subhepatic, and pelvic drains, and externalization of the distal tip of the Kehr tube (Fig. 5).
Fig. 5.
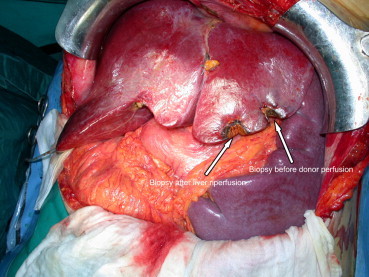
Reperfused liver, biopsy after reperfusion is shown and splenomegaly. Personal experience.
Fig. 5 Fegato trapiantato dopo la riperfusione, particolare della biopsia post-riperfusione e splenomegalia. Esperienza personale.
Living-donor liver transplantation
The first living-donor liver transplantation (LDLT) was performed by Raia in Brazil in 1987, but the recipient did not survive the immediate postoperative phase. While the first successful living-donor liver transplantation was carried out by Strong in Australia in 1989. More than 10 years have passed since this transplant method was introduced, and it has now become a well-defined procedure, strongly supported by clinical findings.
In terms of organ and recipient survival, current results obtained with LDLT are similar to or even better than those reported with cadaver liver transplantations. In some series, especially those involving pediatric patients, 82–88% of the patients were still alive one year after transplantation.
One of the advantages of this approach is that an ideal donor can be selected, and in this case functional recovery of the transplanted organ is immediate and optimal. In addition, the timing of the transplantation can be selected to allow optimal preparation of the recipient, and finally LDLT reduces mortality among patients on transplant waiting lists.
However, removing a liver from a living-donor is a particularly demanding procedure, for the surgeon and for the healthcare center, and there is a real risk of donor morbidity and mortality. Living-donor hepatic resection is based on the physiological assumption that the liver is able to regenerate itself.
The living donor has to be evaluated to determine his/her clinical and psychological fitness for organ donation. The first technical phase is aimed at determining how much parenchyma is required to support the patient's metabolic needs. The most reliable method is calculation of the graft/recipient's body weight (GRBW) ratio, which should ideally be 0.8 or higher.
In our center, we use multislice computed tomography with dedicated reconstruction software (MEVIS) to evaluate the vascular and biliary anatomy of the liver and to estimate its volume. Three-dimensional hepatic reconstruction software is being developed in a research project conducted by the Department of Surgery of Kyoto University Hospital together with the MeVis Research Center in Germany. This software was first developed in 1994 by MeVis in collaboration with several academic hospitals in Germany. This system for vascular reconstruction is used by our transplant center in collaboration with the Institute of Radiology. The complete computer-assisted study includes two parts. The first involves analysis of the image obtained, and it in turn includes various phases: segmentation of the liver, segmentation and structural analysis of the portal and hepatic veins, calculation of vascular territories, volumetric analysis of the liver and the designated territories. The second part involves surgical planning and analysis of risk. It includes definition of the resection lines (which are proposed automatically and can also be modified manually); analysis of risk with calculation of the volume of the hepatic parenchyma; volumetric analysis of the territories at risk; evaluation of 3-D images correlated with two-dimensional CT slices; and presentation of intraoperative options, such as the transection surface. Various resection strategies can be explored and the risk of each analyzed. This allows the team to select the best surgical approach for each case, and an appropriate image can be prepared for use during surgery. Thus far, we have used this system in nine cases of LDLT, and it has allowed us to provide all recipients with an adequate hepatic graft (GBWR of 0.8–1.0). In general, 0.8 is considered the lowest GBWR that will allow a good chance of transplantation success. The living donor must always be left with at least 30% of the original volume of the liver parenchyma. In the majority of LDLT involving adult donors and recipients, the graft consists in the right half of the liver composed of segments V, VI, VII, VIII.
Removal of the right hemiliver: V–VIII segments
The procedure begins with bilateral subcostal incisions that are extended along the median line to the xyphoid apophysis. The peritoneum is opened, and the round ligament divided between ties. The falciform ligament is sectioned with the electric scalpel, proceeding upward to the anterior wall of the suprahepatic vena cava. The abdominal retractor is inserted.
The common trunk of the middle and left hepatic veins is exposed, and the caval opening of the right hepatic vein is identified. The triangular and right coronary ligament is divided to mobilize the right lobe. The left lobe should not be mobilized; otherwise, rotation of the left half of the liver can occur postoperatively, causing venous outflow problems.
At this point, intraoperative cholangiography and cholecystectomy are performed. The cystic duct is isolated and cannulated, and the biliary anatomy is subjected to a thorough radiological examination.
Intraoperative sonography is done to identify the course of the middle and right hepatic veins, their openings into the vena cava, the presence and dimensions of accessory hepatic veins draining the right lobe, the parenchymal resection plane, and above all the veins draining the V and VIII segments that empty into the middle hepatic vein.
The right lobe is completely freed from the retrohepatic caval plane by dividing between ties the accessory hepatic veins coming from the right lobe and from the right half of the caudate lobe, which are present on the right lateral and anterior walls of the vena cava. The accessory veins running along the left edge and draining the left part of the caudate lobe are left intact.
The mobilization proceeds cranially until the right side of the hepato-caval ligament is reached; the latter is isolated and divided between Kelly clamps and the cut surfaces are oversewn. At this point, the right hepatic vein becomes accessible and it can be isolated and encircled with a vessel loop (Fig. 6).
Fig. 6.

Living-donor liver transplantation. Retrohepatic caval dissection. Personal experience.
Fig. 6Trapianto di fegato da donatore vivente. Preparazione della vena cava retroepatica. Esperienza personale.
The hilar structures are then isolated (Fig. 7). After the peritoneal leaflet to the porta hepatis has been opened, blunt dissection is carried out to identify the anatomical elements. The right branch of the hepatic artery generally runs behind the main bile duct. The vessel is isolated and the bifurcation is identified, in particular arterial branches directed toward the IV segment, which must be preserved. In around 30% of all cases, arterial flow to the IV segment is provided by branches arising from the right hepatic artery.
Fig. 7.
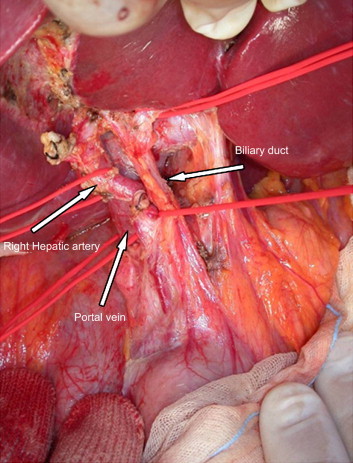
Living-donor liver transplantation, right epatectomy. Isolation of vascular and biliary elements. Personal experience.
Fig. 7 Trapianto di fegato da donatore vivente, epatectomia destra. Isolamento delle strutture ilari. Esperienza personale.
With a lateral approach, the portal vein, its bifurcation, and the right portal branch are isolated; after the presence of any branches directed toward the IV segment has been excluded, the full circumference of right portal branch is freed at its origin.
Isolation of the bile duct requires extreme caution to avoid damaging its blood supply. The right hepatic duct must be sectioned 2–3 mm from the bifurcation. This will leave a stump that is easy to suture without narrowing the donor bile duct. When multiple ducts merge near the hepato-caval junction, the bile duct must not be sectioned in an attempt to create a single orifice shared by all of the ducts. This can cause damage to the donor bile duct. Instead, the ducts should be divided separately although this precaution will naturally require more complicated reconstruction in the recipient.
Parenchymal phase
Right before the parenchymal phase, the right arterial and portal branches can be clamped briefly (1–2 min) to visualize the ischemic demarcation line dividing the right and left hemilivers.
The standard technique for parenchymal transection calls for the use of an ultrasonic dissector (CUSA) and a radiofrequency scalpel (Tissuelink) or bipolar forceps with a nozzle at the tip for normal-saline irrigation. During the entire parenchymal transection phase, the graft is normally perfused. The transection begins at the anterior border of the liver and proceeds simultaneously in a cranial direction and toward the hilum. All vessels and bile ducts over 2 mm in diameter should be sutured on both sides and divided.
Veins >5 mm in diameter that drain the V and VIII segments and empty into the middle hepatic vein must be identified for subsequent reconstruction with the venous graft in the recipient.
The hepatic transection phase requires approximately 2 h of highly meticulous work to limit blood loss to less than 500 cc of blood loss and achieve optimal bilistasis. The right graft remains attached exclusively to the vascular pedicles (Fig. 8). Before the vessels are clamped, and the graft removed, low-dose (40 U/Kg) heparin is administered to the donor. The vessels on the right are sectioned only when it is absolutely certain that the left hemiliver is being adequately perfused.
Fig. 8.
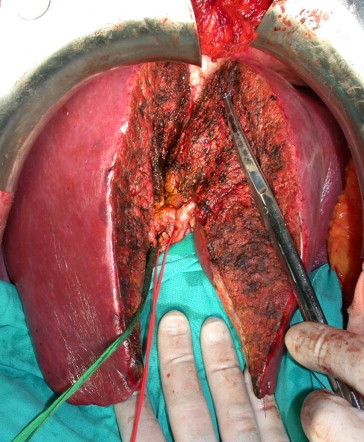
Right epatectomy for living-donor liver transplantation. Completed parenchymal transection. Personal experience.
Fig. 8 Trapianto di fegato da donatore vivente, epatectomia destra. Sezione del parenchima completata. Esperienza personale.
Clamps are applied in the following order:
-
1.
The right branch of the hepatic artery is clamped with a fine bull-dog forceps and sectioned. The stump must be sufficiently long so that it can be sutured without compromising the anatomy of the bifurcation.
-
2.
In clamping the right portal vein, the clamp should not be placed too close to the bifurcation, where it could interfere with portal flow to the left. The right portal branch is divided.
-
3.
Clamping and sectioning of any accessory hepatic veins maintained for reconstruction.
-
4.
Partial clamping of the vena cava with a small Satinsky clamp and of the right hepatic vein, which is sectioned. At least 2 mm of vascular wall should be left above the clamp for subsequent suturing.
-
5.
The graft is then removed from the operative field and placed on the table, where it is perfused exclusively via the portal pathway with perfusion solution at 4 °C.
The right arterial branch should not be used for the perfusion to avoid the risk of any damage to the intima. When the perfusion has been terminated, the graft is weighed so that the exact GRWR can be calculated.
The vascular stumps of the donor are sutured beginning with the right hepatic vein (5/0 monofilament) and any accessory hepatic veins that might be present. The right portal branch is then closed with 5/0 or 6/0 monofilament sutures. Ligation of the right arterial stump. Suturing of the biliary stump. When all bleeding has been controlled, fibrin glue is applied to the hepatic resection area. Two drainage tubes are placed, one in Winslow's foramen, the other in the right hepatic space. The falciform ligament is then reconstructed, and the round ligament is anchored to the abdominal wall to prevent rotation of the residual left hemiliver. The operation ends with layered closure of the abdominal wall.
Recipient procedure
One of the main advantages of living-donor liver transplantation is that the graft is exposed to a limited period of cold ischemia (less than 1–2 h) because the donor and recipient procedures are performed simultaneously.
The procedure consists essentially in hepatectomy with preservation of the inferior vena cava and orthotopic implantation of the graft. Veno-venous by-pass is hardly ever used. Preparation of the hilar structures involves preservation of long segments of the artery and the portal vein with their primary intrahepatic branches. The vessels of the graft are always very short, and their calibers may be different from those of the recipient and in the reconstructive phase, technical possibilities must be maximized. The bile duct is identified and isolated down to its bifurcation. Care should be taken to preserve its axial blood supply. The distal-most segments of the right and left hepatic ducts are sectioned between ties. The hepatic artery and portal vein are also divided equally in the distal-most segments of the right and left branches. The hepatectomy ends with the clamping of the common trunk of the middle and left hepatic veins and partial clamping of the vena cava near the orifice of the right hepatic vein. The liver is then removed, and the common trunk is closed with 5/0 monofilament sutures.
The stump of the hepatic vein is measured, and if it is smaller than the right hepatic vein of the graft, venoplasty is performed on the cuff of the recipient hepatic vein with an incision on the vena cava. This widening procedure facilitates venous drainage and improves the stability of the graft's attachment to the caval axis.
The graft is removed from its sterile container and positioned in the hepatic space. The ostium of the recipient hepatic vein is anastomosed to that of the graft with running 5/0 monofilament sutures.
If there are any accessory inferior hepatic veins with diameters of 5 mm or more, they are anastomosed to the wall of the vena cava. Reconstruction of the venous branches draining segments V and VIII should be considered if their diameters are at least 5 mm and the GRWR is borderline. In this case, congestion and parenchymal damage caused by impaired venous outflow can cause liver failure similar to that observed in the small-for-size graft syndrome. It is virtually impossible to anastomose these vessels directly to the wall of the vena cava, which is too far from the resection area where these vessels emerge. The distance must therefore be bridged with a venous interposition graft.
The portal anastomosis is created with the traditional end-to-end technique using 5/0 monofilament sutures. The reconstruction method is selected based on the diameter and length of the recipient portal vein and the presence or absence of graft anomalies.
Once the portal anastomosis has been completed, the graft is ready for reperfusion. Declamping begins with the hepatic vein-caval anastomosis followed by the portal anastomosis. This brief period of parenchymal reperfusion proceeds in a backwards direction, eliminating the sudden portal inflow that would occur if the portal vein were declamped first.
Interrupted 7/0 monofilament sutures are used to create an end-to-end anastomosis between the right branch of the hepatic artery of the graft and the proper hepatic artery of the recipient.
If the recipient bile duct and the right bile duct of the graft are well vascularized, the biliary reconstruction can be done with a traditional end-to-end duct-to-duct anastomosis. Alternatively an end-to-side anastomosis can be created between the bile duct of the graft and a Roux-en-Y jejunal loop. In either case, a small internal–external biliary drain should always be inserted to protect the anastomosis and reduce the risk of complications (Fig. 9).
Fig. 9.
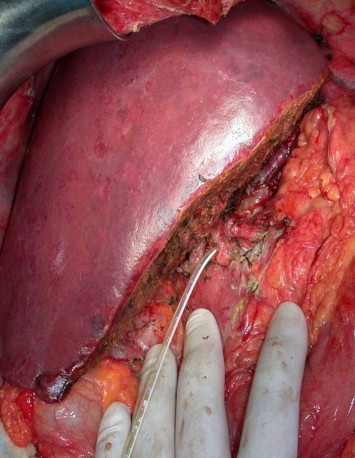
Living-donor liver transplantation at the end of the procedure. Personal experience.
Fig. 9 Trapianto di fegato da donatore vivente al termine della procedura. Esperienza personale.
Fibrin glue is applied to the resection area, drainage tubes are positioned, and the procedure ends with layered closure of the surgical wound.
Split-liver transplantation
The split-liver approach involves division of the liver into two functionally independent parts that can be used for two different recipients. The organ can be divided after it has been removed from a deceased donor (ex situ split-liver) or while it is still inside a live donor and being vascularized (in situ split-liver).
Anatomically speaking, there are two ways to split a liver, and they are used for different purposes. The first is used for a pediatric liver transplantation. A left lateral segmentectomy is done to obtain a right graft composed of segments I, IV, V, VI, VII, and VIII, which will be used for an adult recipient, and the left graft (segments II and III) is used for the pediatric patient. The second procedure is used to provide grafts for two adult recipients. It calls for right hepatectomy to obtain a right graft consisting of segments V, VI, VII, and VIII and a left graft composed of segments I, II, III, and IV.
The adult/pediatric split-liver technique is now well standardized. It produces convincing results, and its use has significantly reduced waiting lists among pediatric patients. In contrast, the adult/adult splitting technique is still in the developmental stage.
Surgical technique
Splitting is used when the deceased donor is young, hemodynamically stable and with normal liver function parameters.
A jugulo-pubic incision is made, the sternum is sectioned, the pericardium is opened, and the peritoneum and abdominal cavity are opened. Sectioning of the round and falciform ligaments. The liver is examined (color, consistency, margins) and the overall size as well as that of the left lateral segment are noted. The other abdominal organs are also carefully explored to exclude conditions that would constitute contraindications to the donation, and an in situ split is performed.
The infrarenal segment of the aorta distal to the bifurcation is identified for later cannulization and perfusion; the inferior vena cava is also prepared for venous outflow after clamping. The subdiaphragmatic aorta is isolated and prepared for later clamping. At this point, vascular access ports are prepared for the perfusion; if the donor is hemodynamically unstable, rapid perfusion is started, and the liver is removed in toto.
The proper hepatic artery is then isolated down to the bifurcation, and the left arterial branch is isolated. The vessels are examined for anomalies, in particular the presence of a left hepatic artery arising from the left gastric artery, which is isolated and preserved. The left bile duct is isolated carefully to avoid damaging its blood supply. The bile duct can be sectioned with a cold blade during this phase or later in the procedure.
One of the key steps in the splitting process involves full-length isolation of the round ligament all the way to the Rex recess and the left branch of the portal vein. The isolation is carried out along the right edge of the ligament. The right branches of the recess of Rex are carefully dissected and sutured on both sides with 5/0 monofilament transfixion sutures. The process continues in the same manner all the way to the left branch of the portal vein, which is isolated and encircled.
On the lower aspect of the liver, the venous ligament is freed, proceeding upward. This allows further freeing of the left branch of the portal vein. The latter is sectioned, providing access to the left hepatic vein, which is isolated and encircled with a vessel loop.
At this point, resection of the hepatic parenchyma begins. An incision is made with an electric scalpel in Glisson's capsule. The resection line follows the course of the falciform ligament on the anterior aspect of the liver and the venous ligament on the lower surface.
Transection of the parenchyma can be accomplished with:
-
•
Kelly forceps
-
•
Ultrasonic scalpel
-
•
Harmonic scalpel
-
•
Bipolar forceps
It begins at the anterior margin of the liver, and transfixion sutures are placed in each tiny vascular or biliary element; at the hilar plate, the left bile duct is sectioned. At this point, the loop of the left hepatic vein can be displaced anteriorly, along the course of the ductus venosus; this reveals the exact line to follow in completing the parenchymal transection. When the transection is complete, there will be two separate liver grafts joined only by vascular elements.
This is followed by cannulization of the aorta, clamping, in situ perfusion of all the abdominal organs, and removal en bloc of the pancreas, duodenum, and liver.
The vascular elements will be sectioned “in situ” in the cadaver or on the table. The left hepatic vein is divided at its insertion into the vena cava, the left branch of the portal vein right before the bifurcation, the left hepatic artery in most cases remains attached to the celiac trunk, and the right branch is divided at its origin. On the table, the two grafts thus obtained are perfused via the portal branch and packaged separately under sterile conditions.
Early and late complications of liver transplantation
Liver transplantation can be associated with numerous complications due to the technical difficulties involved in the surgical procedure itself, the use of immunosuppressant therapy, acute and chronic rejection processes, and even the recurrence of the liver disease (tumors, viral infections) that prompted the transplantation in the first place.
These complications can be divided into hepatic and non-hepatic subgroups.
The hepatic complications include:
-
•
Acute or chronic rejection [95,96]
-
•
Thrombosis of the hepatic artery (Fig. 10) [97,98] or portal vein [99,100]
-
•
Anastomotic and non-anastomotic biliary complications (stenosis, fistulas) [101]
-
•
Recurrence of the disease that prompted the transplantation (viral infection, PBC, SBC, alcohol, autoimmune cirrhosis, Budd–Chiari syndrome, primary liver tumors)
-
•
De novo viral infections
-
•
Massive hemorrhagic necrosis
-
•
Primary-non-function (PNF) [102–104], a multifactorial disorder characterized by functional failure of the transplanted organ. It leads to death or re-transplantation within 10 days of the original transplantation
-
•
PDF (Primary dysfunction), a multifactorial disorder similar to PNF. It leads to death or re-transplantation 11 or more days after the original transplantation
Fig. 10.

Hepatic artery dissection. The anastomosis was performed to the supraceliac aorta. Personal experience.
Fig. 10Dissecazione dell'arteria epatica. Anastomosi arteriosa eseguita sull'aorta sopraceliaca. Esperienza personale.
Non-hepatic complications include:
-
•
Intraoperative death (due to massive hemorrhage, hemodynamic imbalance, arrhythmias)
-
•
Extrahepatic infections [105]: bacterial, viral, fungal, parasitic [106–109]
-
•
Gastrointestinal complications [110]: gastrointestinal hemorrhage, pancreatitis, intestinal perforation
-
•
Cardiovascular complications: myocardial infarction, arrhythmias
-
•
Cerebrovascular complications [111,112]: intracranial hemorrhage, ischemic stroke, cerebral edema encephalitis
-
•
Tumors: De novo hepatic or non-hepatic tumors, tumors transmitted by the donor, lymphoproliferative disease
-
•
Urinary-tract complications [113,114]: renal failure (acute and chronic), urinary-tract infection
-
•
Pulmonary complications [115]: pulmonary embolism, acute respiratory distress syndrome, pneumonia [116–118]
-
•
Social complications: Non-compliance with immunosuppressant regimen, psychosocial adaptation difficulties, suicide
-
•
Bone-marrow depression
-
•
Multi-organ failure
Immunosuppressant therapy
For years, the problem of immunosuppression was the main obstacle to progress in the field of liver transplantation and its routine use. The first regimens used to prevent liver transplant rejection were based on the use of azathioprine, corticosteroids, and anti-thymocyte globulins. The results were disappointing, and rejection rates were high. The introduction of cyclosporine in 1980 [4] substantially reduced these rates from 15% (reported by various groups) to 2–5% [119].
Cyclosporine and tacrolimus (FK 506), which was first used in clinical practice in the 1990s [120–122], are inhibitors of calcineurin, a serine-threonine phosphatase involved in the activation of various transcription factors. In activated T lymphocytes, the inhibition of calcineurin blocks the transcription of various cytokines, including IL-2, which plays a fundamental role in activating the immune response. Tacrolimus is 10–100 times more potent than cyclosporine A in suppressing the immune response. The doses to be administered are selected based on blood levels of the drugs, which need to be monitored at regular intervals. Both drugs are metabolized at the hepatic level by the P450 IIIA cytochrome system, and reactions with other drugs can occur that increase (erythromycin, fluconazole, verapamil, cimetidine) or reduce (phenobarbital, phenytoin, carbamazepine) their levels in the blood. These drugs also have multiple side effects. Their nephrotoxicity is due to dose-dependent damage to the renal tubule as well as to vasospastic effects on the renal artery. Other side effects include arterial hypertension, glucose intolerance, neurological symptoms (tremor), and cyclosporine also causes gingival hyperplasia and hirsutism [123].
Rapamycin [124,125] is a structural analog of tacrolimus and it shares the same targets, but it acts during a later phase of lymphocyte activation. It can cause bone-marrow suppression, and its action must be closely monitored with white blood cell counts. Rapamycin also interferes with lipid metabolism, and signs of dyslipidemia are a common side effect [126–128].
Mycophenolate mofetil (MMF) [129] inhibits the proliferation of activated T lymphocytes by blocking purine metabolism. Its main side effect is gastrointestinal (diarrhea) [130].
Azathioprine is a derivative of mercaptopurine, and it acts as a structural analog and antimetabolite. It is metabolized by the enzyme xanthine oxidase, which is the molecular target of the gout medication, allopurinol, and co-administration of the two drugs can cause serious azathioprine toxicity with severe bone-marrow suppression.
Immunoglobulins directed against the lymphocytes (ALG) and thymocytes (ATG) and monoclonal antibodies against T lymphocytes (OKT3, basiliximab zenapax) are used in many centers to induce immunosuppression and to treat acute rejection events that are unresponsive to boluses of cortisone [131,132].
The corticosteroids are the first class of hormones whose lymphocytolytic effects were described [133]. They interact with the immune system at various levels, reducing the number and size of lymphoid cells, inhibiting the production of inflammatory mediators such as PAF, leukotrienes, and prostaglandins; inhibiting monocyte and neutrophil chemotaxis; and producing lympho- and neutropenia, not through direct cytotoxicity but by altering the diffusion of these cell populations. They are common components of combined immunosuppressant regimens and are also administered as intravenous boluses to treat acute rejection events.
Glucocorticoids have a number of side effects, particularly when they are used for long periods: glucose intolerance, hypertension, osteoporosis, muscle-mass reduction, weight gain with central obesity, moon facies, striae rubrae, psychosis, cataract, glaucoma, and even iatrogenic Cushing syndrome [134,135].
All immunosuppressant drugs increase the risk for all types of infection (bacterial, viral, fungal) and for neoplastic disease, hematological forms (lymphoma) and solid tumors as well.
The immunosuppressant regimen used for liver transplant recipients in our center calls for the use of a calcineurin inhibitor (cyclosporine or FK 506) combined with MMF and steroids, which are tapered and finally discontinued within one year after transplantation. Other drugs such as sirolimus and azathioprine are used when toxic effects are caused by other drugs, mainly the calcineurin inhibitors, and they are prescribed on a case-by-case basis.
Results
Data furnished by the ELTR indisputably support the therapeutic validity of liver transplantation. Before 1985, the one- and five-year survival rates were 34% and 21%, respectively. Today, those figures have risen to 85% and 70%.
Few studies have examined the question of long-term survival after liver transplantation [136]. Busuttil et al. recently analyzed a large series of 3200 OLTs performed at the University of California – Los Angeles between 1984 and 2001 [137]. Their data indicate that the long-term results are also excellent, with survival rates of 81% at one year, 72% at five years, 68% at 10 years, and 64% at 15 years.
When the series was divided into two groups based on the year the transplantation was performed, even more encouraging results were observed in the patients who underwent transplantation between 1992 and 2001. The one-, five-, and 10-year survival rates of 83%, 75%, and 71%, respectively, clearly reflect substantial technical improvements in the field of liver transplantation. An analysis of patients based on age and underlying disease revealed several significant differences.
-
•
Pediatric patients have better survival rates than other age groups (survival rates of 86%, 82%, and 79% at one, five, and 10 years among patients 1–18 years of age compared with rates of 77%, 65%, and 58%, respectively, among those over age 55).
-
•
Survival rates in cases of non-urgent transplantation are clearly higher than those for patients who receive emergency transplantations.
-
•
Among pediatric patients, those with biliary atresia have the best post-transplant survival rates (82%, 79%, and 78% at one, five, and 10 years, respectively).
-
•
In adults, the best results are seen among patients with primary biliary cirrhosis (82%, 77%, and 68% at one, five, and 10 years, respectively); sclerosing cholangitis (85%, 76%, and 70%); and alcoholic cirrhosis (84%, 77%, and 70%).
-
•
Survival among recipients with HBV-related cirrhosis are lower than those for transplantations performed for cholestatic disease but higher than those for HCV-related cirrhosis, which are 81%, 68%, and 62% at one, five, and 10 years, respectively.
-
•
As one would imagine, the worst outcomes are associated with transplantations performed for neoplastic disease (survival rates of 68%, 43%, and 36% at one, five, and eight years, respectively).
-
•
As for HCC, since the introduction of the Milan Criteria [68], results have been similar to those obtained in patients with non-tumoral pathology (survival rates of 85% at one year and 70% at five years), and recurrence rates are less than 15%. Certain centers have experimented with the extension of these criteria. Gondolesi et al. [69], for example, reported a series of 36 LDLTs, 33% of which involved recipients with HCC who failed to satisfy the Milan Criteria. The results were excellent in terms of disease-free survival rates (82% at one year and 74% at two years) and overall survival rates (75% at one year and 60% at two years). Use of the UCSF Criteria has been associated with five-year survival rates of 50% [7].
Footnotes
This work was supported by the Interuniversity Organ Transplantation Consortium, Rome, Italy Director Prof. R. Cortesini.
References
- 1.Starzl T.E., Marchiono T.L., Von Kaulia K.N. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl T.E., Iwatsuki S., Van Thiel D.H. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolles K., Williams R., Neuberger J., Calne R.Y. The Cambridge and King's College Hospital experience of liver transplantation, 1968–1983. Hepatology. 1984;4(1 Suppl.):50S–55S. doi: 10.1002/hep.1840040715. [DOI] [PubMed] [Google Scholar]
- 4.Calne R.Y., Rolles K., White D.J.G. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 5.Raia S., Nery J.R., Mies S. Liver transplantation from live donors. Lancet. 1989;4:497. doi: 10.1016/s0140-6736(89)92101-6. [DOI] [PubMed] [Google Scholar]
- 6.Strong R.W., Lynch S.V.G., Ong T.H. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. doi: 10.1056/NEJM199005243222106. [DOI] [PubMed] [Google Scholar]
- 7.Broelsch C.E., Emond J.C., Whitington P.F. Application of reduced-size liver transplants as split grafts, auxiliary hortotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–377. doi: 10.1097/00000658-199009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annual report of the US Scientific Registry for Transplant Recipients and the Organ Procurement and Transplantation Network Transplant Data: 1988–1994. Richmond, USA: United Network for Organ Sharing and the Division of Organ Transplantation, Bureau of Health Resources Development.
- 9.Alter M.J. Epidemiology of hepatitis C. Hepatology. 1997;269(Suppl.):S62–S65. [Google Scholar]
- 10.Di Bisceglie A.M., Goodman Z.D., Ishak K.G., Hoofnagle J.H., Melpolder J.C., Alter M.J. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T., Bedossa P., Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 12.Fattovich G., Giustina G., Degos F. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 13.Di Bisceglie A.M. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26(Suppl.):S34–S38. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 14.Poynard T., Leroy V., Cohard M. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778–789. doi: 10.1002/hep.510240405. [DOI] [PubMed] [Google Scholar]
- 15.Carithers R.L., Jr., Emerson S.S. Therapy of hepatitis C: meta-analysis of interferon alfa-2b trials. Hepatology. 1997;26(Suppl.):S83–S88. doi: 10.1002/hep.510260715. [DOI] [PubMed] [Google Scholar]
- 16.Forman L.M., Lewis J.D., Berlin J.A., Feldman H.I., Lucey M.R. The association between Hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–896. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 17.Consensus conference: indications for liver transplantation, January 19 and 20, 2005, Lyon-Palais Des Congres. Text of recommendations (long version) Liver Transpl. 2006;12:998–1011. doi: 10.1002/lt.20765. [DOI] [PubMed] [Google Scholar]
- 18.Perrillo R.P., Schiff E.R., Davis G.L. A randomised, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. N Engl J Med. 1990;323:295–301. doi: 10.1056/NEJM199008023230503. [DOI] [PubMed] [Google Scholar]
- 19.Hoofnagle J.H., Di Bisceglie A.M., Waggoner J.G., Park Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology. 1993;104:1116–1121. doi: 10.1016/0016-5085(93)90281-g. [DOI] [PubMed] [Google Scholar]
- 20.Terrault N.A., Holland C.C., Ferrell L. Interferon alpha for recurrent hepatitis B infection after liver transplantation. Liver Transpl Surg. 1996;2:132. doi: 10.1002/lt.500020209. [DOI] [PubMed] [Google Scholar]
- 21.Rayes N., Seehofer D., Hopf U. Comparison of famciclovir and lamivudine in the long-term treatment of hepatitis B infection after liver transplantation. Transplantation. 2001;71(1):96–101. doi: 10.1097/00007890-200101150-00016. [DOI] [PubMed] [Google Scholar]
- 22.Krawitt E.L. Autoimmune hepatitis. N Engl J Med. 1996;334:897–903. doi: 10.1056/NEJM199604043341406. [DOI] [PubMed] [Google Scholar]
- 23.Hoofnagle J.H., Kresina T., Fuller R.K. Liver transplantation for alcoholic liver disease: executive statement and recommendations. Summary of a National Institutes of Health workshop, held December 6–7, 1996, Bethesda, Maryland. Liver Transpl Surg. 1997;3:347–350. doi: 10.1002/lt.500030324. [DOI] [PubMed] [Google Scholar]
- 24.Poupon R.E., Lindor K.D., Cauch-Dudek K., Dickson E.R., Poupon R., Heathcote E.J. Combined analysis of randomised controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 25.Tinmouth J., Tomlinson G., Heathcote E.J., Lilly L. Benefit of transplantation in primary biliary cirrhosis between 1985–1997. Transplantation. 2002;72(2):224–227. doi: 10.1097/00007890-200201270-00012. [DOI] [PubMed] [Google Scholar]
- 26.Dickson E.R., Grambsch P.M., Fleming T.R., Fischer L.D., Langworthy A. Prognosis in primary biliary cirrhosis: model for decision making. Hepatology. 1989;10:1. doi: 10.1002/hep.1840100102. [DOI] [PubMed] [Google Scholar]
- 27.Lee Y.M., Kaplan M.M. Primary sclerosing cholangitis. N Engl J Med. 1995;332:924–933. doi: 10.1056/NEJM199504063321406. [DOI] [PubMed] [Google Scholar]
- 28.Ricci P., Therneau T.M., Malinchoc M. A prognostic model for the outcome of liver transplantation in patients with cholestatic liver disease. Hepatology. 1997;25:672–677. doi: 10.1002/hep.510250330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickson E.R., Murtaugh P.A., Wiesner R.H. Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Elmagd K.M., Malinchoc M., Dickson E.R. Efficacy of hepatic transplantation in patients with primary sclerosing cholangitis. Surg Gynecol Obstet. 1993;177:335–344. [PMC free article] [PubMed] [Google Scholar]
- 31.Ohi R., Ibrahim M. Biliary atresia. Semin Pediatr Surg. 1992;1:115–124. [PubMed] [Google Scholar]
- 32.Alagille D. Alagille syndrome today. Clin Invest Med. 1996;19:325–330. [PubMed] [Google Scholar]
- 33.Caroli J. Diseases of the intrahepatic biliary tree. Clin Gastroenterol. 1973;2:147–161. [PubMed] [Google Scholar]
- 34.Marcus N., Teckman J.H., Perlmutter D.H. Alpha1-antitrypsin deficiency: from genotype to childhood disease. J Pediatr Gastroenterol Nutr. 1998;27:65–74. doi: 10.1097/00005176-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Devenport M., Kerkar N., Mieli V.G., Mowat A.P., Howard E.R. Biliary atresia: the King's College Hospital experience (1974–1995) J Pediatr Surg. 1997;32:479–485. doi: 10.1016/s0022-3468(97)90611-4. [DOI] [PubMed] [Google Scholar]
- 36.Otte J.B., Ville-de-Goyet J., Reding R. Pediatric liver transplantation: from the full-size liver graft to reduced, split, and living related liver transplantation. Pediatr Surg Int. 1998;13:308–318. doi: 10.1007/s003830050328. [DOI] [PubMed] [Google Scholar]
- 37.Woodie E.S., Millis J.M., So S.K. Liver transplantation in the first three months of life. Transplantation. 1998;66:606–609. doi: 10.1097/00007890-199809150-00010. [DOI] [PubMed] [Google Scholar]
- 38.Sandler A.D., Azarow K.S., Superina R.A. The impact of a previous Kasai procedure on liver transplantation for biliary atresia. J Pediatr Surg. 1997;32:416–419. doi: 10.1016/s0022-3468(97)90594-7. [DOI] [PubMed] [Google Scholar]
- 39.Niederau C., Fischer R., Purschel A., Stremmel W., Haussinger D., Strohmeyer G. Long-term survival in patients with hereditary hemochromatosis. Gastroenterology. 1996;110:1107–1119. doi: 10.1053/gast.1996.v110.pm8613000. [DOI] [PubMed] [Google Scholar]
- 40.Niederau C., Fischer R., Sonnenberg A., Stremmel W., Trampisch H.J., Strohmeyer G. Survival and causes of death in cirrhotic and noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 41.Poulos J.E., Bacon B.R. Liver transplantation for hereditary hemochromatosis. Dig Dis Sci. 1996;14:316–322. doi: 10.1159/000171562. [DOI] [PubMed] [Google Scholar]
- 42.Farrell F.J., Nguyen M., Woodley S. Outcome of liver transplantation in patients with hemochromatosis. Hepatology. 1994;20:404–410. [PubMed] [Google Scholar]
- 43.Westra W.H., Hruban R.H., Baughman K.L. Progressive hemochromatotic cardiomyopathy despite reversal of iron deposition after liver transplantation. Am J Clin Pathol. 1993;99:39–44. doi: 10.1093/ajcp/99.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Shilsky M.L. Wilson's disease: genetic basis of copper toxicity and natural history. Semin Liver Dis. 1996;16:83–95. doi: 10.1055/s-2007-1007221. [DOI] [PubMed] [Google Scholar]
- 45.Schilsky M.L. Treatment of Wilson disease: what are the relative roles of penicillamine, trientine and zinc supplementation? Curr Gastroenterol Rep. 2001;3:54–59. doi: 10.1007/s11894-001-0041-4. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher G., Platz K.P., Mueller A.R. Liver transplantation: treatment of choice for hepatic and neurological manifestation of Wilson's disease. Clin Transpl. 1997;11:217–224. [PubMed] [Google Scholar]
- 47.Schilsky M.L., Scheinberg I.H., Sternlieb I. Liver transplantation for Wilson's disease: indications and outcome. Hepatology. 1994;19:583–587. doi: 10.1002/hep.1840190307. [DOI] [PubMed] [Google Scholar]
- 48.Perlmutter D.H. Alpha-1-antitrypsin deficiency: biochemistry and clinical manifestations. Ann Med. 1996;28:385–394. doi: 10.3109/07853899608999097. [DOI] [PubMed] [Google Scholar]
- 49.Filipponi F., Soubrade O., Labrousse F. Liver transplantation for end-stage liver disease associated with alpha-1-antitrypsin deficiency in children: pretransplant natural history, timing and results of transplantation. J Hepatol. 1994;20:72–78. doi: 10.1016/s0168-8278(05)80469-6. [DOI] [PubMed] [Google Scholar]
- 50.Burke A., Lucey M.R. Non-alcoholic fatty liver disease, non alcoholic steatohepatitis and orthotopic liver transplantation. Am J Transplant. 2004;4:686–693. doi: 10.1111/j.1600-6143.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 51.Contos M.J., Cales W., Sterling R.K. Development of non-alcoholic fatty liver disease after orthotopic liver transplantation for cryptogenetic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 52.Trey C., Lipworth L., Chalmers I.C. Fulminant hepatic failure: presumable contribution of halothane. N Engl J Med. 1968;279:798–801. doi: 10.1056/NEJM196810102791504. [DOI] [PubMed] [Google Scholar]
- 53.Lee W.M. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 54.Williams R. Classification, etiology, and considerations of outcome in acute liver failure. Semin Liver Dis. 1996;16:343–348. doi: 10.1055/s-2007-1007247. [DOI] [PubMed] [Google Scholar]
- 55.Mas A., Rodès J. Fulminant hepatic failure. Lancet. 1997;349:1081–1085. doi: 10.1016/S0140-6736(96)08054-3. [DOI] [PubMed] [Google Scholar]
- 56.Ellis A.J., Saleh M., Smith H., Portmann B., Gimson A., Williams R. Late-onset hepatic failure: clinical features, serology and outcome following transplantation. J Hepatol. 1995;23:363–372. doi: 10.1016/0168-8278(95)80193-6. [DOI] [PubMed] [Google Scholar]
- 57.O'Grady J.G., Gimson A.E.S., O'Brien C.J., Pucknell A., Huges R.D., Williams R. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology. 1988;94:1186–1192. doi: 10.1016/0016-5085(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 58.O'Grady J.G., Alexander G.J.M., Hayllar K.M., Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 59.Isoniemi H., Koivusalo A.M., Repo H., Ilonen I., Höckerstedt K. The effect of albumin dialysis on cytokine levels in acute liver failure and need for liver transplantation. Transplant Proc. 2005;37:1088–1090. doi: 10.1016/j.transproceed.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 60.Bismuth H., Samuel D., Castaing D., Williams R., Pereira S.P. Liver transplantation in Europe for patients with acute liver failure. Semin Liver Dis. 1997;16:415–425. doi: 10.1055/s-2007-1007254. [DOI] [PubMed] [Google Scholar]
- 61.Ellis A., Wendon J. Circulatory, respiratory, cerebral, and renal derangements in acute liver failure: pathophysiology and management. Semin Liver Dis. 1996;16:379–388. doi: 10.1055/s-2007-1007251. [DOI] [PubMed] [Google Scholar]
- 62.Bosch F.X., Ribes J. Epidemiology of liver cancer in Europe. Can J Gastroenterol. 2000;14(7):621–630. doi: 10.1155/2000/815454. [DOI] [PubMed] [Google Scholar]
- 63.Okuda K. Clinical and public health challenges of cancer. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J Gastroenterol Hepatol. 2002 Apr;17(4):401–405. doi: 10.1046/j.1440-1746.2002.02734.x. [review] [DOI] [PubMed] [Google Scholar]
- 64.Moradpour D., Blum H.E. Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17(5):477–483. doi: 10.1097/00042737-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Busuttil R.W., Farmer D.G. The surgical treatment of primary hepatobiliary malignancy. Liver Transpl Surg. 1996;2:114–130. [PubMed] [Google Scholar]
- 66.Bruix J., Sherman M., Llovet J.M. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 67.Varela M., Sanchez W., Bruix J., Gores G.J. Hepatocellular carcinoma in the setting of liver transplantation. Liver Transpl. 2006;12:1028–1036. doi: 10.1002/lt.20833. [DOI] [PubMed] [Google Scholar]
- 68.Mazzaferro V., Regalia E., Doci R. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 69.Gondolesi G.E., Roayaie S., Muñoz L. Adult living donor transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg. 2004;239:142–149. doi: 10.1097/01.sla.0000109022.32391.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cillo U., Vitale A., Bassanello M. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao F.Y., Ferrel L., Bass N.M. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 72.Pichlmayr R., Weimann A., Klempnauer J. Surgical treatment in proximal bile duct cancer: a single centre experience. Ann Surg. 1996;224:628–638. doi: 10.1097/00000658-199611000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwatsuki S., Todo S., Marsh J.W. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimoda M., Farmer D.G., Colquhoun S.D. Liver transplantation for cholangiocellular carcinoma: analysis of a single-center experience and review of the literature. Liver Transpl. 2001;7(12):1023–1033. doi: 10.1053/jlts.2001.29419. [DOI] [PubMed] [Google Scholar]
- 75.De Vreede I., Steers J.L., Burch P.A. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 76.Lehnert T. Liver transplantation for metastatic neuroendocrine carcinoma: an analysis of 103 patients. Transplantation. 1998;66(10):1307–1312. doi: 10.1097/00007890-199811270-00007. [DOI] [PubMed] [Google Scholar]
- 77.O'Grady J.G. Treatment options for other hepatic malignancies. Liver Transpl. 2000;6(6 Suppl. 2):S23–S29. doi: 10.1053/jlts.2000.18687. [DOI] [PubMed] [Google Scholar]
- 78.Murray K.F., Carithers R.L., Jr. AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41(6):1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 79.Pugh R.N.H., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–648. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 80.Malinchoc M., Kamath P.S., Gordon F.D., Peine C.J., Rank J., ter Borg P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 81.Lucey M.R., Brown K.A., Everson G.T. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628–637. doi: 10.1002/lt.500030613. [DOI] [PubMed] [Google Scholar]
- 82.Wiesner R., Edwards E., Freeman R. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 83.Freeman R.B., Wiesner R.H., Edwards E., Harper A., Merion R., Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 84.Starzl T.E., Todo S., Gordon R.D. Liver transplantation in older patients. New Engl J Med. 1987;316:484–485. doi: 10.1056/NEJM198702193160814. [DOI] [PubMed] [Google Scholar]
- 85.Herrero J.I., Lucena J.F., Quiroga J. Liver transplant recipients older than 60 years have lower survival and higher incidence of malignancy. Am J Transplant. 2003;3:1407–1412. doi: 10.1046/j.1600-6143.2003.00227.x. [DOI] [PubMed] [Google Scholar]
- 86.Nair S., Verma S., Thuluvath P.J. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 87.Bouscarat F., Samuel D., Simon F., Debat P., Bismuth H., Saimot A.G. An observational study of 11 French liver transplant recipients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1994;19:854–859. doi: 10.1093/clinids/19.5.854. [DOI] [PubMed] [Google Scholar]
- 88.Neff G.W., Bonham A., Tzakis A.G. Orthotopic liver transplantation in patients with human immunodeficiency virus and end-stage liver disease. Liver Transpl. 2003;9:239–247. doi: 10.1053/jlts.2003.50054. [DOI] [PubMed] [Google Scholar]
- 89.Schvarcz R., Rudbeck G., Soderdahl G., Stahle L. Interaction between nelfinavir and tacrolimus after orthoptic liver transplantation in a patient coinfected with HIV and hepatitis C virus (HCV) Transplantation. 2000;69:2194–2195. doi: 10.1097/00007890-200005270-00041. [DOI] [PubMed] [Google Scholar]
- 90.Roland M.E., Stock P.G. Review of solid-organ transplantation in HIV-infected patients. Transplantation. 2003;75:425–429. doi: 10.1097/01.TP.0000046943.35335.18. [DOI] [PubMed] [Google Scholar]
- 91.Tector A.J., Mangus R.S., Chestovich P. Use of extended criteria livers decreases wait time for liver transplantation without adversely impacting posttransplant survival. Ann Surg. 2006;244(3):439–450. doi: 10.1097/01.sla.0000234896.18207.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cameron A.M., Ghobrial R.M., Yersiz H. Optimal utilization of donor grafts with extended criteria: a single-center experience in over 1.000 liver transplants. Ann Surg. 2006;243:748–755. doi: 10.1097/01.sla.0000219669.84192.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ginanni Corradini S., Elsei W., De Marco R. Preharvest donor hyperoxia predicts good early graft function and longer graft survival after liver transplantation. Liver Transpl. 2005;11:140–151. doi: 10.1002/lt.20339. [DOI] [PubMed] [Google Scholar]
- 94.Amin M.G., Wolf M.P., Tenbrook M.A. The underutilization of expanded criteria donor grafts for deceased donor liver transplantation under the MELD system: a decision analysis. Liver Transpl. 2004;10:1468–1475. doi: 10.1002/lt.20304. [DOI] [PubMed] [Google Scholar]
- 95.Fisher L.R., Henley K.S., Lucey M.R. Acute cellular rejection after liver transplantation: variability, morbidity, and mortality. Liver Transpl Surg. 1995;1:10–15. doi: 10.1002/lt.500010104. [DOI] [PubMed] [Google Scholar]
- 96.Kouwenhoven E.A., Ijzermans J.N.M., De Bruin R.W.F. Etiology and pathophysiology of chronic transplant dysfunction. Transpl Int. 2000;13:385–401. doi: 10.1007/s001470050721. [DOI] [PubMed] [Google Scholar]
- 97.Tzakis A.G., Gordon R.D., Byers W. Clinical presentation of hepatic artery thrombosis after liver transplantation in the cyclosporine era. Transplantation. 1985;40:667–671. doi: 10.1097/00007890-198512000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abbasoglu O., Levy M.F., Vodapally M.S. Hepatic artery stenosis after liver transplantation: incidence, presentation, treatment, and long-term outcome. Transplantation. 1997;630:250–255. doi: 10.1097/00007890-199701270-00013. [DOI] [PubMed] [Google Scholar]
- 99.Settmacher U., Nussler N.C., Glanemann M. Venous complications after orthotopic liver transplantation. Clin Transpl. 2000;14:235–241. doi: 10.1034/j.1399-0012.2000.140309.x. [DOI] [PubMed] [Google Scholar]
- 100.Buell J.F., Funaki B., Cronin D.C. Long-term venous complications after full-size and segmental pediatric liver transplantation. Ann Surg. 2002;236(5):658–666. doi: 10.1097/00000658-200211000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rerknimitr R., Sherman S., Fogel E.L. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic finding and results of therapy. Gastrointest Endosc. 2002;55:224–231. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 102.Bzeizi K.I., Jalan R., Plevris J.N. Primary graft dysfunction after liver transplantation: from pathogenesis to prevention. Liver Transpl Surg. 1997;3:137–148. doi: 10.1002/lt.500030206. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez F.X., Rimola A., Grande L. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565–573. doi: 10.1002/hep.1840200304. [DOI] [PubMed] [Google Scholar]
- 104.Nanashima A., Pillay P., Verran D.J. Analysis of initial poor graft function after orthotopic liver transplantation: experience of an Australian single liver transplantation center. Transplant Proc. 2002;34:1231–1235. doi: 10.1016/s0041-1345(02)02639-8. [DOI] [PubMed] [Google Scholar]
- 105.Winston D.J., Emmanouilides C., Busuttil R.W. Infections in liver transplant recipients. Clin Infect Dis. 1995;21:1077–1091. doi: 10.1093/clinids/21.5.1077. [DOI] [PubMed] [Google Scholar]
- 106.George D.L., Arnow P.M., Fox A.S. Bacterial infection as a complication of liver transplantation: epidemiology and risk factors. Respir Infect Dis. 1991;13:387–396. doi: 10.1093/clinids/13.3.387. [DOI] [PubMed] [Google Scholar]
- 107.Dummer S., Kusne S. Liver transplantation and related infections. Semin Respir Infect. 1993;8:191–198. [PubMed] [Google Scholar]
- 108.Kusne S., Dummer J.S., Singh N. Infections after liver transplantation: an analysis of 101 consecutive cases. Medicine. 1988;67:132–143. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kusne S., Torre-Cisneros J., Manez R. Factors associated with invasive lung aspergillosis and the significance of positive aspergillus culture after liver transplantation. J Infect Dis. 1992;166:1379–1383. doi: 10.1093/infdis/166.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sterling R.K. Gastrointestinal complications in liver transplant recipients. Medscape Gastroenterol. 2001;3(1):1–7. [Google Scholar]
- 111.Singh N., Yu V.L., Gayowski T. Central nervous system lesions in adult liver transplant recipients: clinical review with implications for management. Medicine. 1994;73:110–118. doi: 10.1097/00005792-199403000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Bronster D.J., Emre S., Mor E. Neurologic complications of orthotopic liver transplantation. Mt Sinai J Med. 1994;61:63–69. [PubMed] [Google Scholar]
- 113.Mc Caulley J., Van Thiel D., Starzl T.E. Acute and chronic renal failure after liver transplantation. Nephron. 1990;55:121. doi: 10.1159/000185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gonwa T.A., Klintmalm G.B., Levy M. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361–365. [PubMed] [Google Scholar]
- 115.O'Brien J.D., Ettinger N.A. Pulmonary complications of liver transplantation. Clin Chest Med. 1996;17:99–114. doi: 10.1016/s0272-5231(05)70301-4. [DOI] [PubMed] [Google Scholar]
- 116.Aggarwal S., Kang Y., Freeman J.A. Postperfusion syndrome: hypotension after reperfusion of the transplanted liver. J Crit Care. 1993;8:154–160. doi: 10.1016/0883-9441(93)90021-c. [DOI] [PubMed] [Google Scholar]
- 117.Doyle H.R., Marino R., Miro A. Adult respiratory distress syndrome secondary to end-stage liver disease. Successful outcome following liver transplantation. Transplantation. 1993;55:292–296. doi: 10.1097/00007890-199302000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaparala R., Kramer D.J., Mro A. Comparison of clinical score, bronchoalveolar lavage and protected brush specimens for the diagnosis of bacterial pneumonia in critically ill patients with liver disease. Am Rev Respir Dis. 1993;147(Suppl.):S38. [Google Scholar]
- 119.Starzl T.E., Iwatsuki S., Shaw B.W., Jr. Liver transplantation in the cyclosporin era. Prog Allergy. 1986;38:366. doi: 10.1159/000318481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Starzl T.E., Makowka L., Todo S. FK506: a potential breakthrough in immunosuppression. Transplant Proc. 1987;19(Suppl. 6):3. [PMC free article] [PubMed] [Google Scholar]
- 121.European FK506 multicentre Liver Study Group Randomised trial comparing tacrolimus (FK506) and cyclosporine in prevention of liver allograft rejection. Lancet. 1994;344:423. [PubMed] [Google Scholar]
- 122.The US Multicenter FK506 Liver Study Group A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 123.Henry M.L. Cyclosporine and tacrolimus (FK506): a comparison of efficacy and safety profiles. Clin Transpl. 1999;13:209–220. doi: 10.1034/j.1399-0012.1999.130301.x. [DOI] [PubMed] [Google Scholar]
- 124.Vezina C., Kudelski A., Sehgal S.N. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 125.Sehgal S.N., Baker H., Vezina C. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J Antibiot. 1975;28:727–732. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 126.Sehgal S.N. Rapamune (sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17(6):660–665. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 127.Sehgal S.N. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action, immunosuppressive effects results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 128.Neuhaus P., Klupp J., Langrehr J.M. mTOR inhibitors: an overview. Liver Transpl. 2001;7(6):473–484. doi: 10.1053/jlts.2001.24645. [DOI] [PubMed] [Google Scholar]
- 129.Wu J.C. Mycophenolate mofetil: molecular mechanism of action. Perspect Drug Discov Des. 1994;2:185–204. [Google Scholar]
- 130.Allison A.C., Eugui E.M. Purine metabolism and immunosuppressive affects of mycophenolate mofetil (MMF) Clin Transplant. 1996;10:77–84. [PubMed] [Google Scholar]
- 131.Larick J.W. Potential of monoclonal antibodies as pharmacological agents. Pharmacol Rev. 1989;41:539. [PubMed] [Google Scholar]
- 132.Ortho Multicenter Transplant Study Group Randomised trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N Engl J Med. 1985;313:337. doi: 10.1056/NEJM198508083130601. [DOI] [PubMed] [Google Scholar]
- 133.Starzl T.E., Marchiono T.L., Rowlands D.T. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stegall M.D., Everson G.T., Schroter G. Prednisone withdrawal late after liver transplantation reduces diabetes, hypertension, and hypercholesterolemia without causing graft loss. Hepatology. 1997;25:173. doi: 10.1002/hep.510250132. [DOI] [PubMed] [Google Scholar]
- 135.Reding R. Steroid withdrawal in liver transplantation: benefits, risks, and unanswered questions. Transplantation. 2000;70(3):405–410. doi: 10.1097/00007890-200008150-00001. [DOI] [PubMed] [Google Scholar]
- 136.Jain A., Reyes J., Kashyap R. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232(4):490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Busuttil R.W., Farmer D.G., Yersiz H. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905–918. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]


