Abstract
Focal nodular hyperplasia (FNH) is the second most common benign tumor of the liver, after hemangioma. It is generally found incidentally and is most common in reproductive-aged women, but it also affects males and can be diagnosed at any age. Patients are rarely symptomatic, but FNH sometimes causes epigastric or right upper quadrant pain. The main clinical task is to differentiate it from other hypervascular hepatic lesions such as hepatic adenoma, hepatocellular carcinoma, or hypervascular metastases, but invasive diagnostic procedures can generally be avoided with the appropriate use of imaging techniques. Magnetic resonance (MR) imaging is more sensitive and specific than conventional ultrasonography (US) or computed tomography (CT), but Doppler US and contrast-enhanced US (CEUS) can greatly improve the accuracy in the diagnosis of FNH. Once a correct diagnosis has been made, in most cases there is no indication for surgery, and treatment includes conservative clinical follow-up in asymptomatic patients.
Keywords: Focal nodular hyperplasia, Diagnosis, Management, Ultrasonology, Contrast-enhanced ultrasonography
Sommario
L'iperplasia nodosa focale (FNH) è il tumore epatico benigno più frequente dopo l'angioma. E' di solito rilevata in modo occasionale, per lo più nelle donne in età fertile, anche se si può riscontrare in entrambi i sessi e ad ogni età. I soggetti portatori raramente sono sintomatici, nonostante l'FNH possa essere causa di dolore ai quadranti addominali superiori. Nella gestione dell'FNH il problema clinico principale è rappresentato dalla difficoltà di differenziarla dalle altre lesioni epatiche ipervascolarizzate, come l'adenoma, l'epatocarcinoma o le metastasi ipervascolari, tuttavia utilizzando le opportune tecniche diagnostiche è oggi possibile evitare il ricorso ad esami invasivi. La Risonanza Magnetica (RM) ha una sensibilità e una specificità superiori rispetto a quelle dell'ecografia convenzionale e della Tomografia Assiale Computerizzata (TC) per la diagnosi di FNH. L'uso dell'angioecografia perfusionale (CEUS) e dell'eco-Doppler può tuttavia aumentare in modo significativo la confidenza diagnostica nell'identificazione e nella caratterizzazione dell'FNH con ultrasuoni. Una volta posta con certezza la diagnosi, nella maggior parte dei casi non ci sono indicazioni al trattamento chirurgico e nei soggetti asintomatici è raccomandato un follow-up clinico.
Definition and epidemiology
The term focal nodular hyperplasia (FNH), which was coined by Edmondson in 1958 [1], refers to a relatively common benign hepatic tumor. Focal nodular hyperplasia accounts for 8% of all primary liver tumors and 25% of those that are benign. In the latter category, it is second in frequency only to hepatic hemangioma [2]. Focal nodular hyperplasia is often an incidental finding, and its prevalence in the general population has been estimated at 0.9–3.0% [3,4]. Until the mid-1980s, FNH was diagnosed with a frequency similar to that reported for hepatic adenoma [2,5], but since 1989 there has been a significant increase in the number of cases of FNH while the number of adenomas has remained unchanged. This trend does not appear to be related to improvements in diagnostic techniques [5].
Focal nodular hyperplasia is more common in females (female/male ratio: 8/1) [4]. The vast majority of cases (82–91%) are diagnosed in women [6]. Roughly 20% of all cases are characterized by multiple tumors [2,7]. In female patients, most of the lesions are identified between the third and fourth decades of life, as demonstrated in numerous studies conducted over a long period of time [2,5,8]. In men, FNH is diagnosed significantly later in life. The lesions are smaller, but they display more severe morphological atypia [9], and for this reason FNH in males is more likely to be managed surgically [9].
Etiology
There are currently no unequivocal data on the cause of FNH, and various hypotheses have been advanced. For years, the tumors were attributed in large part to the use of hormone preparations, in particular, oral contraceptives [10,11]. This conclusion was based on the markedly higher frequency of the tumors among women and the fact that up to 85% of all women with FNH (depending on the series being analyzed) had histories of oral contraceptive use [8]. It has recently been disproved, however, by a large study conducted in France. The authors of this study examined a total of 216 women and found that the incidence of FNH was similar among those who used oral contraceptives and those who did not [12]. At this point, it seems clear that oral contraceptives do not play a direct role in the pathogenesis of FNH, although the trophic effects of estrogen–progestin therapy could conceivably promote tissue hyperplasia around a cicatricial lesion in the liver [13]. The use of birth control pills by women with FNH has also been associated with intralesional hemorrhage [14,15] and a rare case of spontaneous rupture of an FNH nodule that caused acute intraabdominal bleeding [16]. Today, most investigators regard FNH as a hyperplastic reaction to the presence of a localized vascular malformation (congenital or acquired) [13,17]. More specifically, compensatory liver-cell hypertrophy is thought to be triggered by altered distribution of oxygenated blood within the hepatic parenchyma, thus giving rise to the initial developmental nucleus of the FNH nodule. Additional support for this hypothesis has been provided by the demonstration that bile-duct proliferation like that observed in FNH nodules is a late but persistent response to ischemia in the human liver [18]. In addition, the extracellular matrix in FNH nodules has a composition similar to that found in the portal tracts, and it has been shown to contain vitronectin deposits, which are a reflection of local vascular disturbances [19].
Macroscopic and microscopic structural features
Focal nodular hyperplasia develops within hepatic parenchymal tissue that is otherwise histologically normal. Macroscopically, the tumor is characterized by the presence of depressed, grayish-white scar, which is generally (but not always) located at the center of the lesion, with fibrous septa radiating outward, toward the periphery of the tumor (Fig. 1). This stellate scar is found in only 50% of all FNH nodules, but its presence is regarded as pathognomonic [4]. The lesion itself is usually lighter in color than the tissue that surrounds it, and it sometimes has a yellowish hue. FNH nodules are generally not capsulated; their margins are well defined, and they are sometimes lobulated [2]. Subcapsular localizations are not uncommon, and in some cases (5–20%) the tumor is pedunculated. Apart from the central scar, the most common macroscopic feature (present in around 80% of all cases) [4] is the presence of multiple pseudolobules with fibrovascular and ductular areas that radiate from the perilobular septa [20]. Around 1–2% of all FNH nodules contain areas of calcification [21]. Histologically, FNH contains all the cell types found in normal hepatic parenchyma, but their structural organization is anomalous. The lesion includes a rearrangement of structurally normal hepatocytes and Kupffer cells with foci of bile-duct proliferation. The triglyceride and glycogen contents of the hepatocytes are often increased, and the cells are arranged in numerous spherical aggregates consisting of flat, thickened laminae. Earlier studies suggested that the hepatocytes in FNH nodules were clonal in origin [22], but later studies based on inactivation of the X-chromosome demonstrated that the cells were actually polyclonal, which indicated that the lesions are probably reactive [23]. The bile ducts within the nodules present signs of active, exuberant proliferation, and they are not connected to the biliary tree. The ducts themselves (especially those in the peripheral regions of the fibrous septa) are surrounded by an intense inflammatory infiltrate. Within the nodule, the number of progenitor cells is increased [24], probably as a result of the overexpression of transforming growth factor-alpha that occurs in FNH and is particularly evident at the level of the proliferating bile ducts [25]. Occasionally, the periseptal zones display evidence of fatty degeneration [26], bile formation, and Mallory bodies. Specific assays for copper may reveal deposits of the metal within the nodules.
Fig. 1.
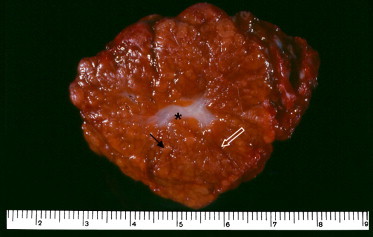
Gross pathologic features of a resected specimen of FNH. Yellowish nodules (straight black arrow) surrounded by multiple fibrous septa (white arrow) and a central scar (*) are visible. Note that there is no fibrous capsule at the interface of the lesion and the liver.
The vascular features of the lesion, which are quite peculiar, play a primary role in diagnosis of these nodules. There is a hypertrophic feeding artery that develops centripetally and terminates in the central scar. Its branches radiate outward, running through the fibrous septa. Their walls are thickened secondary to fibromuscular hyperplasia of the intima with concentric or eccentric restrictions of the lumen. Capillarization of the sinusoids has also been reported along with the absence of portal vessels and the central hepatic veins. The arterial and capillary network does not communicate with the portal circulation and nodular blood drains directly into the hepatic venules with a variable amount of arterovenous shunting [27].
The telangiectatic form of nodular hyperplasia is a histologic variant [4] found in 9.5–19% of all cases, depending on the series being examined [4,28]. The main feature that distinguishes it from classic FNH is the presence of hypertrophy of the tunica media of the arteries in the absence of intimal proliferation. In 10–20% of all cases, the presentation is atypical. These forms rarely include a fibrous scar, and they may contain zones of hyperplasia, adenomatous changes and even large-cell dysplasia [4].
Associated conditions
Focal nodular hyperplasia (particularly cases characterized by multiple lesions) can be associated with hepatic hemangiomas, an association found in 23% of the FNH patients examined in a French study [29]. The coexistence of FNH and hepatic adenomas [30,31], which are included in the differential diagnosis of FNH, is much less common (3.6%). This association seems to be promoted by the presence of vascular anomalies, thrombotic events, or localized arterovenous shunts [4,32]. The association between FNH and fibrolamellar hepatocellular carcinoma is still the subject of some debate. Some authors maintain that the latter tumors are the malignant counterparts of FNH [33], but thus far there has been no proof that FNH nodules undergo malignant transformation. There have also been reports of rare associations of FNH with other types of vascular anomalies [28,34], which lend support to the hypothesis that multiple FNH nodules occur within a syndromal context [7] related to irregular arterial support in the liver with areas of localized hypoperfusion. These associated anomalies include congenital absence of the portal vein [35,36], inflammatory pseudotumors of the liver [37], hepatic-vein thrombosis [38], and intrahepatic arterovenous shunting [39]. FNH has occasionally been found in association with extrahepatic pathology such as cerebrovascular anomalies [40], sickle-cell anemia [41], cystic dysplasia of the kidney [35], Klippel–Trenaunay syndrome [42], cerebral meningiomas and astrocytomas [28], and hereditary hemorrhagic telangiectasia [43]. Focal nodular hyperplasia has also been described in patients with glycogen-storage disease [44] although the latter condition is much more frequently associated with hepatic adenomas. There have also been reports of FNH nodules adjacent to a hemangioma and an adenoma (as well as a hydatid cyst) in the same liver [45].
Clinical aspects
In the vast majority of cases, focal nodular hyperplasia is initially asymptomatic. In some patients (mainly those with large nodules), FNH causes vague, nonspecific abdominal pain (related to pressure exerted on Glisson's capsule or, if the nodule is superficial, on adjacent organs), dyspepsia, feelings of fullness or epigastric discomfort [5]. The results of liver function tests are typically within normal limits [46], as are α-fetoprotein levels [5]. Mildly elevated levels of γGT alone can, however, be found in about half of all cases [5]. Cases of spontaneous regression of FNH nodules have been reported in the literature, including one of 16 patients enrolled in an ultrasound study conducted in Italy [47]. In another study based on computed tomography (CT) and magnetic resonance (MR) imaging follow-up of 18 FNH nodules [48], six remained stable over time, two increased in volume, and 10 regressed spontaneously. There have also been reports of the recurrence of multiple FNH nodules after surgical resection [49], an event that was attributed to a “progressive” variant of the disease.
FNH nodules generally show no malignant transformation tendency [50]. The complications they cause include intralesional hemorrhage, which occurs in 2–3% of all cases [5]. (Such complication is much more frequently encountered in hepatic adenoma.) Obstruction of the hepatic vein is even less common [51], and there has even been one report of Kasabach–Merritt syndrome (characterized by thrombocytopenia, hemolytic anemia, disseminated intravascular coagulation secondary to vascular malformations) that was attributed to FNH [52].
Sonographic features
The histologic features that distinguish FNH from other focal hepatic lesions are also important in the ultrasound examination: the presence of a feeding arteriole that develops centripetally and is enclosed within the central scar and the radiating pattern of the intralesional arteries [53–55]. On B-mode studies (Fig. 2), the appearance of FNH is nonspecific and variable [56]. In approximately 75–80% of cases, the lesion appears isoechoic or mildly hypoechoic with respect to the surrounding hepatic parenchyma [57,58]. Hyperechoic nodules are less common. When the nodule is isoechoic, displacement of vascular structures may be the only sign of its presence. As the nodule increases in size, its echostructure becomes increasingly inhomogeneous. In some cases, the lobulated profile of the nodule can be appreciated (Fig. 2b), and the margins may be quite clear or poorly defined (Fig. 2a). Some nodules also present a hypoechoic halo (Fig. 3) that represents perilesional tissues (parenchyma or blood vessels) compressed by the nodule. It is usually more evident when the surrounding parenchyma is steatotic [59]. The central scar can be visualized in a limited percentage of cases: the reported figures range from 19% to 47% [47,60,61]. It usually appears mildly hyperechoic on B-mode scans (Fig. 4).
Fig. 2.
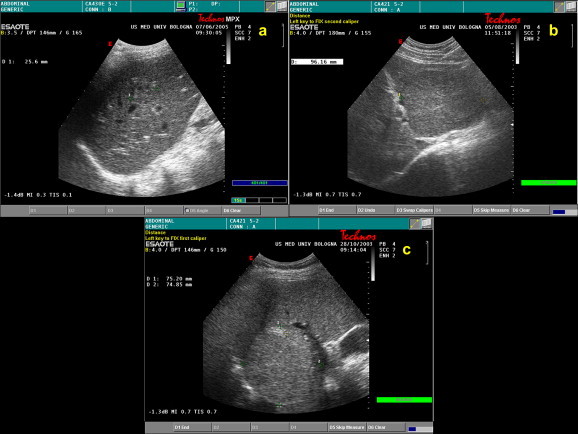
FNH features on conventional B-mode ultrasound: (a) gray-scale sonogram shows a 25-mm inhomogeneous FNH nodule with poorly delimited border; (b) large, partially exophytic, lobulated FNH nodule, 96 mm in diameter, in the left lobe of the liver. The lesion appears isoechoic to the surrounding tissue; (c) partially exophytic, hyperechoic FNH nodule (75 mm in diameter) found in the VIII hepatic segment.
Fig. 3.
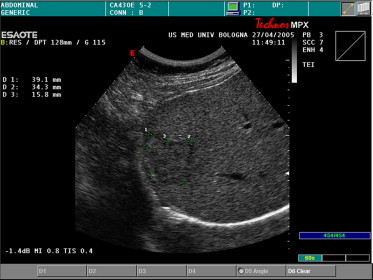
A 40-mm FNH nodule located in the VI hepatic segment. A thin hypoechoic rim can be seen surrounding the inhomogeneous isoechoic lesion.
Fig. 4.
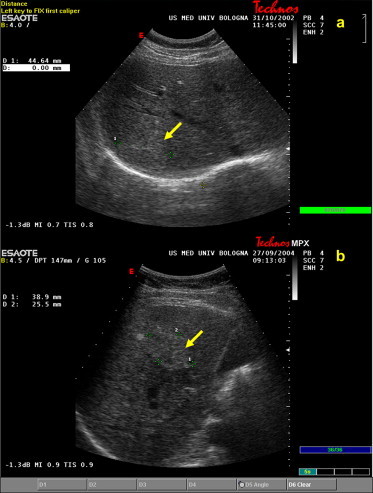
Hypoechoic central fibrous scars (yellow arrow) detected in two FHN nodules: (a) 45 mm in the VII segment and (b) 39 mm in the V hepatic segment.
Color and power Doppler studies of FNH nodules provide sufficient data to reach a diagnosis (65–70% of the studies performed), but in around one-third of all cases [53] they fail to reveal the typical stellate or spoke-wheel distribution of arterial vessels (Fig. 5), which arise from a hypertrophic central feeding artery that generally increases in caliber as the blood supply increases [58]. In rare cases, hypertrophy can also be detected at the level of the hepatic artery (Fig. 6), which has a tortuous, corkscrew-like appearance consistent with that of the feeding artery. In most cases, spectral analysis will reveal arterial signals in and around the nodule (Fig. 7). Flow through the central artery is pulsatile with a high peak systolic frequency (>1 kHz) and low impedance, which correspond to a resistance index (RI) (calculated as peak systolic frequency shift minus end-diastolic frequency shift divided by peak systolic frequency shift) of less than 0.65 (typically within range: 0.50–0.55) [62]. This is significantly lower than the RI of the hepatic artery, and it probably reflects the dystrophic changes in the nodular arteries. Measurement of impedance on arterial tracings during spectral analysis is important for distinguishing FNH nodules from malignant lesions, such as hepatocellular carcinoma or liver metastases. Large metastatic? lesions may have radiating vascular patterns similar to those of FNH [63,64]. In all three types of lesions, spectral tracings may present high peak systolic frequency values, but the RI of an FNH nodule is generally lower than that of a malignant lesion, which is usually >0.70 [65]. In rare cases, spectral analysis will reveal continuous intralesional flow, which (when present) represents venous drainage [66]. This finding complicates the differential diagnosis considerably as intranodular venous flow is typical of hepatic adenoma. In contrast, FNH nodules often show large venous vessels in the periphery, along the margins.
Fig. 5.
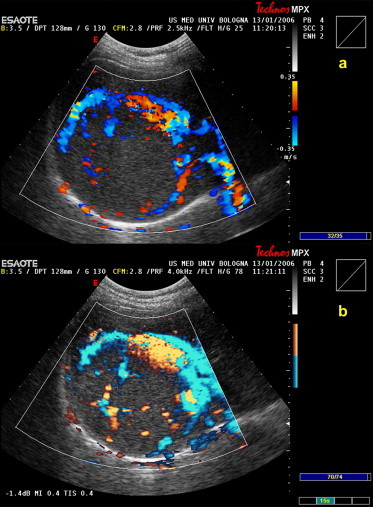
(a) Conventional color and (b) power Doppler of an 85-mm FNH nodule located in the VII–VIII hepatic segments reveals a spoke-wheel pattern corresponding to arteries radiating toward the periphery.
Fig. 6.
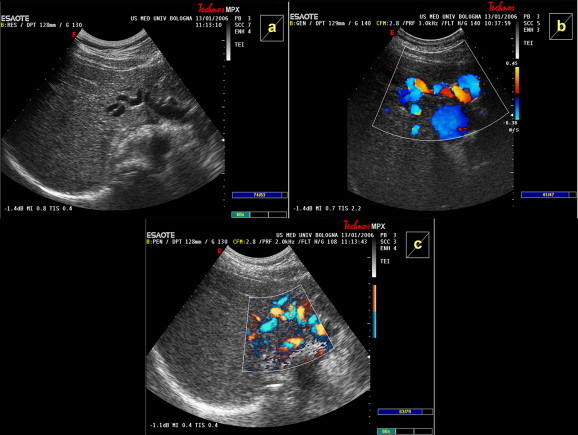
Prominent hypertrophic hepatic artery with features suggestive of a feeding artery. The artery is well visualized on (a) conventional US (b) color and (c) power Doppler in a patient with FNH.
Fig. 7.
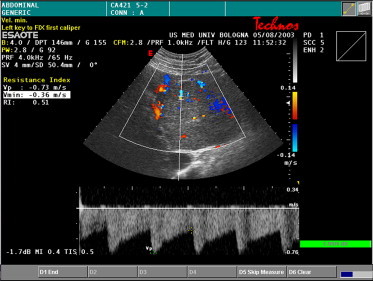
Doppler spectral analysis of an intranodular feeding artery shows an arterial pulsatile arterial waveform with high peak flow (1.73 kHz) and low resistance (RI = 0.51).
The recent use of ultrasound contrast agents has proved to be very helpful in the sonographic characterization of these nodules [56,67–72]. A study of 61 focal lesions [67] was conducted to determine whether use of a first-generation contrast agent (Levovist®, Schering AG) could increase the sensitivity of the power Doppler examination for identifying the typical vascular pattern of FNH (Fig. 8). The feeding arteriole was identified in a significantly higher percentage of cases in the contrast-enhanced examination (98% of all cases versus 85% when power Doppler was used without contrast enhancement). This difference was particularly evident when the nodule being examined was located in the left lobe of the liver, where motion artifacts are a greater problem. After injection of Levovist®, FNH nodules exhibit homogeneous enhancement and constant late-phase uptake of the contrast agent, which reflect the peculiar distribution of the arterial vessels within the nodule and distinguish them from malignant tumors [69]. For the diagnosis of FNH, this approach has proved to be both sensitive (83%) and specific (98%) [70].
Fig. 8.
The characteristic “spoke-wheel” pattern of an FNH nodule is seen on power Doppler after administration of the first-generation ultrasound contrast agent, Levovist®.
Additional improvements are seen when contrast-enhanced US (CEUS) is used with harmonic imaging and second-generation contrast agents. Its use in FNH patients was evaluated in a recent study [71], whose results have yet to be validated in other series. In 23 of the 24 FNH nodules examined, second-generation CEUS revealed marked hyperechogenicity in both the arterial phase (8–22 s after injection of the contrast agent) and the early portal phase (12–30 s after injection).
We recently studied 55 FNH nodules with CEUS using the second-generation contrast agent, SonoVue® (Bracco S.p.A., Milano, Italy) (Fig. 9). Fifty-two of these lesions exhibited typical hyperechogenicity in the arterial phase and the characteristic spoke-wheel pattern of vascularization (personal data). In the other three nodules (all belonging to the same patient), typical contrast enhancement was not observed in the early phase, and they were identified as FNH nodules only after surgical resection. This anomalous behavior has no definite explanation, but was observed in only one patient and thus appears related to patient- rather than lesion-specific abnormality. Contrast enhancement of the liver began 17.2 s after the SonoVue injection (range: 11–27 s), and peak signal intensity (which increased evidence of the hypervascularity of the lesion compared with the surrounding parenchyma) was observed after a mean interval of 23 s (range: 15–42). In the late-phase, most of the lesions became isoechoic to the surrounding parenchyma 37 s after the SonoVue injection (range: 20–65 s). A few remained mildly hyperechoic or became slightly hypoechoic. These findings of ours are consistent with those that have emerged from previous studies [71,72], and they provide confirmation based on a larger series of lesions of previous descriptions of the CEUS features of FNH: intense, homogeneous enhancement in the arterial phase (present in 97.7% of the patients we examined) and isoechogenicity or weak hyperechogenicity in the portal and parenchymal phases (95.4% of our cases). None of the lesions we studied presented frank hypoechogenicity in the late phase, a feature that is more suggestive of a malignant lesion.
Fig. 9.
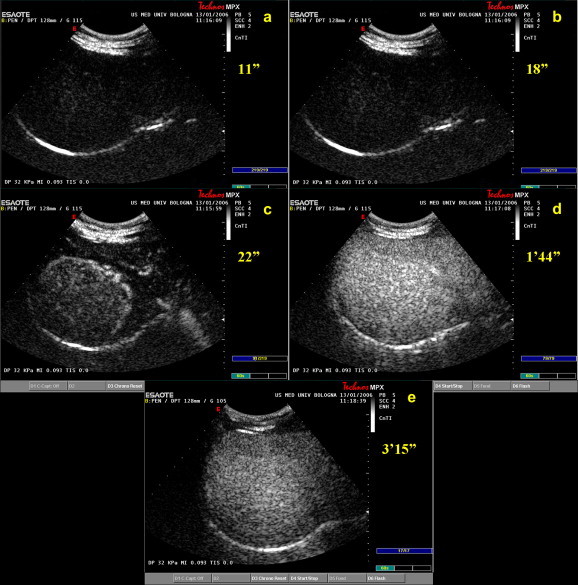
US appearance of an FNH nodule (85 mm) after administration of a second-generation contrast agent (SonoVue®): (a) baseline image (11 s after contrast injection); (b) early arterial phase (18 s after injection); (c) late arterial phase (22 s after injection); (d) portal phase (104 s after injection); and (e) delayed venous phase imaging (195 s after injection).
Radiologic imaging techniques
Many authors maintain that magnetic resonance imaging (MRI) is the imaging method of choice for the study of focal nodular hyperplasia [5,73], especially for distinguishing these nodules from hepatic adenomas. Compared with the surrounding hepatic parenchyma, 94–100% of FNH nodules appear uniformly isointense or hypointense on T1-weighted MRI and isointense or mildly hyperintense on T2-weighted images (Fig. 10) [58,74]. The isointensity of the FNH nodule in sequences acquired before the administration of the contrast agent reflects the presence of structurally normal hepatocytes in these lesions, and this feature plays a key role in differentiating these nodules from other focal lesions. Even when an FNH nodule appears hypointense, the difference with respect to the surrounding parenchyma is minimal. The central scar can be identified in 50–70% of moderate-to-large-sized nodules and a much lower percentage of small lesions (<3 cm). It appears hypointense in T1 and hyperintense in T2 due to the presence of blood vessels, bile ducts, and edema-inducing phenomena [66,75], and these findings clearly differentiate it from the lesional tissue around it. When the MRI study is performed with a conventional vascular contrast agent like gadolinium, the nodule displays intense, uniform enhancement in the arterial phase followed by isointensity in the late phase [59,76]. In contrast, the central scar is characterized by relative hypointensity in the arterial phase, but it can become hyperintense in the late phase. The latter phenomenon is caused by persistence of the contrast medium within the lax mesenchymal tissue of the scar.
Fig. 10.
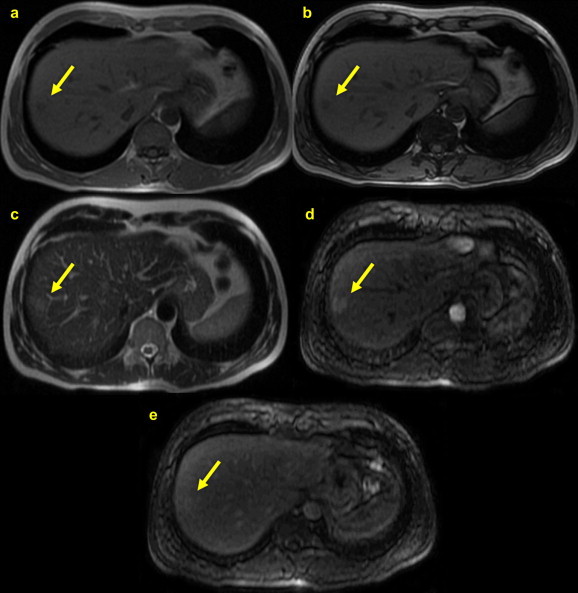
MRI appearance of FNH: (a) T1-weighted in-phase image; (b) T1 out-of-phase image; (c) In a T2-weighted image, the FNH nodule (yellow arrow) appears isointense to the surrounding liver parenchyma; (d) during the arterial phase of gadolinium-enhanced imaging, the entire nodule (except the central scar) displays intense, homogeneous enhancement; and (e) during the late phase, the FNH lesion becomes isointense to the surrounding parenchyma.
Recent reports have analyzed the use of new contrast agents in MRI [77], including superparamagnetics that are specific for Kupffer cells (e.g., ferucarbotran) as well as hepatobiliary agents like mangafodipir. After administration of the latter agent, FNH nodules appear isointense or mildly hyperintense in T1-weighted images whereas malignant hepatic lesions are hypointense because they lack structurally normal hepatocytes [59,78].
Computed tomography (CT) can be a valid alternative to MRI in the assessment of focal nodular hyperplasia (Fig. 11). Before the administration of the contrast agent, FNH nodules typically appear isodense or mildly hypodense to the surrounding parenchyma. A hypodense central scar and the fibrous septa that radiate from it can be visualized in about one-third of all cases [60]. After the injection of the contrast medium, the richly vascularized nodule displays rapid, transient enhancement in the early arterial phase (20–30 s after injection) and isodensity in the portal phase (70–90 s after injection) [79,80]. During the late parenchymal phase (up to 5 min after the injection of the contrast medium), the FNH remains isodense to the surrounding parenchyma. As on MRI, the central scar may remain hyperdense even during the late phase. This reflects the slow wash-out of contrast medium from the myxomatous stroma that makes up the scar [79]. Brancatelli and coworkers examined 78 subjects with FNH and were able to visualize the central scar on CT in 65% of the large nodules and 35% of the smaller lesions [80].
Fig. 11.
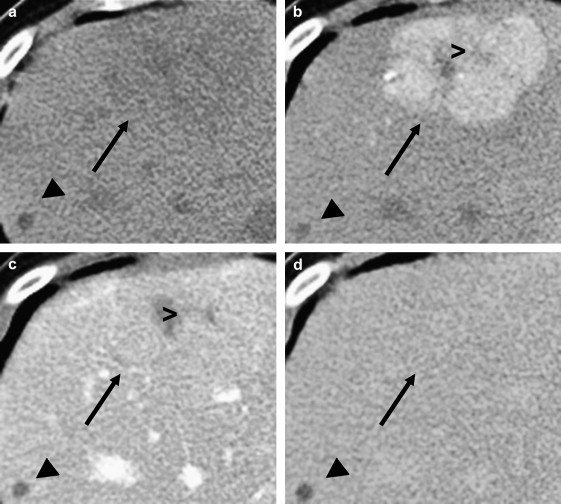
CT appearance of FNH: (a) on a precontrast CT image, the FNH nodule (black arrow) is only slightly hypoattenuating compared with the surrounding liver tissue; (b) contrast-enhanced CT image obtained during the arterial phase shows homogeneous enhancement of the lesion; the central scar (>) remains hypoattenuating; (c) in the portal; and (d) late phases, the lesion becomes isoattenuating with respect to the surrounding liver. A hepatic cyst (◀) is also present.
In selected cases, scintigraphy and angiography (ideally with Lipiodol enhancement) can also be used for diagnosis of FNH [81]. The latter study reveals a hypervascular formation with a central origin and centrifugal development. In a high percentage of cases (70–80%), scintigraphy with 99Tc reveals normal or increased uptake of the colloid at the level of the lesion, which reflects the high concentration of Kupffer cells in these nodules.
Differential diagnosis
The diagnosis of FNH is based on the demonstration in imaging studies of the central scar, but this can also be seen as a zone of fibrosis in fibrolamellar hepatocellular carcinoma [82], hepatic adenoma, and intrahepatic cholangiocarcinoma. A second element that is fundamental to the diagnosis of FNH is the presence on color and power Doppler of the spoke-wheel pattern of vascularization with arterial flow characterized by low resistance and high peak flow. Unfortunately, these features may not be easy to document in nodules measuring less than 4 cm. Therefore, in certain cases, uncertainties can arise in differentiating FNH nodules from other types of focal hepatic lesions [83]. From a clinical point of view, it is extremely important to distinguish between FNH and hepatic adenomas. In the latter case, spontaneous rupture with intratumoral hemorrhage or hemoperitoneum occurs in a substantial percentage of cases, and these tumors are also subject to malignant degeneration [7,8]. In these cases, the diagnostic accuracy of ultrasound-guided fine-needle biopsy is quite high (94.7%) [84], but it may be inconclusive, especially when the amount of tissue collected is limited. In fact, cytology alone may not be sufficient to distinguish FNH from well-differentiated hepatocellular carcinoma or adenoma. Uncertainties are especially likely to arise in atypical variants that lack a central scar or other typical imaging findings [85], and in these cases, the best solution is histologic analysis of an ultrasound-guided biopsy specimen. To reach the nodule, the needle has to pass through at least 1 cm of normal hepatic parenchyma.
Management
Once the nature of an FNH nodule has been established, no specific treatment is required if the patient is asymptomatic, and some authors advocate a “wait and see” approach [5,73]. This generally involves ultrasound examinations every six months for the first three years after diagnosis. The frequency can be reduced once the disease has stabilized with no change in nodule size or number. Surgical resection should be considered if there is evidence of progressive growth or particularly large lesions (>10 cm in diameter), symptoms of compression, or an increased risk of hemorrhage related to trauma [86] (e.g., large subcapsular or exophytic nodules). Subcapsular nodules can also be resected laparoscopically [87]. It appears that low doses of estrogen are safe to use in FNH patients, but if there is no strong indication for it, hormone therapy should generally be discontinued. There are a number of reports of pregnancies carried successfully to term in women with FNH [12,88]. The nodules should be closely monitored, however, since they may increase significantly in size during pregnancy.
Acknowledgements
The authors would like to thank Dr. Michelangelo Fiorentino for furnishing the image Fig. 1.
References
- 1.Edmondson H.A. Armed Forces Institute of Pathology; Washington, DC: 1958. Tumors of the liver and intrahepatic bile ducts. (Atlas of tumor pathology). section 7, part 25. [Google Scholar]
- 2.Craig J.R., Peters R.L., Edmondson H.A. Armed Forces Institute of Pathology; Washington, DC: 1989. Tumors of the liver and intrahepatic bile ducts. (Atlas of tumor pathology). 2nd series, part 26. [Google Scholar]
- 3.Karhunen P.J. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183–188. doi: 10.1136/jcp.39.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen B.N., Flejou J.F., Terris B., Belghiti J., Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441–1454. doi: 10.1097/00000478-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Cherqui D., Rahmouni A., Charlotte F. Management of focal nodular hyperplasia and hepatocellular adenoma in young women: a series of 41 patients with clinical, radiological, and pathological correlations. Hepatology. 1995;22:1674–1681. [PubMed] [Google Scholar]
- 6.Ishak K.G., Goodman Z.D., Stocker J.T. Armed Forces Institute of Pathology; Washington, DC: 2001. Tumors of the liver and intrahepatic bile ducts. (Atlas of tumor pathology). 3rd series, part 31. [Google Scholar]
- 7.International Working Party Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983–993. doi: 10.1016/0270-9139(95)90324-0. [DOI] [PubMed] [Google Scholar]
- 8.Reddy K.R., Kligerman S., Levi J. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–178. [PubMed] [Google Scholar]
- 9.Luciani A., Kobeiter H., Maison P. Focal nodular hyperplasia of the liver in men: is presentation the same in men and women? Gut. 2002;50:877–880. doi: 10.1136/gut.50.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott L.D., Katz A.R., Duke J.H., Cowan D.F., Maklad N.F. Oral contraceptives, pregnancy, and focal nodular hyperplasia of the liver. JAMA. 1984;251:1461–1463. [PubMed] [Google Scholar]
- 11.Pain J.A., Gimson A.E., Williams R., Howard E.R. Focal nodular hyperplasia of the liver: results of treatment and options in management. Gut. 1991;32:524–527. doi: 10.1136/gut.32.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu D., Kobeiter H., Maison P. Oral contraceptive use and focal nodular hyperplasia of the liver. Gastroenterology. 2000;118:560–564. doi: 10.1016/s0016-5085(00)70262-9. [DOI] [PubMed] [Google Scholar]
- 13.Wanless I.R., Mawdsley C., Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194–1200. doi: 10.1002/hep.1840050622. [DOI] [PubMed] [Google Scholar]
- 14.Stauffer J.Q., Lapinski M.W., Honold D.J., Myers J.K. Focal nodular hyperplasia of the liver and intrahepatic haemorrhage in young women on oral contraceptives. Ann Intern Med. 1975;83:301–306. doi: 10.7326/0003-4819-83-3-301. [DOI] [PubMed] [Google Scholar]
- 15.Brunt E.M., Flye M.W. Infarction in focal nodular hyperplasia of the liver. A case report. Am J Clin Pathol. 1991;95:503–506. doi: 10.1093/ajcp/95.4.503. [DOI] [PubMed] [Google Scholar]
- 16.Kleespies A., Settmacher U., Neuhaus P. Spontaneous rupture of hepatic focal nodular hyperplasia – rare cause of acute intraabdominal bleeding. Zentralbl Chi. 2002;127:326–328. doi: 10.1055/s-2002-31555. [DOI] [PubMed] [Google Scholar]
- 17.Kondo F. Benign nodular hepatocellular lesions caused by abnormal hepatic circulation: etiological analysis and introduction of a new concept. J Gastroenterol Hepatol. 2001;16:1319–1328. doi: 10.1046/j.1440-1746.2001.02576.x. [DOI] [PubMed] [Google Scholar]
- 18.Shimamatsu K., Wanless I.R. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343–350. doi: 10.1002/hep.510260214. [DOI] [PubMed] [Google Scholar]
- 19.Scoazec J.Y., Flejou J.F., D'Errico A. Focal nodular hyperplasia of the liver: composition of the extracellular matrix and expression of cell–cell and cell–matrix adhesion molecules. Hum Pathol. 1995;26:1114–1125. doi: 10.1016/0046-8177(95)90274-0. [DOI] [PubMed] [Google Scholar]
- 20.Fischer H.P., Lankes G. Morphologic correlation between liver epithelium and mesenchyme allows insight into histogenesis of focal nodular hyperplasia (FNH) of the liver. Virchows Arch B Cell Pathol. 1991;60:373–380. doi: 10.1007/BF02899569. [DOI] [PubMed] [Google Scholar]
- 21.Caseiro-Alves F., Zins M., Mahfouz A.-E. Calcification in focal nodular hyperplasia: a new problem for differentiation from fibrolamellar hepatocellular carcinoma. Radiology. 1996;198:889–892. doi: 10.1148/radiology.198.3.8628888. [DOI] [PubMed] [Google Scholar]
- 22.Gaffey M.J., Iezzoni J.C., Weiss L.M. Clonal analysis of focal nodular hyperplasia of the liver. Am J Pathol. 1996;148:1089–1096. [PMC free article] [PubMed] [Google Scholar]
- 23.Paradis V., Laurent A., Flejou J.F., Vidaud M., Bedossa P. Evidence for the polyclonal nature of focal nodular hyperplasia of the liver by the study of X-chromosome inactivation. Hepatology. 1997;26:891–895. doi: 10.1002/hep.510260414. [DOI] [PubMed] [Google Scholar]
- 24.Roskams T., De Vos R., Desmet V. ‘Undifferentiated progenitor cells’ in focal nodular hyperplasia of the liver. Histopathology. 1996;28:291–299. doi: 10.1046/j.1365-2559.1996.d01-438.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaff Z., Hsia C.C., Sarosi I., Tabor E. Overexpression of transforming growth factor-alpha in hepatocellular carcinoma and focal nodular hyperplasia from European patients. Hum Pathol. 1994;25:644–651. doi: 10.1016/0046-8177(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 26.Eisenberg L.B., Warshauer D.M., Woosley J.T., Cance W.G., Bunzendahl H., Semelka R.C. CT and MRI of hepatic focal nodular hyperplasia with peripheral steatosis. J Comput Assist Tomogr. 1995;19:498–500. doi: 10.1097/00004728-199505000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Fukukura Y., Nakashima O., Kusaba A., Kage M., Kojiro M. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J Hepatol. 1998;29:470–475. doi: 10.1016/s0168-8278(98)80067-6. [DOI] [PubMed] [Google Scholar]
- 28.Wanless I.R., Albrecht S., Bilbao J. Multiple focal nodular hyperplasia of the liver associated with vascular malformations of various organs and neoplasia of the brain: a new syndrome. Mod Pathol. 1989;2:456–462. [PubMed] [Google Scholar]
- 29.Mathieu D., Zafrani E.S., Anglade M.C., Dhumeaux D. Association of focal nodular hyperplasia and hepatic hemangioma. Gastroenterology. 1989;97:154–157. doi: 10.1016/0016-5085(89)91429-7. [DOI] [PubMed] [Google Scholar]
- 30.Friedman L.S., Gang D.L., Hedberg S.E., Isselbacher K.J. Simultaneous occurrence of hepatic adenoma and focal nodular hyperplasia: report of a case and review of the literature. Hepatology. 1984;4:536–540. doi: 10.1002/hep.1840040330. [DOI] [PubMed] [Google Scholar]
- 31.Grange J.D., Guechot J., Legendre C., Giboudeau J., Darnis F., Poupon R. Liver adenoma and focal nodular hyperplasia in a man with high endogenous sex steroids. Gastroenterology. 1987;93:1409–1413. doi: 10.1016/0016-5085(87)90273-3. [DOI] [PubMed] [Google Scholar]
- 32.Laurent C., Trillaud H., Lepreux S., Balabaud C., Bioulac-Sage P. Association of adenoma and focal nodular hyperplasia: experience of a single French academic center. Comp Hepatol. 2003;2:6. doi: 10.1186/1476-5926-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecchio F.M., Fabiano A., Ghirlanda G., Manna R., Massi G. Fibrolamellar carcinoma of the liver: the malignant counterpart of focal nodular hyperplasia with oncocytic change. Am J Clin Pathol. 1984;81:521–526. doi: 10.1093/ajcp/81.4.521. [DOI] [PubMed] [Google Scholar]
- 34.Haber M., Reuben A., Burrell M., Oliverio P., Salem R.R., West A.B. Multiple focal nodular hyperplasia of the liver associated with hemihypertrophy and vascular malformations. Gastroenterology. 1995;108:1256–1262. doi: 10.1016/0016-5085(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 35.Guariso G., Fiorio S., Altavilla G. Congenital absence of the portal vein associated with focal nodular hyperplasia of the liver and cystic dysplasia of the kidney. Eur J Pediatr. 1998;157:287–290. doi: 10.1007/s004310050812. [DOI] [PubMed] [Google Scholar]
- 36.Kinjo T., Aoki H., Sunagawa H., Kinjo S., Muto Y. Congenital absence of the portal vein associated with focal nodular hyperplasia of the liver and congenital choledochal cyst: a case report. J Pediatr Surg. 2001;36:622–625. doi: 10.1053/jpsu.2001.22303. [DOI] [PubMed] [Google Scholar]
- 37.Sakai M., Ikeda H., Suzuki N. Inflammatory pseudotumor of the liver: case report and review of the literature. J Pediatr Surg. 2001;36:663–666. doi: 10.1053/jpsu.2001.22316. [DOI] [PubMed] [Google Scholar]
- 38.Schilling M.K., Zimmermann A., Redaelli C., Seiler C.A., Buchler M.W. Liver nodules resembling focal nodular hyperplasia after hepatic venous thrombosis. J Hepatol. 2000;33:673–676. doi: 10.1034/j.1600-0641.2000.033004673.x. [DOI] [PubMed] [Google Scholar]
- 39.Lalonde L., Van Beers B., Trigaux J.P., Delos M., Melange M., Pringot J. Capsule and mosaic pattern of hepatocellular carcinoma: correlation between CT and MR imaging. Gastrointest Radiol. 1992;17:154–156. doi: 10.1007/BF01888558. [DOI] [PubMed] [Google Scholar]
- 40.Goldin R.D., Rose D.S. Focal nodular hyperplasia of the liver associated with intracranial vascular malformations. Gut. 1990;31:554–555. doi: 10.1136/gut.31.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heaton N.D., Pain J., Cowan N.C., Salisbury J., Howard E.R. Focal nodular hyperplasia of the liver: a link with sickle cell disease? Arch Dis Child. 1991;66:1073–1074. doi: 10.1136/adc.66.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bathgate A., MacGilchrist A., Piris J., Garden J. Multiple focal nodular hyperplasia in Klippel–Trenaunay syndrome. Gastroenterology. 1999;117:284–285. doi: 10.1016/s0016-5085(99)70596-2. [DOI] [PubMed] [Google Scholar]
- 43.Buscarini E., Danesino C., Plauchu H. High prevalence of hepatic focal nodular hyperplasia in subjects with hereditary hemorrhagic telangiectasia. Ultrasound Med Biol. 2004;30:1089–1097. doi: 10.1016/j.ultrasmedbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Takamura M., Mugishima H., Oowada M., Harada K., Uchida T. Type Ia glycogen storage disease with focal nodular hyperplasia in siblings. Acta Paediatr Jpn. 1995;37:510–513. doi: 10.1111/j.1442-200x.1995.tb03365.x. [DOI] [PubMed] [Google Scholar]
- 45.Di Carlo I., Urrico G.S., Ursino V., Russello D., Puleo S., Latteri F. Simultaneous occurrence of adenoma, focal nodular hyperplasia, and hemangioma of the liver: are they derived from a common origin? J Gastroenterol Hepatol. 2003;18:227–230. doi: 10.1046/j.1440-1746.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- 46.Meyers W.C., Jones R.S. Textbook of liver and biliary surgery. Lippincott; Philadelphia: 1990. Disorders of the liver; pp. 86–126. [Google Scholar]
- 47.Di Stasi M., Caturelli E., De Sio I., Salmi A., Buscarini E., Buscarini L. Natural history of focal nodular hyperplasia of the liver: an ultrasound study. J Clin Ultrasound. 1996;24:345–350. doi: 10.1002/(SICI)1097-0096(199609)24:7<345::AID-JCU3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 48.Leconte I., Van Beers B.E., Lacrosse M. Focal nodular hyperplasia: natural course observed with CT and MRI. J Comput Assist Tomogr. 2000;24:61–66. doi: 10.1097/00004728-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Sadowski D.C., Lee S.S., Wanless I.R., Kelly J.K., Heathcote E.J. Progressive type of focal nodular hyperplasia characterized by multiple tumors and recurrence. Hepatology. 1995;21:970–975. [PubMed] [Google Scholar]
- 50.Bennett W.F., Bova J.G. Review of hepatic imaging and a problem-oriented approach to liver masses. Hepatology. 1990;12(4 Pt 1):761–775. doi: 10.1002/hep.1840120423. [DOI] [PubMed] [Google Scholar]
- 51.Arrivè L., Dahan H., Tubiana J.M. Hepatic vein obstruction in a case of focal nodular hyperplasia. AJR Am J Roentgenol. 1999;173:857. doi: 10.2214/ajr.173.3.10470963. [DOI] [PubMed] [Google Scholar]
- 52.Mahfouz A.E., Rahmouni A., Terem C. MRI of intralesional hemolysis in focal nodular hyperplasia of the liver. J Comput Assist Tomogr. 1999;23:684–686. doi: 10.1097/00004728-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Golli M., Mathieu D., Anglade M.C., Cherqui D., Vasile N., Rahmouni A. Focal nodular hyperplasia of the liver: value of color Doppler US in association with MR imaging. Radiology. 1993;187:113–117. doi: 10.1148/radiology.187.1.8451397. [DOI] [PubMed] [Google Scholar]
- 54.Wang L.Y., Wang J.H., Lin Z.Y. Hepatic focal nodular hyperplasia: findings on color Doppler ultrasound. Abdom Imaging. 1997;22:178–181. doi: 10.1007/s002619900167. [DOI] [PubMed] [Google Scholar]
- 55.Uggowitzer M.M., Kugler C., Mischinger H.J. Echo-enhanced Doppler sonography of focal nodular hyperplasia of the liver. J Ultrasound Med. 1999;18:445–451. doi: 10.7863/jum.1999.18.7.445. [DOI] [PubMed] [Google Scholar]
- 56.Shirkhoda A., Farah M.C., Bernacki E., Madrazo B., Roberts J. Hepatic focal nodular hyperplasia: CT and sonographic spectrum. Abdom Imaging. 1994;19:34–38. doi: 10.1007/BF02165858. [DOI] [PubMed] [Google Scholar]
- 57.Saul S.H. Masses of the liver. In: Sternberg S.S., editor. Diagnostic surgical pathology. 2nd ed. Raven; New York: 1994. pp. 1517–1580. [Google Scholar]
- 58.Buetow P.C., Pantongrag-Brown L., Buck J.L., Ros P.R., Goodman Z.D. Focal nodular hyperplasia of the liver: radiologic–pathologic correlation. Radiographics. 1996;16:369–388. doi: 10.1148/radiographics.16.2.8966294. [DOI] [PubMed] [Google Scholar]
- 59.Hussain S.M., Terkivatan T., Zondervan P.E. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT, and pathologic analysis. Radiographics. 2004;24:3–17. doi: 10.1148/rg.241035050. [DOI] [PubMed] [Google Scholar]
- 60.Shamsi K., De Schepper Degryse H., Deckers F. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging. 1993;18:32–38. doi: 10.1007/BF00201698. [DOI] [PubMed] [Google Scholar]
- 61.Bartolozzi C., Lencioni R., Paolicchi A., Moretti M., Armillotta N., Pinto F. Differentiation of hepatocellular adenoma and focal nodular hyperplasia of the liver: comparison of power Doppler imaging and conventional color Doppler sonography. Eur Radiol. 1997;7:1410–1415. doi: 10.1007/s003300050308. [DOI] [PubMed] [Google Scholar]
- 62.Uggowitzer M., Kugler C., Machan L. Power Doppler imaging and evaluation of the resistive index in focal nodular hyperplasia of the liver. Abdom Imaging. 1997;22:268–273. doi: 10.1007/s002619900187. [DOI] [PubMed] [Google Scholar]
- 63.Numata K., Tanaka K., Mitsui K., Morimoto M., Inoue S., Yonezawa H. Flow characteristics of hepatic tumors at color Doppler sonography: correlation with arteriographic findings. AJR Am J Roentgenol. 1993;160:515–521. doi: 10.2214/ajr.160.3.8381573. [DOI] [PubMed] [Google Scholar]
- 64.Gaiani S., Casali A., Serra C. Assessment of vascular patterns of small liver mass lesions: value and limitation of the different Doppler ultrasound modalities. Am J Gastroenterol. 2000;95:3537–3546. doi: 10.1111/j.1572-0241.2000.03372.x. [DOI] [PubMed] [Google Scholar]
- 65.Gaiani S., Piscaglia F., Serra C., Bolondi L. Hemodynamics in focal nodular hyperplasia. J Hepatol. 1999;31:576. doi: 10.1016/s0168-8278(99)80057-9. [DOI] [PubMed] [Google Scholar]
- 66.Kehagias D., Moulopoulos L., Antoniou A. Focal nodular hyperplasia: imaging findings. Eur Radiol. 2001;11:202–212. doi: 10.1007/s003300000575. [DOI] [PubMed] [Google Scholar]
- 67.Uggowitzer M., Kugler C., Groll R. Sonographic evaluation of focal nodular hyperplasias (FNH) of the liver with a transpulmonary galactose-based contrast agent (Levovist) Br J Radiol. 1998;71:1026–1032. doi: 10.1259/bjr.71.850.10211062. [DOI] [PubMed] [Google Scholar]
- 68.Bleuzen A., Tranquart F. Incidental liver lesions: diagnostic value of cadence contrast pulse sequencing (CPS) and SonoVue. Eur Radiol. 2004;14(Suppl. 8):P53–P62. doi: 10.1007/s10406-004-0079-0. [DOI] [PubMed] [Google Scholar]
- 69.Von Herbay A., Voght C., Haussinger D. Late-phase pulse inversion sonography using the contrast agent Levovist: differentiation between benign and malignant focal liver lesions of the liver. AJR Am J Roentgenol. 2002;179:1273–1279. doi: 10.2214/ajr.179.5.1791273. [DOI] [PubMed] [Google Scholar]
- 70.Dill-Macky M.J., Burns P.N., Khalili K., Wilson S.R. Focal hepatic masses: enhancement patterns with SH U 508A and pulse-inversion US. Radiology. 2002;222:95–102. doi: 10.1148/radiol.2221010092. [DOI] [PubMed] [Google Scholar]
- 71.Dietrich C.F., Schuessler G., Trojan J., Fellbaum C., Ignee A. Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol. 2005;78:704–707. doi: 10.1259/bjr/88181612. [DOI] [PubMed] [Google Scholar]
- 72.Xu H.X., Liu G.J., Lu M.D. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261–272. doi: 10.1002/jcu.20234. [DOI] [PubMed] [Google Scholar]
- 73.Herman P., Pugliese V., Machado M.A. Hepatic adenoma and focal nodular hyperplasia: differential diagnosis and treatment. World J Surg. 2000;24:372–376. doi: 10.1007/s002689910059. [DOI] [PubMed] [Google Scholar]
- 74.Vilgrain V., Flejou J.F., Arrive L. Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology. 1992;184:699–703. doi: 10.1148/radiology.184.3.1509052. [DOI] [PubMed] [Google Scholar]
- 75.Mortelè K.J., Praet M., Van Vlierberghe H., Kunnen M., Ros P.R. CT and MR imaging findings in focal nodular hyperplasia of the liver: radiologic-pathologic correlation. AJR Am J Roentgenol. 2000;175:687–692. doi: 10.2214/ajr.175.3.1750687. [DOI] [PubMed] [Google Scholar]
- 76.Mathieu D., Rahmouni A., Anglade M.C. Focal nodular hyperplasia of the liver: assessment with contrast-enhanced Turbo FLASH MR imaging. Radiology. 1991;180:25–30. doi: 10.1148/radiology.180.1.2052704. [DOI] [PubMed] [Google Scholar]
- 77.Hussain S.M., Zondervan P.E., IJzermans J.N., Schalm S.W., de Man R.A., Krestin G.P. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023–1039. doi: 10.1148/radiographics.22.5.g02se061023. [DOI] [PubMed] [Google Scholar]
- 78.Ba-Ssalamah A., Schima W., Schmook M.T. Atypical focal nodular hyperplasia of the liver: imaging features of nonspecific and liver-specific MR contrast agents. AJR Am J Roentgenol. 2002;179:1447–1456. doi: 10.2214/ajr.179.6.1791447. [DOI] [PubMed] [Google Scholar]
- 79.Carlson S.K., Johnson C.D., Bender C.E., Welch T.J. CT of focal nodular hyperplasia of the liver. AJR Am J Roentgenol. 2000;174:705–712. doi: 10.2214/ajr.174.3.1740705. [DOI] [PubMed] [Google Scholar]
- 80.Brancatelli G., Federle M.P., Grazioli L., Blachar A., Peterson M.S., Thaete L. Focal nodular hyperplasia: CT findings with emphasis on multiphasic helical CT in 78 patients. Radiology. 2001;219:61–68. doi: 10.1148/radiology.219.1.r01ap0361. [DOI] [PubMed] [Google Scholar]
- 81.Kondo S., Nishikawa M., Takami S. Diagnostic value of lipiodol injection in focal nodular hyperplasia of the liver. Am J Gastroenterol. 1991;86:779–781. [PubMed] [Google Scholar]
- 82.Kinnard M.F., Alavi A., Rubin R.A., Lichtenstein G.R. Nuclear imaging of solid hepatic masses. Semin Roentgenol. 1995;30:375–395. doi: 10.1016/s0037-198x(05)80024-0. [DOI] [PubMed] [Google Scholar]
- 83.Hamrick-Turner J.E., Shipkey F.H., Cranston P.E. Fibrolamellar hepatocellular carcinoma: MR appearance mimicking focal nodular hyperplasia. J Comput Assist Tomogr. 1994;18:301–304. [PubMed] [Google Scholar]
- 84.Livraghi T., Belli P., Garavaglia G.M., Matricardi L., Torzilli G., Vettori C. Focal nodular hyperplasia of the liver: diagnostic role of smear cytology versus microhistology following ultrasound guided fine needle biopsy. J Intervent Radiol. 1993;8:155–159. [Google Scholar]
- 85.Fabre A., Audet P., Vilgrain V. Histologic scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology. 2002;35:414–420. doi: 10.1053/jhep.2002.31103. [DOI] [PubMed] [Google Scholar]
- 86.Ott R., Hohenberger W. Focal nodular hyperplasia and liver cell adenoma: operation or observation? Zentralbl Chir. 1998;123:145–153. [PubMed] [Google Scholar]
- 87.Descottes B., Lachachi F., Sodji M. Early experience with laparoscopic approach for solid liver tumors: initial 16 cases. Ann Surg. 2000;232:641–645. doi: 10.1097/00000658-200011000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathieu D., Kobeiter H., Cherqui D., Rahmouni A., Dhumeaux D. Oral contraceptive intake in women with focal nodular hyperplasia of the liver. Lancet. 1998;352:1679–1680. doi: 10.1016/S0140-6736(05)61451-1. [DOI] [PubMed] [Google Scholar]


