Abstract
Purpose
We examined breast nodules with three-dimensional (3D) sonography and power-Doppler to identify new parameters that might be useful in differentiating benign and malignant lesions.
Materials and methods
Breast nodules in 34 women were examined with a Voluson-GE 730 scanner and a 7.5 MHz linear-array dedicated 3D probe. Each nodule was examined in the B-mode, and its vascular characteristics were evaluated with power-Doppler; 3D reconstruction was used in both studies. All examinations were performed by the same operator, who was unaware of the case characteristics. The examiner classified each lesion as benign or malignant based on B-mode appearance, margin characteristics, infiltration, and blood vessel distribution on power-Doppler; lesion volume was also calculated for T staging. Results were compared with those of biopsies, which were performed on all nodules after the sonographic examination.
Results
Biopsy findings revealed that 29 nodules were benign and 5 malignant. Based on the 3D sonographic examination, 27 lesions were considered benign, and 7 were classified as malignant. Two of the latter diagnoses were false-positives; there were no false negatives (specificity: 93.1%, sensitivity: 100%, accuracy: 94.1%).
Conclusions
3D sonography can be used to calculate lesional mass for T1 staging of malignant breast nodules. It can also reveal wall irregularities in benign lesions that are missed on two-dimensional (2D) scans and the limits of infiltration of malignant lesions. The 3D power-Doppler examination provides a panoramic full-length view of blood vessels supplying the nodule, and the number of vessels visualized with this approach is higher than that observed on 2D studies.
Keywords: 3D sonography, Breast nodules, Power-Doppler
Sommario
Scopo
Lo scopo di questo studio è stato quello di valutare le lesioni mammarie benigne e maligne con esame ecografico 3D per mettere in evidenza i rapporti delle lesioni con i tessuti circostanti e altri segni che potessero meglio differenziare tra loro i due tipi di lesioni.
Materiali e metodi
Sono state studiate 34 pazienti non consecutive affette da lesioni mammarie benigne (29) e maligne (5). È stato effettuato l'esame ecografico con un apparecchio Voluson-GE con sonda lineare 6–12 MHz dedicata alla ricostruzione 3D e l'esame power-Doppler con ricostruzione 3D per mettere in evidenza la vascolarizzazione delle lesioni. Tutte le 34 pazienti sono state sottoposte successivamente ad agoaspirato ecoguidato con prelievo di materiale per l'esame citopatologico, che è stato considerato il gold standard.
Risultati
Oltre al calcolo del volume delle lesioni sono stati valutati da tre operatori differenti i parametri “forma-ecostruttura”, “contorni-margini”, “infiltrazione”, “vasi al power-Doppler”. Si sono avuti due falsi positivi e la mancanza di falsi negativi nella diagnosi differenziale benigno/maligno che portano la metodica, nella nostra esperienza, a una specificità di 93,1, a una sensitività di 100 per un'accuratezza globale di 94,1.
Conclusioni
L'esame ecografico 3D è un esame di II livello che attraverso la possibilità di calcolare la massa della lesione, consente una migliore stadiazione T delle lesioni maligne; è utile inoltre per un più sicuro giudizio di benignità e/o malignità a causa della ottimale evidenziazione delle pareti delle lesioni e della loro eventuale infiltrazione nel tessuto circostante. L'esame 3D della neoangiogenesi, inoltre, sfrutta la capacità del power-Doppler tridimensionale di evidenziare la panoramicità dei vasi.
Introduction
Ultrasonography plays a highly important role in the diagnosis of breast nodules. It is particularly useful in distinguishing solid and cystic masses and for characterizing malignancy. Technological advances in recent years have provided a number of types of sonographic software, which can be helpful in characterizing breast nodules that would otherwise require invasive diagnostic methods (e.g., those for color and power-Doppler, II harmonics studies, elastosonography, and three-dimensional ultrasound). New programs are continually being proposed, but it is not always clear whether they offer real advantages in clinical settings. The opinions expressed in the literature are often discordant.
The program that allows three-dimensional (3D) reconstruction of soft tissues is now widely used for the study of superficial tissues, and it can therefore be applied to the study of breast lesions. It is a second-level examination that furnishes full-thickness images of focal lesions. These images are then elaborated to highlight the sonographic characteristics of the lesion and reveal new diagnostically useful elements.
The objective of the present study was to characterize a series of benign and malignant breast nodules with 3D ultrasound and 3D power-Doppler, in particular, the relation between the lesions and the surrounding tissues and all features that can be used to differentiate between the two types of lesions.
Materials and methods
We studied 34 non-consecutive patients with breast nodules (benign in 29 cases, malignant in 5). The focal lesions were identified by mammography followed by B-mode sonography, which was carried out with a Siemens Antares scanner and a 10–13 MHz transducer. The nodules were then re-examined with 3D ultrasound. For this examination, we used a GE Voluson scanner equipped with a broad-based (5 × 6 cm) linear-array 6–12 MHz transducer (Fig. 1). This transducer was used to perform the B-mode sonography and power-Doppler assessment of the vascular characteristics of the lesion (both with 3D reconstruction). The transducer was placed on the skin overlying the nodule, and the acoustic array automatically swept through the selected region of interest with no movement of the transducer. The 3D images thus acquired were subsequently elaborated to evaluate the volume of the lesion, its degree of vascularization, and its relation with surrounding tissues.
Fig. 1.
Three-dimensional transducer.
Each of the 34 breast lesions was subjected to ultrasound-guided needle aspiration for cytological diagnosis (gold-standard). Patients whose lesions were malignant underwent dedicated magnetic resonance imaging (MRI) of the breast (GE-Ovation-0.35 T) with paramagnetic contrast enhancement (Magnevist–Schering) to exclude the presence of multifocal tumors.
Results
The results of the 3D sonographic examinations are shown in Table 1. In addition to lesion volumes (used for the preoperative T staging of malignant tumors) (Fig. 2), we also evaluated the following parameters: shape-echo structure, margins (Fig. 3), infiltration, and vascular characteristics on power-Doppler. All readings were made by the same operator. Later, the recorded images were reviewed and re-interpreted by two radiologists, each with over 20 years of experience in breast sonography. In all 34 cases (including the two false-positives described below) and for all parameters, the three examiners were fully concordant on the results.
Table 1.
Series of breast lesions studied with 3D sonography
| Case | Volume (3D cm3) | B-mode | Margins (3D) | Infiltration (3D) | Vascularization (3D) | B | M | BIRAD | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AMC | 0.18 | B | B | B | B | B | Cyst C2 | |
| 2 | AN | 0.78 | B | B | B | B | B | Cyst C2 | |
| 3 | BR | 0.07 | B | B | B | B | B | Papilloma C3 | |
| 4 | BDA | 0.73 | B | B | B | B | B | Cyst C2 | |
| 5 | CM | 0.17 | B | B | B | B | B | Septated cyst C2 | |
| 6 | CA | 0.18 | B | B | B | B | B | Mastopathy C2 | |
| 7 | CC | 0.10 | B | B | B | B | B | Mastopathy C2 | |
| 8 | GD | 0.35 | B | B | B | B | B | Debris-filled duct C2 | |
| 9 | GA | 0.11 | B | B | B | B | B | Debris-filled cyst C2 | |
| 10 | GS | 0.15 | B | B | B | B | B | Fibroadenoma C2 | |
| 11 | LL | 0.44 | B | B | B | B | B | Hamartoma C2 | |
| 12 | MAM | 0.10 | B | B | B | B | B | Fibroadenoma C2 | |
| 13 | MML | 0.39 | B | B | B | B | B | Debris-filled cyst C2 | |
| 14 | ME | 0.31 | B | B | B | B | B | Calcifications dysplastic C1 | |
| 15 | MF | 0.31 | B | B | B | B | B | Fibroadenoma C2 | |
| 16 | NC | 1.7 | B | B | B | B | B | Fibroadenoma C2 | |
| 17 | PLo | 0.19 | B | B | B | M | M | Fibroadenoma FP C3 | |
| 18 | PLu | 1.3 | B | B | B | B | B | Fibroadenoma C2 | |
| 19 | PS | 0.45 | B | B | B | B | B | Cyst C2 | |
| 20 | PS | 3.46 | B | B | B | B | B | Cyst C2 | |
| 21 | Mastopathy C2 | ||||||||
| 22 | SR | 4 | B | B | B | B | B | Fibroadenoma proliferating C3 | |
| 23 | SS | 0.15 | B | B | B | B | B | Intraductal papilloma C3 | |
| 24 | TML | 0.9 | B | B | B | B | B | Cyst C2 | |
| 25 | TR | 0.31 | B | B | B | B | B | Fibroadenoma C2 | |
| 26 | TS | 0.26 | B | B | B | B | B | Debris-filled cyst C2 | |
| 27 | VA | 0.18 | B | B | M | B | M | Fibroadenoma FPC3 | |
| 28 | BB | 0.1 | B | B | B | B | B | Cyst C2 | |
| 29 | FR | 1.3 | B | B | B | B | B | Intraductal papilloma C3 | |
| 30 | BP | 0.2 | M | M | M | M | M | Ca C5 | |
| 31 | GE | 1.37 | M | M | M | M | M | Ca C5 | |
| 32 | MP | 0.31 | M | M | M | M | M | Ca C5 | |
| 33 | TI | 0.32 | M | M | M | M | M | CaC5 | |
| 34 | SB | 2.79 | M | M | M | M | M | Ca C5 |
Fig. 2.
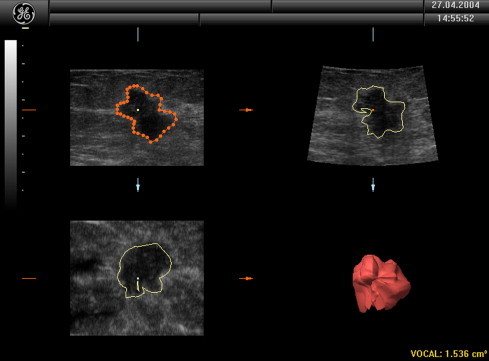
Calculation of the volume.
Fig. 3.
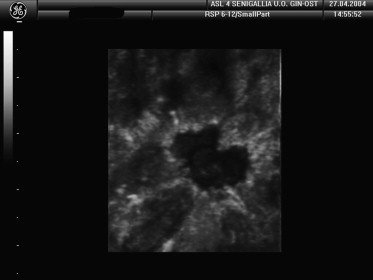
Breast nodules with irregular, spiculated margins typical of malignancy.
The first parameter (referred to simply as “B-mode” in Table 1) included shape (roundish vs. oval) and internal echo structure (anechoic, hypoechoic, hyperechoic, more or less homogeneous), and on the basis of these findings, the lesion was classified as benign (B) or malignant (M). The same classification (B vs. M) was made based on margin characteristics, which are much easier to visualize than on two-dimensional (2D) studies. Infiltration was defined as the presence of irregular “spiculated” margins. In 2D studies, infiltration is evaluated indirectly based on the presence or absence of posterior attenuation, but 3D studies allow clear visualization of the anterior and lateral margins of the lesion and their relation with healthy tissues surrounding the nodule. The enhanced visualization is so striking that it can lead to overestimation of margin irregularities and consequently false-positive diagnoses of malignancy (case no. 27).
Power-Doppler evaluation of the appearance of blood vessels included single and multiple poles. This increased the risk of false-positive diagnosis of malignancy due to overestimation of the number of vessels supplying the nodule (case no. 17). The presence of “anarchic” vascularization was an extremely interesting finding in terms of establishing whether or not the lesion was malignant (Fig. 4).
Fig. 4.
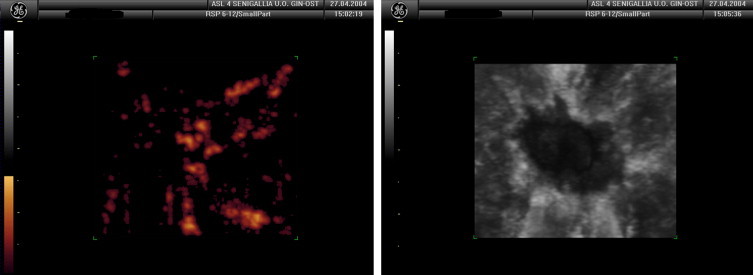
Power-Doppler reveals the “anarchic” vascularization of a malignant breast lesion.
In our hands, the method yielded two false-positives and no false negative results, which gives it a specificity of 93.1%, sensitivity of 100%, accuracy of 94.1%, positive and negative predictive values of 72% and 100%, respectively.
Discussion
Breast ultrasound is now considered an indispensable complement to mammography [1,2]. Together, they provide an accurate method for identifying malignant breast tumors [3] although there is room for improvement of both. The addition of digitalization and computer-aided diagnosis has improved the diagnostic prevision of mammography [4]. As for ultrasound, its diagnostic applications have expanded following the introduction of high-frequency multifocal transducers and color and power-Doppler techniques. It plays an indisputable role in the diagnosis of breast disease, particularly in the presence of mammographically dense breast tissue [5]. The introduction of 3D sonography allows three-dimensional visualization of breast lesions [6–9], and this facilitates estimates of the lesion volume, which are complicated and imprecise with 2D studies. In addition, the automatic scanning option reduces the operator-dependence of the ultrasound examination, a factor that has always limited the potential of this imaging modality, especially in the study of breasts. The images can also be re-elaborated after the examination has been completed and the patient is no longer present. The margins of the lesion can be thoroughly explored and characterized, along with the surrounding tissue. This facilitates the distinction between local infiltration and simple compression of perilesional tissues. The 3D study of the lesion's vascularization provides a panoramic view of the vessels, which can thus be quantified for a more reliable judgment on the presence of neoangiogenesis in benign and malignant lesions. The false-positive results that emerged for two lesions in our series were probably caused by overestimation of margin irregularity in one case and the presence of numerous vascular poles in the other. In one of the two, case no. 17, the fibroadenoma presented signs of proliferation (cytopathological class C3). The fact that this lesion was probably increasing in size may have been responsible for the poorly delimited margins (Fig. 5), which were interpreted by all three observers as a sign of malignancy. The second case was that of a fibroadenoma in a hyperglandular breast with fibrocystic mastopathy, and the examination was carried out during the premenstrual period, which is not ideal. As a result, the tissues surrounding the tumor presented “hypertrophic” vascularization, which was erroneously interpreted as hypervascularization (Fig. 6). In the diagnosis of breast lesions, a risk of false positivity is obviously preferable to that of false negativity. At worst, it can lead to additional testing (biopsy for microhistology and/or magnetic resonance imaging), which is in fact appropriate in several borderline situations [10–12]. The two nodules that produced false-positive results in our series both proved to be C3 in microhistology and MRI revealed characteristics associated with benign tumors. In contrast, the six lesions that were definitely classified as malignant were also diagnosed as malignant based on MRI and 3D sonographic findings.
Fig. 5.
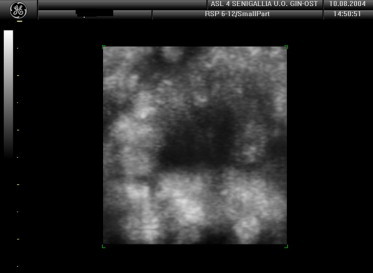
The margins of the mass are not perfectly regular (false-positive).
Fig. 6.
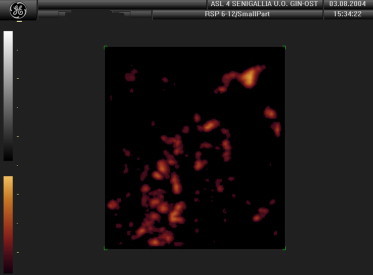
The mass appears to be hypervascularized (false-positive).
Conclusions
Three-dimensional sonography is a second-level examination that allows more precise estimates of lesion volumes, and consequently, more accurate T staging of malignant breast lesions. It is also helpful for differentiating benign and malignant breast lesions since it provides excellent visualization of the walls and margins of the lesion and its relation with surrounding tissues. Three-dimensional studies of neoangiogenesis exploit the ability of power-Doppler to detect slow flow and the extended field of view provided by 3D, which allows full-length visualization of each vessel. The result is a more reliable estimate of the number of vessels actually supplying the breast lesion.
Three-dimensional breast sonography should thus be used for the work-up of patients with breast lesions that are considered “at risk” (e.g., those classified as BIRADS class C3), in particular, for selection of patients who need to have MRI [13–15], and those with genetic predisposition to breast cancer, who require special diagnostic attention [16].
In differentiating benign and malignant breast masses, the 3D examination yielded two false-positive results and no false negatives (sensitivity 100%, specificity 91.4%, overall accuracy 92.6%). It should also be an effective, easy-to-use guide for collection of microhistology specimens and Mammotome breast biopsies since it enhances the operator's perception of the depth of the lesion.
References
- 1.Rahbar G., Sie A.C., Hansen G.C. Benign versus malignant solid breast masses. US differentiation. Radiology. 1999;213:889–894. doi: 10.1148/radiology.213.3.r99dc20889. [DOI] [PubMed] [Google Scholar]
- 2.Stavros A.T., Thickman D., Rapp C.L. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 3.Ciatto S., Rosselli del Turco M., Catarzi S., Morrone D. The contribution of ultrasonography to the differential diagnosis of breast cancer. Neoplasma. 1994;41:341–345. [PubMed] [Google Scholar]
- 4.Houssami N., Ciatto S., Irwig L., Simpson J.M., Macaskill P. The comparative sensitivity of mammography and ultrasound in women with breast symptoms: an age – specific analysis. Breast. 2002;11:125–130. doi: 10.1054/brst.2001.0391. [DOI] [PubMed] [Google Scholar]
- 5.Kolb T., Lichy J., Newhause J. Occult cancer in women with dense breasts: detection with screening US-diagnostic yield and tumor characteristics. Radiology. 1998;207:191–199. doi: 10.1148/radiology.207.1.9530316. [DOI] [PubMed] [Google Scholar]
- 6.Shipley J.A., Duck F.A., Goddard D.A. Automated quantitative volumetric breast ultrasound data-acquisition system. Ultrasound Med Biol. 2005;31:905–917. doi: 10.1016/j.ultrasmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T., Tamaki Y., Sato Y. Three-dimensional ultrasound imaging of breast cancer by a real-time intraoperative navigation system. Breast Cancer. 2005;12:122–129. doi: 10.2325/jbcs.12.122. [DOI] [PubMed] [Google Scholar]
- 8.Meyberg-Solomayer G.C., Kraemer B., Bergmann A. Does 3-D sonography bring any advantage to noninvasive breast diagnostics? Ultrasound Med Biol. 2004;30:583–589. doi: 10.1016/j.ultrasmedbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Cho K.R., Seo B.K., Lee J.Y. A comparative study of 2D and 3D ultrasonography for evaluation of solid breast masses. Eur J Radiol. 2005;54:365–370. doi: 10.1016/j.ejrad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl C.K., Mielcareck P., Klaschik S. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. doi: 10.1148/radiology.211.1.r99ap38101. [DOI] [PubMed] [Google Scholar]
- 11.Liberman L., Morris E.A., Dershaw D.D., Abramson A.F., Tan L.K. Ductal enhancement on MR imaging of the breast. AJR Am J Roentgenol. 2003;181:519–525. doi: 10.2214/ajr.181.2.1810519. [DOI] [PubMed] [Google Scholar]
- 12.Del Maschio A., De Gaspari A., Panizza P. Risonanza Magnetica in senologia. Rad Med. 2002;104:253–261. [PubMed] [Google Scholar]
- 13.Hwang E.S., Kinkel K., Esserman L.J., Lu Y., Weidner N., Hylton N.M. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion and multicentricity. Ann Surg Oncol. 2003;10:381–388. doi: 10.1245/aso.2003.03.085. [DOI] [PubMed] [Google Scholar]
- 14.Orel S.G., Schnall M.D. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 15.Hlawatsch A., Teifke A., Schmidt M., Thelen M. Preoperative assessment of breast cancer: sonography versus MR imaging. AJR Am J Roentgenol. 2002;179:1493–1501. doi: 10.2214/ajr.179.6.1791493. [DOI] [PubMed] [Google Scholar]
- 16.Warner E., Plewes D.B., Hill K.A. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]


