Abstract
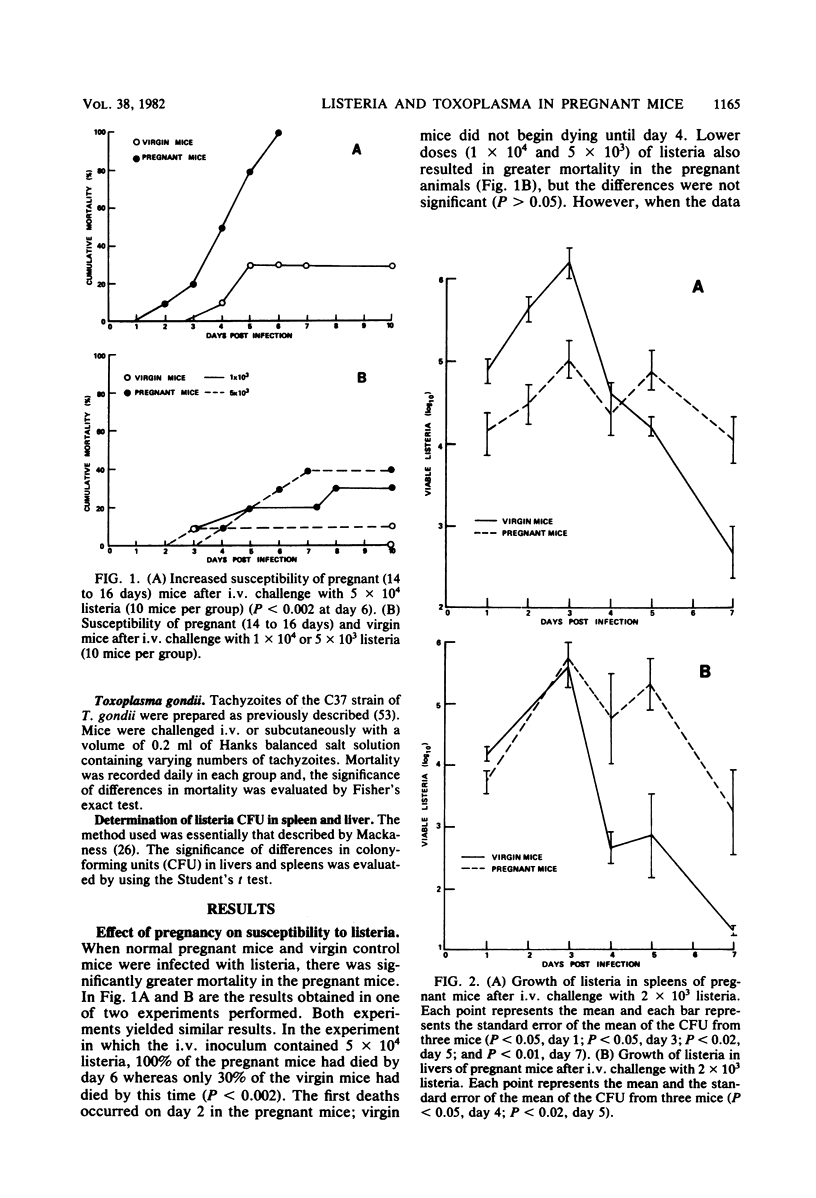
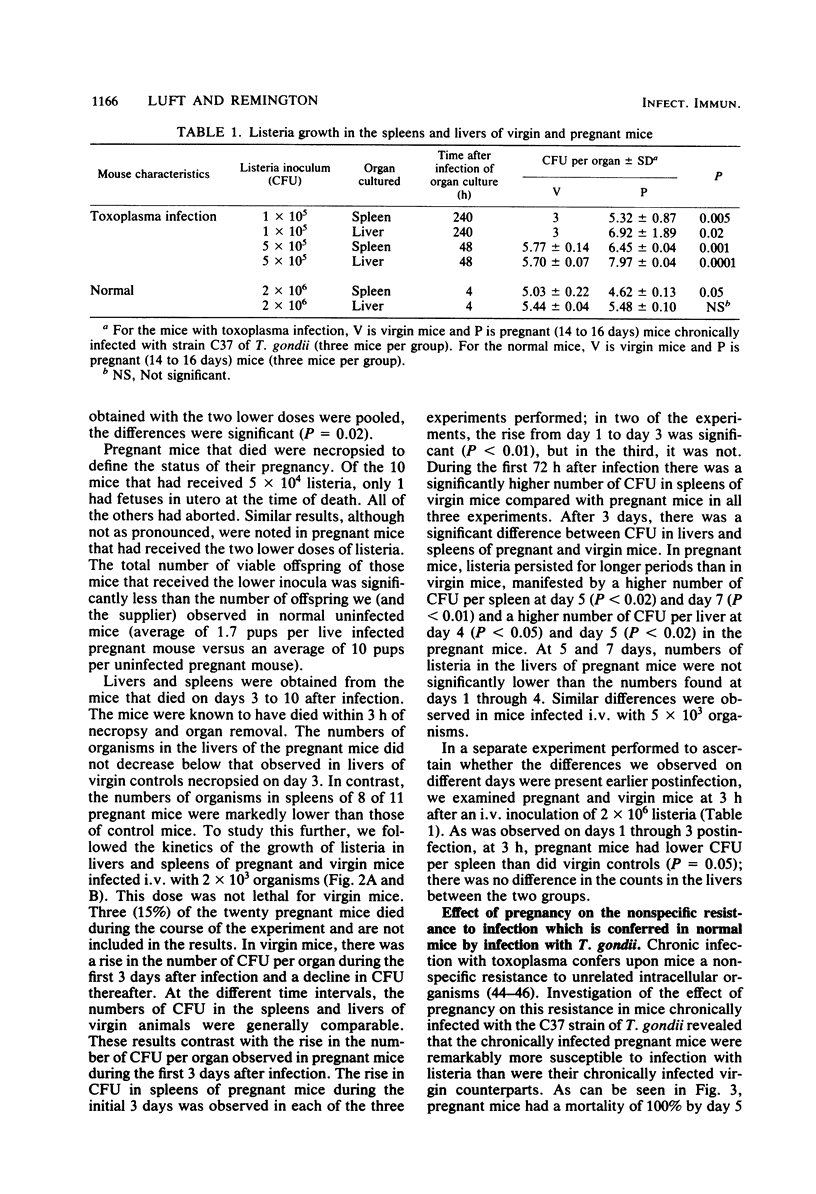
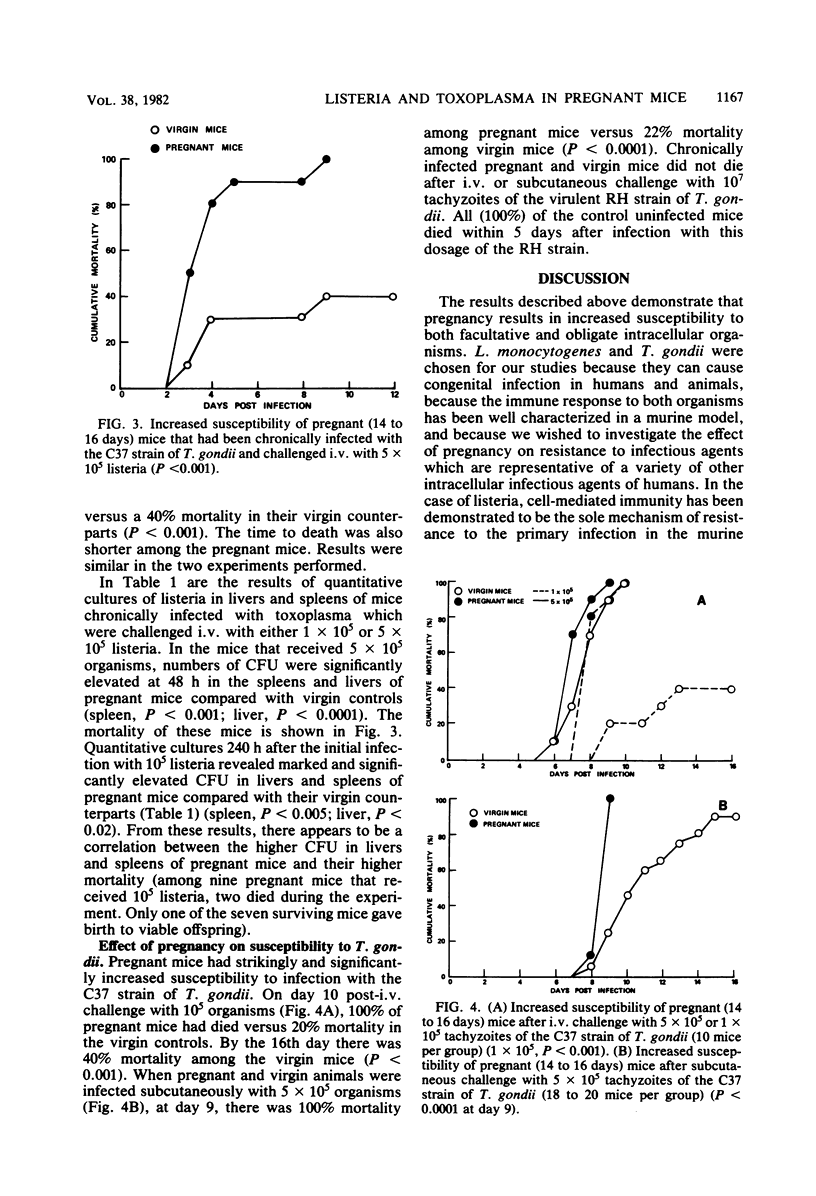
Studies of the effect of pregnancy on the capacity of mice to resist Listeria monocytogenes and Toxoplasma gondii infection revealed significantly diminished resistance of pregnant mice to infection by both agents as measured by mortality. The development of immunity to listeria was assessed by studying the kinetics of listeria growth in the livers and spleens of virgin and pregnant mice infected intravenously with a sublethal dose of listeria. In both virgin and pregnant mice there was a rise in the number of listeria colony-forming units per organ during the first 3 days after infection. Thereafter, there was a decline in colony-forming units in these organs in virgin mice but a persistence of listeria in spleens and livers of pregnant mice. Paradoxically, during the first 3 days after infection, listeria counts in spleens of virgin mice were significantly higher than those in pregnant mice. Nonspecific resistance to listeria conferred by chronic infection with T. gondii was significantly diminished in pregnant mice when measured by mortality and quantitative cultures of listeria in livers and spleens. These studies demonstrate a remarkably decreased resistance of pregnant mice to two intracellular organisms and a diminished capacity of pregnant mice to develop immunity to listeria. This decrease in resistance may play an important role in congenital transmission of these organisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris B. F. Suppressor factor produced by neonatal mouse spleen cells. Cell Immunol. 1981 Aug;62(2):412–424. doi: 10.1016/0008-8749(81)90342-7. [DOI] [PubMed] [Google Scholar]
- BYRD C. L. The influence of infection with Lansing strain poliomyelitis virus on pregnant mice. J Neuropathol Exp Neurol. 1950 Apr;9(2):202–203. doi: 10.1097/00005072-195004000-00010. [DOI] [PubMed] [Google Scholar]
- Bell S. C., Billington W. D. Major anti-paternal alloantibody induced by murine pregnancy is non-complement-fixing IgG1. Nature. 1980 Nov 27;288(5789):387–388. doi: 10.1038/288387a0. [DOI] [PubMed] [Google Scholar]
- Birkeland S. A., Kristoffersen K. Lymphocyte transformation with mitogens and antigens during normal human pregnancy: a longitudinal study. Scand J Immunol. 1980;11(3):321–325. doi: 10.1111/j.1365-3083.1980.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Birkeland S. A., Kristoffersen K. The fetus as an allograft: a longitudinal study of normal human pregnancies studied with mixed lymphocyte cultures between mother-father and mother-child. Scand J Immunol. 1980;11(3):311–319. doi: 10.1111/j.1365-3083.1980.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- CAMPBELL C. H. The susceptibility of mother mice and pregnant mice to the virus of foot-and-mouth disease. J Immunol. 1960 May;84:469–474. [PubMed] [Google Scholar]
- Chaouat G., Voisin G. A., Escalier D., Robert P. Facilitation reaction (enhancing antibodies and suppressor cells) and rejection reaction (sensitized cells) from the mother to the paternal antigens of the conceptus. Clin Exp Immunol. 1979 Jan;35(1):13–24. [PMC free article] [PubMed] [Google Scholar]
- Chaouat G., Voisin G. A. Regulatory T cell subpopulations in pregnancy. I. Evidence for suppressive activity of the early phase of MLR. J Immunol. 1979 Apr;122(4):1383–1388. [PubMed] [Google Scholar]
- Clark D. A., McDermott M. R. Impairment of host vs graft reaction in pregnant mice. I. Suppression of cytotoxic T cell generation in lymph nodes draining the uterus. J Immunol. 1978 Oct;121(4):1389–1393. [PubMed] [Google Scholar]
- Clarke F. M., Morton H., Clunie G. J. Detection and separation of two serum factors responsible for depression of lymphocyte activity in pregnancy. Clin Exp Immunol. 1978 May;32(2):318–323. [PMC free article] [PubMed] [Google Scholar]
- DALLDORF G., GIFFORD R. Susceptibility of gravid mice to Coxsackie virus infection. J Exp Med. 1954 Jan 1;99(1):21–27. doi: 10.1084/jem.99.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Active suppression of B lymphocyte maturation by two different newborn T lymphocyte subsets. J Immunol. 1979 Dec;123(6):2644–2650. [PubMed] [Google Scholar]
- Farber P. A., Glasgow L. A. Factors Modifying Host Resistance to Virus Infection: II. Enhanced Susceptibility of Mice to Encephalomyocarditis Virus Infection During Pregnancy. Am J Pathol. 1968 Sep;53(3):463–481. [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K., Nelson B. M., Arias-Stella J. Immunosuppression and toxoplasmic encephalitis: clinical and experimental aspects. Hum Pathol. 1975 Jan;6(1):97–111. doi: 10.1016/s0046-8177(75)80111-0. [DOI] [PubMed] [Google Scholar]
- GREENBERG M., JACOBZINER H., PAKTER J., WEISL B. A. Maternal mortality in the epidemic of Asian influenza, New York City, 1957. Am J Obstet Gynecol. 1958 Oct;76(4):897–902. doi: 10.1016/0002-9378(58)90027-9. [DOI] [PubMed] [Google Scholar]
- Gehrz R. C., Christianson W. R., Linner K. M., Conroy M. M., McCue S. A., Balfour H. H., Jr Cytomegalovirus-specific humoral and cellular immune responses in human pregnancy. J Infect Dis. 1981 Mar;143(3):391–395. doi: 10.1093/infdis/143.3.391. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lawton A. R. Induction of plasma cell differentiation of human fetal lymphocytes: evidence for functional immaturity of T and B cells. J Immunol. 1977 Oct;119(4):1213–1217. [PubMed] [Google Scholar]
- Hayward A. R., Lydyard P. M. Suppression of B lymphocyte differentiation by newborn T lymphocytes with an Fc receptor for IgM. Clin Exp Immunol. 1978 Dec;34(3):374–378. [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I., Brawn J. Abrogation of cellular immunity to antigenically foreign mouse embryonic cells by a serum factor. Nature. 1969 Nov 29;224(5222):914–915. doi: 10.1038/224914a0. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Kittas C., Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma gondii. Br J Exp Pathol. 1980 Dec;61(6):590–600. [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Delire M., Cambiaso C. L. Circulating immune complexes in normal human pregnancy. Nature. 1977 Apr 7;266(5602):542–543. doi: 10.1038/266542a0. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Sasazuki T. A suppressor T cell in the human mixed lymphocyte reaction. J Exp Med. 1977 Aug 1;146(2):368–380. doi: 10.1084/jem.146.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morito T., Bankhurst A. D., Williams R. C., Jr Studies of human cord blood and adult lymphocyte interactions with in vitro immunoglobulin production. J Clin Invest. 1979 Oct;64(4):990–995. doi: 10.1172/JCI109565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgita R. A. The immunosuppressive role of alpha-fetoprotein during pregnancy. Scand J Immunol. 1976;5(9):1003–1014. doi: 10.1111/j.1365-3083.1976.tb03052.x. [DOI] [PubMed] [Google Scholar]
- Nicklin S., Billington W. D. Macrophage activity in mouse pregnancy. J Reprod Immunol. 1979 Jul;1(2):117–126. doi: 10.1016/0165-0378(79)90012-3. [DOI] [PubMed] [Google Scholar]
- Noonan F. P., Halliday W. J., Morton H., Clunie G. J. Early pregnancy factor is immunosuppressive. Nature. 1979 Apr 12;278(5705):649–651. doi: 10.1038/278649a0. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olding L. B., Murgita R. A., Wigzell H. Mitogen-stimulated lymphoid cells from human newborns suppress the proliferation of maternal lymphocytes actoss a cell-impermeable membrane. J Immunol. 1977 Sep;119(3):1109–1114. [PubMed] [Google Scholar]
- Olding L. B., Oldstone B. A. Thymus-derived peripheral lymphocytes from human newborns inhibit division of their mothers' lymphocytes. J Immunol. 1976 Mar;116(3):682–686. [PubMed] [Google Scholar]
- Olding L. B., Oldstone M. B. Lymphocytes from human newborns abrogate mitosis of their mother's lymphocytes. Nature. 1974 May 10;249(453):161–162. doi: 10.1038/249161a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Persellin R. H., Thoi L. L. Human polymorphonuclear leukocyte phagocytosis in pregnancy. Development of inhibition during gestation and recovery in the postpartum period. Am J Obstet Gynecol. 1979 Jun 1;134(3):250–255. doi: 10.1016/s0002-9378(16)33028-9. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Rola-Pleszczynski M., Frenkel L. D., Fuccillo D. A., Hensen S. A., Vincent M. M., Reynolds D. W., Stagno S., Bellanti J. A. Specific impairment of cell-mediated immunity in mothers of infants with congenital infection due to cytomegalovirus. J Infect Dis. 1977 Mar;135(3):386–391. doi: 10.1093/infdis/135.3.386. [DOI] [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Ruskin J., Remington J. S. Immunity and intracellular infection: resistance to bacteria in mice infected with a protozoan. Science. 1968 Apr 5;160(3823):72–74. doi: 10.1126/science.160.3823.72. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Rengton J. S. Role for the macrophage in acquired immunity to phylogenetically unrelated intracellular organisms. Antimicrob Agents Chemother (Bethesda) 1968;8:474–477. doi: 10.1128/AAC.8.4.474. [DOI] [PubMed] [Google Scholar]
- Sljivić V. S., Clark D. W., Warr G. W. Effects of oestrogens and pregnancy on the distribution of sheep erythrocytes and the antibody response in mice. Clin Exp Immunol. 1975 Apr;20(1):179–186. [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Burton R. C., Barg M., Mitchell G. F. Maternal alloimmunisation in pregnancy. In vitro studies of T cell-dependent immunity to paternal alloantigens. Transplantation. 1978 Apr;25(4):216–220. [PubMed] [Google Scholar]
- Smith R. N., Powell A. E. The adoptive transfer of pregnancy-induced unresponsiveness to male skin grafts with thymus-dependent cells. J Exp Med. 1977 Sep 1;146(3):899–904. doi: 10.1084/jem.146.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W., Matsubayashi H., Akao S. Modification of subclinical toxoplasmosis in mice by cortisone, 6-mercaptopurine and splenectomy. Am J Trop Med Hyg. 1966 Nov;15(6):869–874. doi: 10.4269/ajtmh.1966.15.869. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tomasi T. B., Jr Immune responses during pregnancy. Evidence of suppressor cells for splenic antibody response. J Exp Med. 1979 Oct 1;150(4):898–908. doi: 10.1084/jem.150.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzberg J. E., Krahenbuhl J. L., Remington J. S. Dichotomy between macrophage activation and degree of protection against Listeria monocytogenes and Toxoplasma gondii in mice stimulated with Corynebacterium parvum. Infect Immun. 1975 Nov;12(5):1037–1043. doi: 10.1128/iai.12.5.1037-1043.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than G. N., Csaba I. F., Karg N. J., Szabo D. G., Ambrus M., Bajtai G. Letter: Immunosuppressive effect of pregnancy-associated alpha-glycoprotein. Lancet. 1975 Sep 13;2(7933):515–515. doi: 10.1016/s0140-6736(75)90606-6. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Steele R. W., Vincent M. M., Hensen S. A., Bellanti J. A. Impaired in vitro cell-mediated immunity to rubella virus during pregnancy. N Engl J Med. 1973 Sep 20;289(12):604–606. doi: 10.1056/NEJM197309202891203. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN L., AYCOCK W. L., FEEMSTER R. F. The relation of sex, pregnancy and menstruation to susceptibility in poliomyelitis. N Engl J Med. 1951 Jul 12;245(2):54–58. doi: 10.1056/NEJM195107122450203. [DOI] [PubMed] [Google Scholar]
- Wolf R. L., Lominitzer R., Rabson A. R. An inhibitor of lymphocyte proliferation and lymphokine production released by unstimulated foetal monocytes. Clin Exp Immunol. 1977 Mar;27(3):464–468. [PMC free article] [PubMed] [Google Scholar]
- Yu V. Y., Waller C. A., Maclennan I. C., Baum J. D. Lymphocyte reactivity in pregnant women and newborn infants. Br Med J. 1975 Feb 22;1(5955):428–432. doi: 10.1136/bmj.1.5955.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zon A. A., Eling W. M. Depressed malarial immunity in pregnant mice. Infect Immun. 1980 May;28(2):630–632. doi: 10.1128/iai.28.2.630-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



