Abstract
Transjugular intrahepatic portosystemic shunt (TIPS) has become a widely accepted treatment for complications of portal hypertension. Shunt or hepatic vein stenoses or occlusions are common short- and mid-term complications of the procedure, with a one-year primary patency ranging from 25% to 66%. When promptly identified, shunt stenosis or occlusion may be treated before the recurrence of gastrointestinal bleeding or ascites. The revision is usually successful and the primary-assisted patency of TIPS is approximately 85% at one year.
Doppler sonography is a widely accepted screening modality for TIPS patients, both as a routine follow-up in asymptomatic patients and in those cases with clinically suspected TIPS malfunction.
In a routine US follow-up, a TIPS patient is scheduled for a control 24 h after the procedure, and then after one week, 1 month, 3 months, and at 3-month intervals thereafter. Venography is at present performed solely on the basis of a suspected shunt dysfunction during the sonographic examination.
Color-Doppler sonography is the most reliable method for monitoring the shunt function after TIPS implantation. Several studies have shown that Doppler sonography is a sensitive and relatively specific way to detect shunt malfunction, particularly when multiple parameters are examined. Achieving high sensitivity is optimal so that malfunctioning shunts can be identified and shunt revision can be performed before symptomatic deterioration. Venous angiography is at present indicated only on the basis of US suspicion of shunt compromise. Power-Doppler US and US contrast media can be useful in particular conditions, but are not really fundamental.
Keywords: Tips, Sonography, Doppler US
Sommario
La derivazione portosistemica per via percutanea transgiugulare, nota con l'acronimo TIPS, è diventata una modalità di trattamento delle complicanze dell'ipertensione portale. Stenosi od occlusioni dello shunt o delle vene epatiche sono complicanze a breve e medio termine, con pervietà a un anno compresa tra 25% e 66%. Se la diagnosi di stenosi od occlusione è precoce, il trattamento consente di evitare recidiva di ematemesi o ascite. La revisione dello shunt è generalmente di successo e la pervietà assistita della TIPS è di circa il 5% a un anno.
Le tecniche Doppler sono ampiamente accettate nelle procedure di screening dei pazienti con TIPS, sia nel paziente asintomatico sia in quello con sospetto clinico di malfunzione dello shunt. In una procedura di follow-up la valutazione color-Doppler viene eseguita dopo 24 ore, dopo 7 giorni, dopo 1 mese, dopo 3 mesi e quindi ogni 3 mesi. La venografia viene oramai effettuata soltanto su precisa indicazione dell'esame ecografico. Il color-Doppler è la metodica migliore per il monitoraggio del funzionamento dello shunt dopo l'impianto di una TIPS. Molti studi hanno dimostrato che il Doppler è sensibile e relativamente specifico nell'identificare la malfunzione dello shunt, particolarmente se molteplici parametri vengono esaminati contemporaneamente. L'elevata sensibilità consente di identificare gli shunt malfunzionanti indirizzandoli alla revisione prima del peggioramento delle condizioni. La venografia viene effettuata solo su indicazione della valutazione color- e power-Doppler. L'utilizzo del power-Doppler e del mezzo di contrasto può essere utile in particolari condizioni, ma non è realmente fondamentale.
Introduction
Transjugular intrahepatic portosystemic shunt is widely used for the treatment of complications of portal hypertension [1]. In several randomized trials comparing TIPS with other treatment options, a significant reduction of rebleeding rates [2] and improvement of ascites [2] were reported. Primary indications of TIPS included variceal bleeding, refractory ascites, hypertensive gastropathy, and hepatorenal syndrome in patients awaiting liver transplantation [3] or as a rescue procedure when other treatments have failed [2]. However, a major caveat of TIPS is its progressive stenosis leading to a recurrent increase in portal pressure gradient (PPG) or TIPS dysfunction and to the recurrence of gastrointestinal bleeding or ascites [4]. TIPS dysfunction is very frequent, with an incidence up to 80% in the first year as previously reported [5,6] and it is the most common cause of rebleeding in patients treated with TIPS. Therefore, these patients require close follow-up to identify and to treat shunt dysfunction before the recurrence of gastrointestinal bleeding or ascites [7].
To date, Color-Doppler sonography (CDUS) is a commonly used screening modality for the evaluation of TIPS patients, and it is a routine follow-up tool in asymptomatic patients and in patients with clinically suspected TIPS malfunction [1,2,4,8,9]. A number of studies reported a variety of CDUS criteria with very high sensitivity and specificity to detect TIPS dysfunction [10].
In a routine US follow-up, a TIPS patient is scheduled for a control 24 h after the procedure, and then after 1 week, 1 month, 3 months, and at 3-month intervals thereafter.
Examination technique
Patients should be examined preferably after an overnight fast, with low- or medium-frequency (2.5–3.5 MHz) convex or phased array transducers. Power output has to be set at maximum values, while all other Doppler parameters (including PRF, gain, wall filter, beam focusing and color priority) vary depending on the patient and the vessels to be studied [6].
The sonographic examination includes the complete assessment of the shunt and the vascular evaluation of the liver. The shunt should be first assessed by a conventional US, in order to define correct positioning, complete expansion, and possible narrowing of the stent.
Color- and Power-Doppler allow assessment of shunt patency, which is correctly defined by complete saturation in color, to identify filling defects due to pseudointimal hyperplasia or partial thrombosis and to evaluate the presence of focal color aliasing due to stenosis.
Angle-corrected velocity measurements should be obtained in three segments of the stent: the proximal end (close to the portal vein), the midportion, and the distal end (close to the inferior vena cava). Peak stent velocity, minimum stent velocity, stent velocity gradient (the difference between peak and minimum stent velocity) and stent velocity ratio (ratio of peak to minimum stent velocity) have to be recorded [17].
The post-TIPS sonogram consisted also in the evaluation of patency, flow direction and angle-corrected velocity in the hepatic artery and in the main, right, and left portal veins. The flow reversal in the portal branches distal to the shunt should be recorded as a positive sign. The draining hepatic vein has to be finally investigated, searching for patency or stenosis. A complete sonographic assessment includes also the evaluation of the liver parenchyma and the gallbladder, the determination of the amount of ascites, and the spleen size [1].
The sonogram obtained immediately after the original TIPS placement can be used as a baseline for comparison unless TIPS is revised, in which case the post-revision sonogram will serve as a new baseline.
In selected cases, US contrast media can be used to improve the assessment of TIPS status [11–13], if conventional Doppler US fails to prove normal TIPS patency and function. To date, however, the need for echo enhancers in the assessment of shunt patency is limited to the so called difficult patients, due to bowel gas or obesity. The use of second generation contrast agents doesn't seem to improve significantly the role of contrast-enhanced US.
Functioning shunts
Patent, well-functioning shunts usually show a complete color saturation of the stent, without any evidence of filling defects or focal aliasing.
Spectral Doppler analysis should be performed in three segments of the stent (portal, midportion, and hepatic sites), in order to evaluate the whole length of the shunt. Normal spectral Doppler analysis is usually characterized by a high-velocity turbulent flow, with a gradual velocity shift during respiration. Peak velocities slightly decreased over time on routine follow-up sonograms.
In the past, many investigators [1,2,8–15] have attempted to establish a lower normal limit for shunt velocities. This limit is based on the assumption that a hemodynamically significant stenosis will decrease flow through the shunt and cause a reduced velocity in the non-stenotic portion of the stent. The values range from 50 to 60 cm/s in older reports [14,15] and from 80 to 90 cm/s in more recent papers [8,9]. Another approach to peak shunt velocities is to establish an upper limit, by assuming that a focal stenosis would produce an elevated velocity at this level. Different upper limits have been reported by different authors ranging within 185–220 cm/s [15,16]. Kanterman demonstrated that a range of 90–190 cm/s has a sensitivity of 84% and a specificity of 70%, with a PPV of 82% and NPV of 72% in defining a well-functioning shunt [9]. In our personal experience we have obtained results similar to those published by Kanterman, concerning the focal velocity elevation in the presence of stenosis, but it is not so easy to define correctly a range of velocities [17]. Another issue to be borne in mind is that after TIPS is usually documented an increase of the portal vein velocity due to the effectiveness of shunt as a low-pressure conduit bypassing the hepatic circulation [4]. Therefore, Doppler evaluation of the main portal vein is complementary in the assessment of the shunt itself, and post-TIPS velocities should be compared with pre-TIPS examination. Hepatofugal flow in the portal vein branches is frequently seen after a successful TIPS and reflects a complete diversion of the portal venous flow from the hepatic parenchyma into the shunt [8,9]. In our experience the conversion of flow from retrograde to antegrade is a late sign of shunt malfunction [4].
Another important finding due to the compensatory response to the decreased sinusoidal portal perfusion of the liver, is the hepatic artery flow increase after TIPS procedure. Foshager et al. [15] documented a statistically significant change in the hepatic artery velocity following TIPS, with a mean peak systolic velocity of 79 cm/s before and of 131 cm/s after TIPS placement. Haskal et al. [3] found a significant decline of hepatic artery velocity in the setting of shunt compromise. When utilizing this parameter to assess the TIPS status, we have to consider the high prevalence of normal anatomic variants, the multiplicity of hepatic arteries, the technical difficulties in obtaining accurate routine measurements, and the need to better define normal velocity ranges. The increase of hepatic artery peak systolic velocity should nevertheless be considered as a valid secondary sign of shunt patency.
Finally, assessment of ascites, evaluation of varices and collaterals, and measurement of spleen size are important findings to be recorded in each US examination.
As a matter of fact, the CDUS assessment of TIPS status is a multifactorial analysis, not only including the evaluation of the shunt itself, but also the modification of intra- and extra-hepatic circulation.
Compromised shunts
Early complications of TIPS include shunt thrombosis usually secondary to prolonged catheter manipulation, intimal injury, and failed stent deployment, compromise of hepatic blood supply, biliary hemorrhage and intraperitoneal hemorrhage. The most frequently reported complication after TIPS is in-stent restenosis, occurring in up to 50% of patients at 12 months follow-up. Three models of shunt stenosis or occlusion have been reported. Early stenosis may be caused by bile duct transection, biliary fistulas or shunt thrombosis, mainly due to prolonged catheter manipulation intimal injury, and failed stent deployment; later occlusions are caused by an inflammatory healing response to the stent and proliferation of fibroconnective tissue. Finally, intimal hyperplasia can develop in the hepatic veins above the stent, contributing to shunt dysfunction [2].
Conventional US may be helpful in evaluating the site of deployment and correct expansion of the stent (Fig. 1). Color- and Power-Doppler US define correctly the absence of flow within the stent itself or in the portal vein as an indicator of complete occlusion (Fig. 2). In selected cases, the use of US contrast media may facilitate the diagnosis of occlusion, increasing the operator's diagnostic confidence, mainly in obese or non-collaborating patients. In a series of 31 shunts in 30 patients, echo-enhanced sonography yielded a sensitivity and a specificity of 100%, compared with 100% and 89%, respectively, for unenhanced Doppler sonography in the diagnosis of shunt occlusion [12].
Fig. 1.
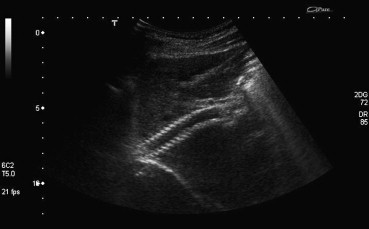
Conventional US. The stent seems to be correctly positioned, well expanded, without evidence of any narrowing.
Fig. 1 Ecografia: stent normale. Lo stent appare correttamente posizionato, ben espanso, senza evidenza di stenosi.
Fig. 2.
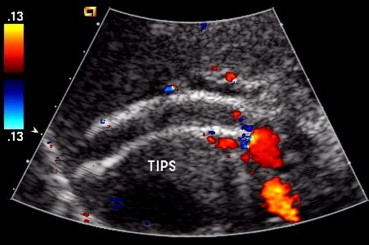
Occluded shunt. The shunt presents homogeneous intraluminal increase in echogenicity, without any color flow within.
Fig. 2 Eco-color-Doppler: stent ostruito. Lo shunt presenta incremento della ecogenicità endoluminale, senza evidenza di segnali di flusso.
Ideally, stent-grafts should address all three types of stenosis, and the graft material should provide specific features to reduce bile leakage and fibroblast proliferation and to favour endothelial lining. Several graft materials have been proposed. Some authors reported the benefits of lining the stent with ePTFE in porcine models, with an increased patency from 8% to 50% at 1 month's follow-up and no bile staining in TIPS stent-grafts. Other materials showed less promising results. Initial studies reported high patency rates with this newly available device. Ideally the increased patency would allow reduction of invasive follow-up and therefore reduce cost. Moreover, the longer durability of the stent-graft seems to improve survival [2] (Fig. 3a–d).
Fig. 3.
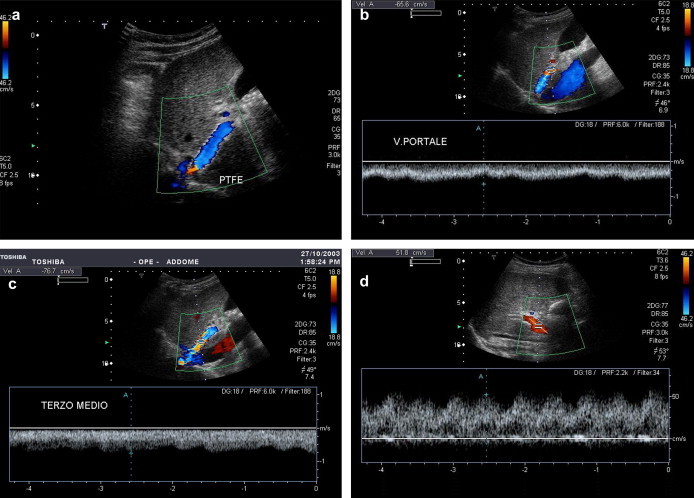
Functioning shunt (a PTFE shunt). The stent seems to be correctly positioned, with hepatofugal flow and complete color-flow saturation (a). Intraluminal velocity increases from portal site (b) through midportion (c) to the hepatic end. Portal vein velocity increases as well from 28 to 52 cm/s (d).
Fig. 3 Shunt ben funzionante (rivestito in PTFE). Lo stent appare correttamente posizionato, con flusso epatofugo e completa saturazione in colore (a). La velocità nello shunt aumenta dal versante portale (b) attraverso il terzo medio (c) sino al versante epatico. La velocità portale aumenta di concerto da 28 a 52 cm/s (d).
A reduced flow velocity within a TIPS has been considered in many preliminary experiences a highly sensitive and specific indicator of shunt compromise [9,10,14,16]. Some of these results were based on relatively small patient series. Many of the reported results are affected by technical artifacts: most of the authors did not attempt to evaluate the entire shunt and usually did not obtain waveforms from the stenotic segment of abnormal shunts. Stenotic shunts, therefore, usually manifested as decreased shunt velocities. In our experience, we have become more successful in identifying and sampling the stenotic segments of the shunt, and thus, found that stenosed shunts are often manifested by elevated peak shunt velocities [4,17].
An increased flow velocity within a TIPS is a highly sensitive and specific indicator of shunt compromise [1,3,4,9].
Three types of shunt stenosis can be distinguished. Rarely, and in most cases soon after TIPS creation, a stenosis is observed at the entrance of the stent into the portal vein (portal site). This type of stenosis is characterized by focal color aliasing with focal velocity elevation at the portal end of the shunt, with a progressive decrease of the velocity along the stent and a decrease of the portal flow velocity.
When a stenosis occurs in the midtract of the shunt (30–35%), focal color aliasing and high-flow velocities can be reached at this level, with a prestenotic decrease of velocity and post-stenotic turbulence, as commonly happens in arterial stenoses [4].
The most common cause of shunt insufficiency (70–80% of cases) is due to intimal hyperplasia at the hepatic end of the shunt or in the draining hepatic vein. In the former case, focal color aliasing with focal velocity elevation can be observed at the distal end of the stent, (Fig. 4a,b) with a progressive decrease of the velocity along the shunt. In the latter case, the shunt is completely color-filled, without evidence of aliasing (but with reduced velocities along the shunt), while focal aliasing with high-flow velocity can be reached in the draining vein.
Fig. 4.
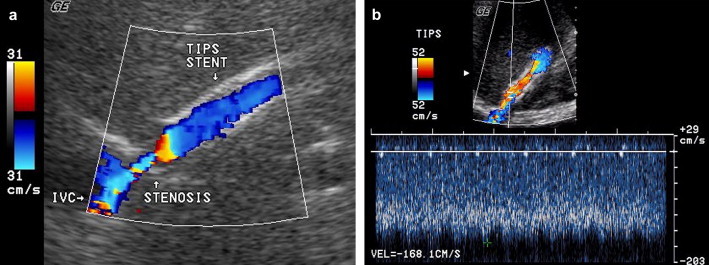
Compromised shunt. Stenosis of the hepatic vein: presence of a focal color aliasing at the hepatic draining vein (a) with focal increase of velocity up to 168 cm/s (b).
Fig. 4 Shunt stenotico. Stenosi del versante epatico dello shunt: presenza di un'area di aliasing focale della vena epatica drenante (a) con consensuale incremento focale della velocità a 168 cm/s (b).
While it is clear that a certain degree of shunt stenosis will place some patients at an increased risk of recurrent bleeding, there is no direct correlation between the focal velocity increase and the elevation of portosystemic gradient at venography .Our experience shows that a decrease greater than 40 cm/s or an increase greater than 60 cm/s may predict abnormal venographic results, with 89% of sensitivity and 94% of specificity [4].
All shunt insufficiency features decrease the portal flow velocity to the pre-TIPS level. Some studies have shown that portal vein velocity and direction of intrahepatic blood flow are the only predictors of TIPS dysfunction [1]. The conversion of left and right portal vein flow from retrograde to antegrade is a late sign of TIPS malfunction and the measurement of MPV allows the detection of a shunt compromise before a change in the portal vein branch flow is possible.
As a compensatory response to decreased liver portal perfusion, hepatic artery flow decreases after the TIPS procedure: temporal decreases in the hepatic artery velocity may be a secondary sign of shunt dysfunction (Fig. 4a,b).
Power-Doppler
Power-Doppler is nowadays a well-established additional tool in a sonographic evaluation of the TIPS patency mainly because its high sensitivity for slow flow may help in the discrimination between complete or partial occlusion. In the former condition, the presence of even a tiny patent lumen can be identified with higher sensitivity. Power-Doppler sonography is useful in defining site, extension and morphology of partial thrombosis (Fig. 5). Recently the introduction of the so called directional Power-Doppler can be also accurate in defining flow variations (direction and/or velocity) correctly addressing spectral Doppler analysis.
Fig. 5.
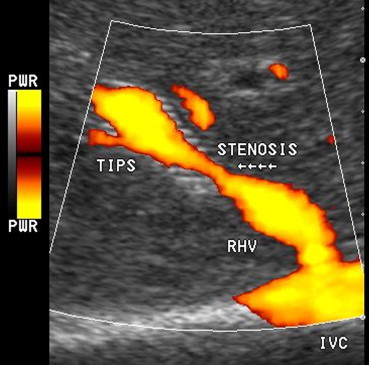
Power-Doppler US. Power-Doppler allows to correctly evaluate morphology and extension of a midshunt stenosis, without any color aliasing at the site of maximum narrowing of the stent lumen.
Fig. 5 Power-Doppler. Il Power-Doppler consente di valutare correttamente morfologia ed estensione di una stenosi al terzo medio dello stent, senza evidenza di aliasing nella sede di massima stenosi.
Conclusions
CDUS is the most reliable tool for the evaluation of shunt function after TIPS placement. Several studies have shown that Doppler sonography is a sensitive and relatively specific tool to detect shunt malfunction, especially when multiple parameters are evaluated. To date, therefore, venous angiography is indicated only when a suspicion of shunt compromise arises at CDUS in order to re-establish shunt function. Conversely, in patients with CDUS criteria indicating good TIPS function, catheterization of the TIPS can be safely avoided. To date, power-Doppler US and US contrast media can be useful in particular conditions, but are not really fundamental diagnostic tools since their role needs to be further investigated.
References
- 1.Abraldes J.G., Gilabert R., Turnes J. Utility of Color Doppler ultrasonography predicting TIPS dysfunction. Am J Gastroenterol. 2005;100:2696–2701. doi: 10.1111/j.1572-0241.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 2.Vignali C., Bargellini I., Grosso M. TIPS with expanded polytetrafluoroethylene-covered stent: results of an Italian multicenter study. AJR Am J Roentgenol. 2005 Aug;185(2):472–480. doi: 10.2214/ajr.185.2.01850472. [DOI] [PubMed] [Google Scholar]
- 3.Haskal Z.J. Transjugular intrahepatic hunt stenosis and thrombosis: shunt biology and stent-grafts. In: Rossi P., Ricci P., Broglia L., editors. Portal hypertension. Springer Verlag; Berlin, Heidelberg: 2000. [Google Scholar]
- 4.Ricci P., Pizzi M., Coniglio M. TIPS: color Doppler follow-up. In: Rossi P., Ricci P., Broglia L., editors. Portal Hypertension. Springer Verlag; Berlin, Heidelberg: 2000. [Google Scholar]
- 5.Casado M., Bosch J., Garcia Pagan J.C. Clinical after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 6.Boyer T.D. Transjugular intrahepatic portosystemic shunt: current status. Gastroenterology. 2003;124:1700–1710. doi: 10.1016/s0016-5085(03)00377-9. [DOI] [PubMed] [Google Scholar]
- 7.Haskal Z.J., Pentecost M.J., Soulen M.C. Transjugular intrahepatic portosystemic shunt stenosis and revision. AJR Am J Roentgenol. 1994;163:439–444. doi: 10.2214/ajr.163.2.8037046. [DOI] [PubMed] [Google Scholar]
- 8.Haskal Z.J., Carroll J.W., Jacobs J.E. Sonography of transjugular intrahepatic portosystemic shunts: detection of elevated portosystemic gradients ad loss of shunt function. J Vasc Interv Radiol. 1997;8:549–556. doi: 10.1016/s1051-0443(97)70607-9. [DOI] [PubMed] [Google Scholar]
- 9.Kanterman R.Y., Darcy M.D., Mileton W.D. Doppler sonography findings associated with transjugular intrahepatic portosystemic shunt malfunction. AJR Am J Roentgenol. 1997;168:467–472. doi: 10.2214/ajr.168.2.9016228. [DOI] [PubMed] [Google Scholar]
- 10.Dodd G.D., Zajko A.B., Oron P.D. Detection of intrahepatic portosystemic shunt dysfunction: value of duplex Doppler sonography. AJR Am J Roentgenol. 1995;164:1119–1124. doi: 10.2214/ajr.164.5.7717217. [DOI] [PubMed] [Google Scholar]
- 11.Furst G., Malms J., Heyer T. Transjugular intrahepatic portosystemic shunts: improved evaluation with echo-enhanced color Doppler sonography. AJR Am J Roentgenol. 1998;170:1047–1054. doi: 10.2214/ajr.170.4.9530057. [DOI] [PubMed] [Google Scholar]
- 12.Skjoldbye B., Wieslander S., Struckmann J. Doppler ultrasound assessment of TIPS patency and function – the need for echo enhancers. Acta Raiologica. 1998;39:675–679. doi: 10.3109/02841859809175495. [DOI] [PubMed] [Google Scholar]
- 13.Leutloff U.C., Richter G.M., Libicher M. Follow-up of TIPSS by Color-coded duplex sonography using an ultrasonic signal enhancer. First results. Radiologe. 1999 Dec;39(12):1072–1077. doi: 10.1007/s001170050604. [DOI] [PubMed] [Google Scholar]
- 14.Chong W.K., Mlisch T.A., Mazer M.J. Transjugular intrahepatic portosystemic shunt: US assessment with maximum flow velocity. Radiology. 1993;189:789–793. doi: 10.1148/radiology.189.3.8234705. [DOI] [PubMed] [Google Scholar]
- 15.Foshager M.C., Ferral H., Finlay D.E., Castaneda-Zuniga W.R., Letourneau J.G. Color Doppler sonography of transjugular intrahepatic portosystemic shunts (TIPS) AJR Am J Roentgenol. 1994;165:1–7. doi: 10.2214/ajr.163.1.8010193. [DOI] [PubMed] [Google Scholar]
- 16.Surratt R.S., Middleton W.D., Darcy M.D., Melson G.L., Brink J.A. Morphologic and hemodynamic findings at sonography before and after creation of transjugular intrahepatic portosystemic shunt. AJR Am J Roentgenol. 1993;160:627–630. doi: 10.2214/ajr.160.3.8430568. [DOI] [PubMed] [Google Scholar]
- 17.Ricci P., Pizzi G., Pizzamiglio M. Noninvasive follow-up in transjugular intrahepatic portosystemic shunt: color-Doppler imaging as valid alternative to venography. Radiology. 1995;197:272. [Google Scholar]


