Abstract
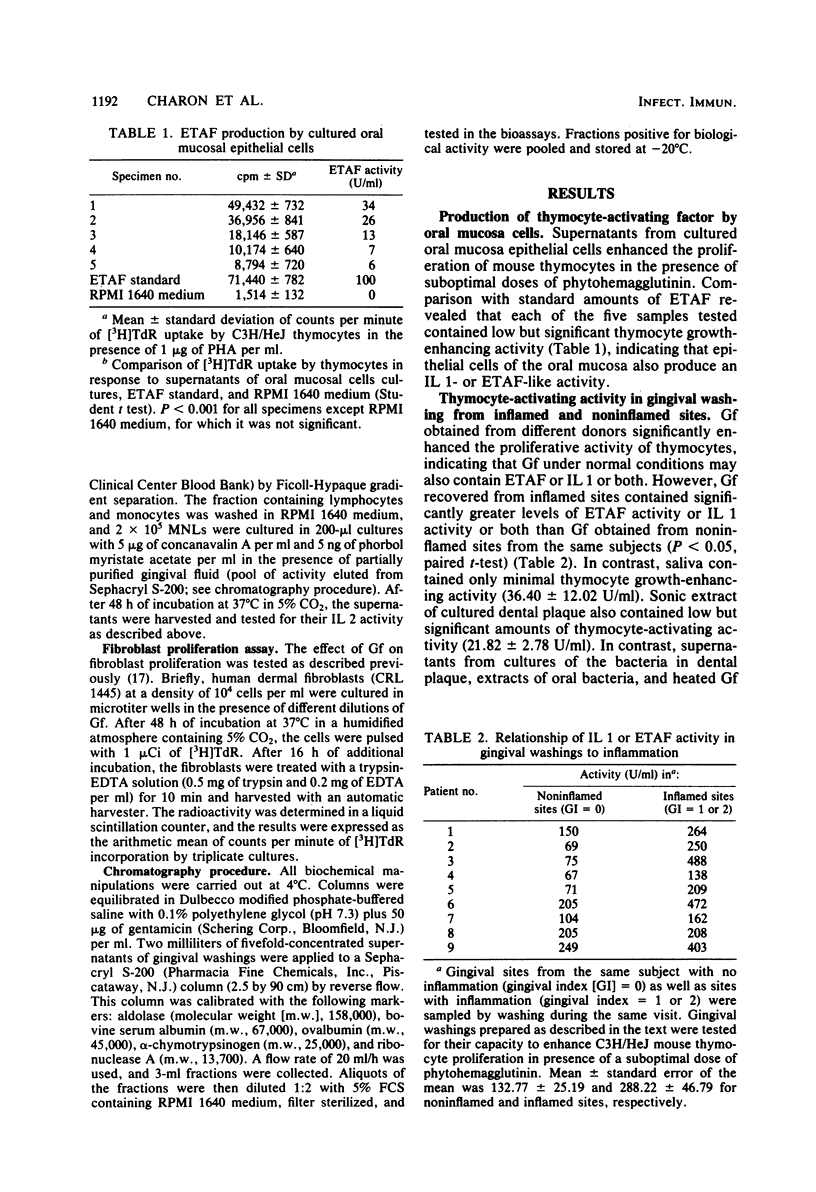
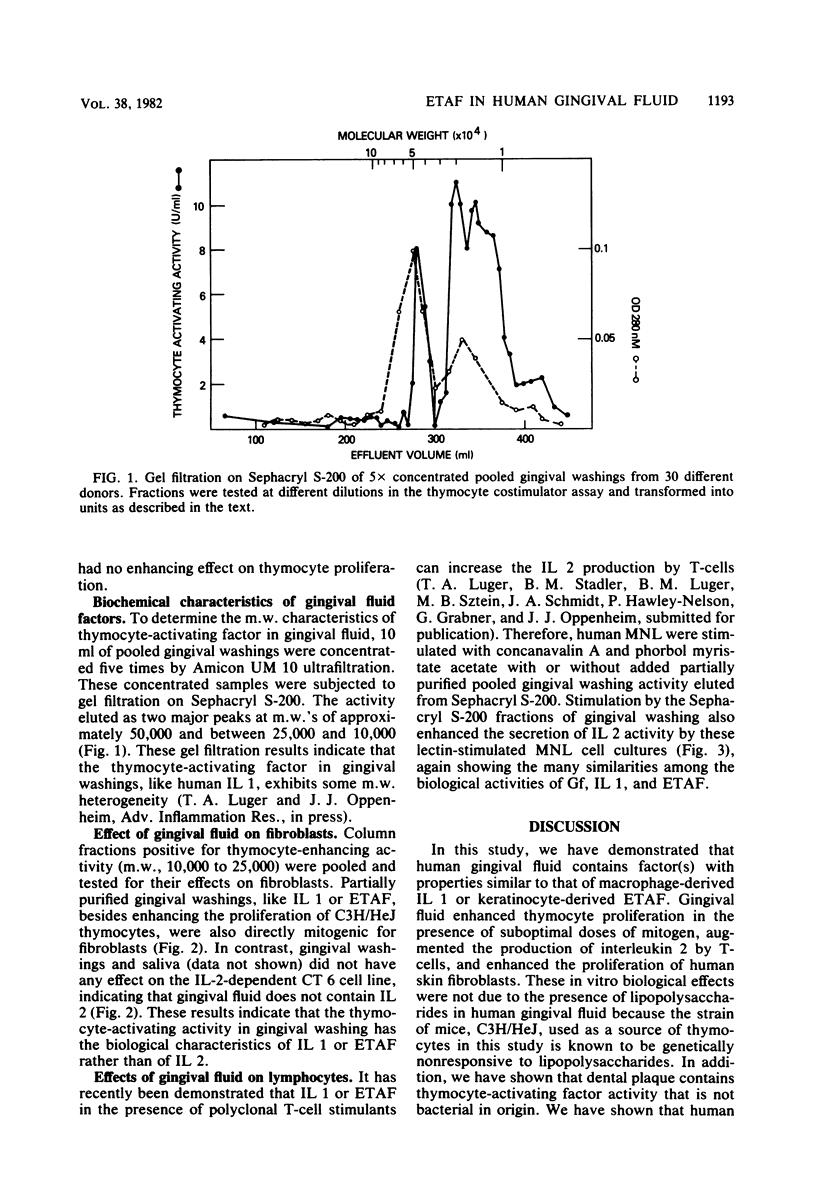
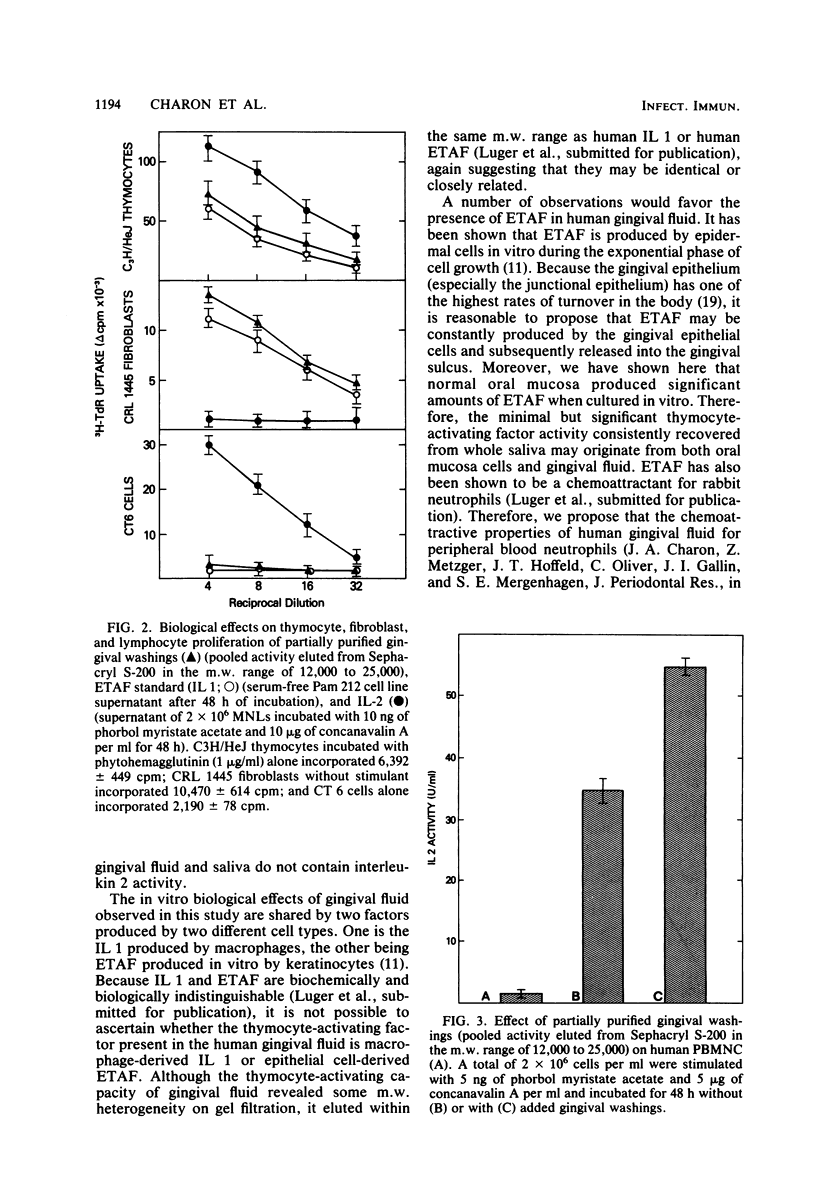
In this study, human gingival fluid from normal subjects was shown to contain a low-molecular-weight factor (molecular weight, 10,000 to 25,000) which augmented murine thymocyte proliferation, enhanced the production of interleukin 2 by T lymphocytes, and augmented the proliferation of fibroblasts. These biochemical and biological properties are characteristic of both macrophage-derived interleukin 1 and epidermal cell-derived thymocyte-activating factor. In addition, we have established that epidermal thymocyte-activating factor or interleukin 1 or both are present to a greater extent in gingival fluid obtained from sites manifesting gingival inflammation. In fact, thymocyte-activating activities were found to be greater in gingival fluid from inflamed than from noninflamed gingival sites from the same subjects. These findings suggest that human gingival fluid contains epidermal thymocyte-activating factor or interleukin 1 or both, which may amplify the inflammatory response in periodontal tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Sullivan J. E., Kung M., Hennings H., Yuspa S. H. Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol. 1980 Aug;75(2):176–182. doi: 10.1111/1523-1747.ep12522602. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Kampschmidt R. F., Pulliam L. A., Upchurch H. F. The activity of partially purified leukocytic endogenous mediator in endotoxin-resistant C3H/HeJ mice. J Lab Clin Med. 1980 Apr;95(4):616–623. [PubMed] [Google Scholar]
- Koopman W. J., Farrar J. J., Fuller-Bonar J. Evidence for the identification of lymphocyte activating factor as the adherent cell-derived mediator responsible for enhanced antibody synthesis by nude mouse spleen cells. Cell Immunol. 1978 Jan;35(1):92–98. doi: 10.1016/0008-8749(78)90129-6. [DOI] [PubMed] [Google Scholar]
- LOE H., SILNESS J. PERIODONTAL DISEASE IN PREGNANCY. I. PREVALENCE AND SEVERITY. Acta Odontol Scand. 1963 Dec;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Katz S. I., Oppenheim J. J. Epidermal cell (keratinocyte)-derived thymocyte-activating factor (ETAF). J Immunol. 1981 Oct;127(4):1493–1498. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Payne W. A., Page R. C., Ogilvie A. L., Hall W. B. Histopathologic features of the initial and early stages of experimental gingivitis in man. J Periodontal Res. 1975 May;10(2):51–64. doi: 10.1111/j.1600-0765.1975.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Schectman L. R., Ammons W. F., Simpson D. M., Page R. C. Host tissue response in chronic periodontal disease. 2. Histologic features of the normal periodontium, and histologic and ultrastructural manifestations of disease in the marmoset. J Periodontal Res. 1972;7(3):195–212. doi: 10.1111/j.1600-0765.1972.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Skapski H., Lehner T. A crevicular washing method for investigating immune components of crevicular fluid in man. J Periodontal Res. 1976 Feb;11(1):19–24. doi: 10.1111/j.1600-0765.1976.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Skougaard M. Turnover of the gingival epithelium in marmosets. Acta Odontol Scand. 1965 Dec;23(6):623–643. doi: 10.3109/00016356509041116. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler B. M., Dougherty S. F., Farrar J. J., Oppenheim J. J. Relationship of cell cycle to recovery of IL 2 activity from human mononuclear cells, human and mouse T cell lines. J Immunol. 1981 Nov;127(5):1936–1940. [PubMed] [Google Scholar]
- Sztein M. B., Vogel S. N., Sipe J. D., Murphy P. A., Mizel S. B., Oppenheim J. J., Rosenstreich D. L. The role of macrophages in the acute-phase response: SAA inducer is closely related to lymphocyte activating factor and endogenous pyrogen. Cell Immunol. 1981 Sep 1;63(1):164–176. doi: 10.1016/0008-8749(81)90037-x. [DOI] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Renggli H. H., Lehner T. A functional comparison of blood and gingival inflammatory polymorphonuclear leucocytes in man. Clin Exp Immunol. 1977 Jan;27(1):152–158. [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Renggli H. H., Lehner T. The isolation and identification of mononuclear cells from the gingival crevice in man. J Periodontal Res. 1976 Sep;11(5):262–268. doi: 10.1111/j.1600-0765.1976.tb00080.x. [DOI] [PubMed] [Google Scholar]


