Abstract
Ultraviolet (UV) radiation is a major environmental factor that affects pigmentation in human skin and can eventually result in various types of UV-induced skin cancers. The effects of various wavelengths of UV on melanocytes and other types of skin cells in culture have been studied but little is known about gene expression patterns in situ following in situe exposure of human skin to different types of UV (UVA and/or UVB). Paracrine factors expressed by keratinocytes and/or fibroblasts that affect skin pigmentation might be regulated differently by UV, as might their corresponding receptors expressed on melanocytes. To test the hypothesis that different mechanisms are involved in the pigmentary responses of the skin to different types of UV, we used immunohistochemical and whole human genome microarray analyses to characterize human skin in situ to examine how melanocyte-specific proteins and paracrine melanogenic factors are regulated by repetitive exposure to different types of UV compared with unexposed skin as a control. The results show that gene expression patterns induced by UVA or UVB are distinct, UVB eliciting dramatic increases in a large number of genes involved in pigmentation as well as in other cellular functions, while UVA had little or no effect on those. The expression patterns characterize the distinct responses of the skin to UVA or UVB, and identify several potential previously unidentified factors involved in UV-induced responses of human skin.
Keywords: ultraviolet radiation, pigmentation, human skin, tanning, regulation
INTRODUCTION
Ultraviolet (UV) radiation is a major environmental factor that affects pigmentation in human skin and can eventually result in various types of UV-induced skin cancers (Gilchrest et al., 1996; 1999; Seline et al., 1996; Noonan et al., 2001; Marr et al., 2004; Yamaguchi et al., 2006; D’Orazio et al., 2006). UVA (320–400 nm) causes immediate pigment darkening (IPD) as well as persistent pigment darkening (PPD) of skin within hours via photooxidation and/or polymerization of existing melanin or melanogenic precursors due to the generation of reactive oxygen species (Maeda and Hatao, 2004). In contrast, UVB (280–320 nm) induces a slower but more stable type of pigmentation termed delayed tanning (DT) which requires the increased synthesis of melanin following the stimulation of tyrosinase activity and the entire melanogenic cascade (Alaluf et al., 2002a; 2002b; Tadokoro et al., 2005). Melanins are uniquely and specifically produced by melanocytes located in the basal layer of the epidermis and are deposited in membrane-bound organelles (termed melanosomes) which are subsequently transferred to neighboring keratinocytes (Yamaguchi et al., 2007; Hearing, 2007). Transcription factors, such as MITF and SOX9, and various melanosomal enzymes, such as tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1) and tyrosinase-related protein 2 (DCT), as well as melanosomal structural components, such as Pmel17 and MART-1, are all well-known melanocyte-specific markers that are involved in UV-induced melanogenesis (Shibahara et al., 2000; Kushimoto et al., 2001; Tadokoro et al., 2005; Passeron et al., 2007).
Melanocyte function is regulated via interactions with neighboring cells in the skin, including keratinocytes in the epidermis and fibroblasts in the underlying dermis. A wide variety of melanogenic autocrine and paracrine factors produced by those cells in the skin have been identified and many of them are in part regulated by UV exposure (Suzuki et al., 1999; Kadekaro et al., 2003; Imokawa, 2004; Yamaguchi et al., 2007). POMC, SCF, HGF, IFN-γ, ET-1, bFGF, IL-1 and GM-CSF are examples of paracrine melanogenic factors, and those factors may be regulated differently by UV, as might their corresponding receptors expressed on melanocytes. However, most studies to date that have characterized responses to UV have been performed using cell culture systems or acute UV exposure of human skin in vivo. Although those studies provide valuable insights into the mechanism of UV-induced pigmentation, they may not accurately reflect the physiological situation in situ where the skin is typically exposed to repetitive suberythemal doses of UV.
It has been previously reported that different wavelengths of UV induce distinct responses in the skin. Solar simulated radiation (SSR), which contains UVA and UVB, stimulates the expression of several important melanocyte-specific markers, such as tyrosinase, TYRP1 and MITF, and increases the production of melanin, whereas UVA alone does not (Schlenz et al., 2005; Miyamura et al., 2007; Wolber et al., 2008). UVB alone significantly increases the melanin content of the skin, and the expression of melanogenic enzymes, but interestingly, UVA can elicit visible tanning of the skin similar to that elicited by UVB. UVA and UVB also have quite distinct deleterious effects on cells which have important consequences for photocarcinogenesis (Black et al., 1997; Wikonkal and Brash, 1999; de Gruijl, 2000). To test the hypothesis that different mechanisms are involved in the pigmentary responses of the skin to different types of UV, we used immunohistochemistry and whole human genome microarray analysis of human skin in situ to characterize how melanocyte-specific proteins and melanogenic signaling pathways are regulated by repetitive exposure to different types of UV (UVA and/or UVB). These results allow important insights into the different mechanisms of skin tanning induced by UVA or UVB, provide a rich database resource of UVA- and/or UVB-responsive genes, and identify potential previously unidentified melanogenic factors involved in the UV-induced pigmentation of human skin.
RESULTS
Tanning responses of human skin to SSR, UVA or UVB
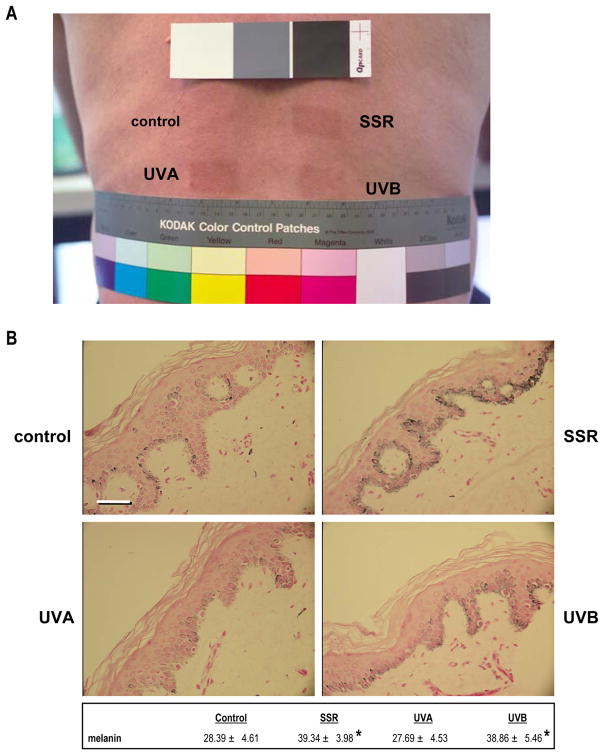
Six subjects with type II–III skin were irradiated 5 times a week for 2 weeks with UVA, UVB or UVA&UVB (termed SSR in this study) at suberythemal doses (0.4 MED in week 1 and 0.5 MED in week 2) that produce comparable levels of visible skin tanning (Fig. 1A). Despite the similar visible tans, Fontana-Masson staining revealed significantly different effects on melanin contents in those skin samples (Fig. 1B). UVB (with or without UVA) significantly increased the melanin content (39% or 37%, respectively) in all 6 subjects, but UVA alone had no significant effect compared to the unirradiated control. That result was consistent with our previous study which used a comparable UV exposure protocol and chemical analysis of melanin content, which showed that repetitive SSR or UVB produced significant increases of eumelanin and pheomelanin in the skin, but UVA alone did not (Wolber et al., 2008). Thus, the mechanism of skin tanning differs significantly following exposure to UVA and/or UVB.
Figure 1.
Tanning responses and Fontana-Masson staining of skin following exposure to UV. A) Photograph of the back of subject 2 showing representative tans elicited by repetitive exposure to SSR (upper right), UVA (bottom left) or UVB (bottom right); unirradiated skin is used as the control (top left). B) Representative specimens stained for melanin content by Fontana Masson staining (subject 2); numbers represent quantitation (mean ± SEM) of melanin density in 10 different images each from 2 biopsies from each of the 4 areas on each of the 6 subjects (n=12 specimens, 120 images for each area). * = p <0.05. Bar = 50 μm.
Overview of the whole human genome microarray data analysis
We hypothesized that such differences between tans elicited in human skin by UVA and/or UVB might result from distinct effects on factors that regulate skin pigmentation. Such factors could be expressed within melanocytes and/or by other cells in the skin, most notably keratinocytes and/or fibroblasts. Therefore, we used the whole human genome microarray analysis of skin biopsies to examine changes in gene expression patterns after 2 weeks of UVA and/or UVB exposure. The raw data was processed using the RosettaResolver™ Software (Rosetta Inpharmatics, Seattle, WA) which compares each treatment sample vs. the respective donor-matched control sample to generate a log ratio and the statistical confidence value (p-value) in the context of the signal strengths and the signal errors for each probe. After filtering based on log ratios and their associated p-values generated by the RosettaResolver™ Software, 21,581 probes were used for further analysis.
One-factor ANOVA analysis was conducted to identify genes differentially regulated among different types of UV exposure. Additionally, t-test analyses were performed for the pair-wise comparisons of skin specimens with the control and different types of UV exposure. All p-values were adjusted based on the Storey FDR correction and the results are shown in Table 1. During the pair-wise analyses, we found that there were few probes differentially expressed between SSR and UVA, SSR and UVB, or UVA and control. Given an adjusted p-value at the 0.05 level, 723 probes were differentially expressed between SSR and control and more than one third of the probes (8,523 out of 21,581) compared were differentially expressed between UVB and control. There were 3,158 probes with adjusted p-values <0.01. Given an adjusted p-value threshold of 0.01, 957 of the 1,239 probes differentially expressed between UVB and control were also differentially expressed in one-factor ANOVA analysis. A summary of the functional analysis of biological processes affected by the different types of UV is shown in Suppl. Fig. 1.
Table 1. Overview of the whole human genome microarray results.
Numbers of discriminatory genes in the different comparisons of skin samples after UV exposure.
| Pair-wise t-test | Number of discriminatory genes * | ||
|---|---|---|---|
| P-value <0.01 | Storey-adjusted P-value<0.01 (<0.05) | ||
| SSR | Control | 1,754 | 0 (783) |
| SSR | UVA | 1,250 | 0 (2) |
| SSR | UVB | 1,192 | 0 (0) |
| UVA | Control | 1,059 | 0 (0) |
| UVA | UVB | 1,590 | 0 (1) |
| UVB | Control | 3,591 | 1,239 (8,523) |
| ANOVA (SSR, UVA, UVB, Control) | 4,050 | 3,158 (7,498) | |
For the 3,158 probes chosen through the ANOVA analysis (with an adjusted p-value <0.01), the fold changes were averaged within each UV group and heat maps were generated. Changes in gene expression greater than two-fold are considered to be biologically important (Claverie, 1999). There were 1,375 probes whose expressions were changed more than two-fold after UV exposure: 184 of them were changed more than two-fold after SSR exposure (87 up-regulated and 97 down-regulated), 1,357 after UVB exposure (683 up-regulated and 674 down-regulated, and none after UVA exposure. Most probes changed by SSR were also changed similarly by UVB alone, but there were a few exceptions to that. Significantly regulated probes are shown in Suppl. Table 2.
Known pigment-related genes were grouped into several gene sets (see Tables 2, 3, and 4 for the genes) and Suppl. Table 1 shows the results of gene set analysis which reveals significant differences in gene expression patterns elicited by SSR compared to the unirradiated control for genes known to be expressed by melanocytes with p-value of less than 0.02 and adjusted p-value of less than 0.1.
Table 2. Expression patterns of melanocyte-specific genes after UVA and/or UVB exposure.
Heat maps were generated on ratios transformed to log base 2 (colors are based on a maximum of 3, a minimum of −1.5). A gene is considered to be significantly differentially expressed (“Discriminatory for”) between treatment (i.e., SSR, UVA, or UVB) and control (denoted as “0”) if its corresponding Storey adjusted p-value is <0.05.
| median (log2 ratio) | ANOVA | Discriminatory for | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Melanocytes | Pigmentary Disease/Function | SSR | UVA | UVB | p-value | p-value (FDR) | SSR vs O | UVA vs O | UVB vs O |
| Pmel17/SILVIHC | melanosome structure/unknown | 2.882 | 0.987 | 2.574 | 0.00000 | 0.00011 | X | X | |
| TYRP1IHC | melanosome function/OCA3 | 2.871 | 0.523 | 2.105 | 0.00000 | 0.00013 | X | X | |
| TYR IHC | melanosome function/OCA1 | 2.487 | 0.748 | 2.179 | 0.00003 | 0.00035 | X | X | |
| MART1IHC | melanosome structure/unknown | 2.405 | 0.896 | 2.164 | 0.00000 | 0.00009 | X | X | |
| KIT IHC | receptor for SCF/UVB melanosis, vitiligo | 2.317 | 0.558 | 1.493 | 0.00001 | 0.00014 | X | X | |
| OA1/GPR143 | receptor for ???/Ocular albinism type 1 | 2.222 | 0.891 | 1.952 | 0.00002 | 0.00025 | X | X | |
| MLPH | melanosome transport/Griscelli Syndrome | 2.163 | 0.642 | 2.067 | 0.00000 | 0.00012 | X | X | |
| EDNRBIHC | receptor for ET1/UVB melanosis, lentigo senilis | 2.144 | 0.611 | 1.639 | 0.00000 | 0.00011 | X | X | |
| DCT IHC | melanogenic enzyme/unknown | 2.104 | 0.839 | 1.307 | 0.00005 | 0.00049 | X | X | |
| PAX3 | transcription factor, Waardenburg syndrome | 2.048 | 0.660 | 1.582 | 0.00005 | 0.00052 | X | X | |
| SOX10 | transcription factor/melanocyte function | 1.604 | 0.614 | 1.281 | 0.00001 | 0.00015 | X | X | |
| P | melanosome function/OCA2 | 0.966 | 0.278 | 0.669 | 0.01168 | 0.01804 | X | ||
| MC1R | receptor for α MSH/skin & hair phenotype | 0.857 | 0.428 | 1.202 | 0.00025 | 0.00146 | X | ||
| MITF IHC | transcription factor/melanocyte function | 0.792 | 0.160 | 0.759 | 0.00293 | 0.00722 | X | ||
| MATP/SLC45A2 | melanosome function/OCA4 | 0.657 | 0.262 | 1.158 | 0.00125 | 0.00414 | X | ||
| WIF1 | melanocyte function/Wnt signaling | 0.560 | 0.211 | 0.937 | 0.00063 | 0.00266 | X | ||
| LYST | Melanosome structure/Chediak Higashi syndrome | 0.285 | 0.092 | 0.055 | 0.51243 | 0.19738 | |||
| MyoVa | melanosome transport/Griscelli syndrome | 0.284 | 0.363 | −0.660 | 0.00066 | 0.00275 | X | ||
| SOX9 | transcription factor/melanocyte function | 0.226 | −0.111 | 0.107 | 0.77804 | 0.25692 | |||
| MET | receptor for HGF/UVA melanosis | 0.208 | −0.080 | −0.202 | 0.04751 | 0.04457 | |||
| FGFRIHC | receptor for bFGF/melanocyte growth | 0.154 | −0.127 | −0.151 | 0.50786 | 0.19625 | |||
| LRP6 | melanocyte function/Wnt signaling | 0.089 | 0.156 | 0.142 | 0.53970 | 0.20359 | X | ||
| FZL | melanocyte function/Wnt signaling | −0.008 | −0.621 | 0.093 | 0.00200 | 0.05473 | |||
| RAB27a | melanosome transport/Griscelli syndrome | −0.040 | −0.149 | −0.248 | 0.00060 | 0.00258 | X | ||
| SLC7A11 IHC | solute carrier/melanin switching | −0.046 | 0.493 | −1.082 | 0.00132 | 0.00429 | X | ||
| NGF-R | receptor for NGF | −0.155 | −0.156 | −0.047 | 0.39043 | 0.16614 | |||
| GSF2RA | receptor for GM-CSF | −0.867 | −0.071 | −1.020 | 0.00000 | 0.00007 | X | X | |
validated by Immunohistochemistry
Table 3. Changes in the expression of genes encoding secreted paracrine factors or their receptors after UVA and/or UVB irradiation.
Heat maps and “Discriminatory for” columns were generated using the same procedure as in Table 2.
| median (log2 ratio) | ANOVA | Discriminatory for | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Fibroblasts | Pigmentary Disease/Function | SSR | UVA | UVB | p-value | p-value (FDR) | SSR vs O | UVA vs O | UVB vs O |
| sSCF | ligand for KIT | 0.362 | 0.060 | −0.515 | 0.05823 | 0.05064 | |||
| HGF | ligand for MET | 0.238 | 0.003 | 0.661 | 0.00049 | 0.00226 | X | ||
| DKK3 IHC | WNT signaling/unknown | 0.225 | 0.068 | 0.260 | 0.39769 | 0.16812 | X | ||
| DKK1 | WNT signaling/skin phenotype | −0.063 | 0.042 | −0.664 | 0.40032 | 0.16864 | X | ||
| bFGF | ligand for FGFR1/melanocyte growth | −0.128 | −0.203 | −0.400 | 0.04256 | 0.04170 | X | ||
|
| |||||||||
| Keratinocytes | Pigmentary Disease/Function | SSR | UVA | UVB | |||||
|
| |||||||||
| mSCF | ligand for KIT/UVB melanosis, lentigo senilis | 0.362 | 0.060 | −0.515 | 0.05823 | 0.05064 | |||
| HGF | ligand for MET | 0.238 | 0.003 | 0.661 | 0.00049 | 0.00226 | X | ||
| p53 | transcription factor/UV responses, apoptosis | −0.099 | −0.067 | −0.058 | 0.84114 | 0.27131 | |||
| bFGF | ligand for FGFR/melanocyte growth | −0.128 | −0.203 | −0.400 | 0.04256 | 0.04170 | X | ||
| PAR-2 | receptor/melanosome transfer | −0.135 | −0.171 | 0.488 | 0.00060 | 0.00258 | X | ||
| ET1 IHC | ligand for EDNRB/UVB melanosis, lentigo senilis | −0.181 | 0.020 | −0.268 | 0.83457 | 0.26989 | |||
| IL-1B | ligand for IL1R/post-inflammatory hyperpigmentation | −0.690 | −0.244 | −1.010 | 0.00249 | 0.00649 | X | ||
| IL-1A | ligand for IL1R/post-inflammatory hyperpigmentation | −0.982 | −0.379 | −0.903 | 0.01334 | 0.01970 | X | ||
| GM-CSF | ligand for GM-CSFR/UVA melanosis | −1.511 | −0.546 | −1.382 | 0.00605 | 0.01169 | X | ||
|
| |||||||||
| Melanocytes | Pigmentary Disease/Function | SSR | UVA | UVB | |||||
|
| |||||||||
| KIT IHC | receptor for SCF/UVB melanosis, vitiligo | 2.317 | 0.558 | 1.493 | 0.00001 | 0.00014 | X | X | |
| EDNRB IHC | receptor for ET1/UVB melanosis, lentigo senilis | 2.144 | 0.611 | 1.639 | 0.00000 | 0.00011 | X | X | |
| MC1R | receptor for α MSH/skin & hair phenotype | 0.857 | 0.428 | 1.202 | 0.00025 | 0.00146 | X | ||
| MET | receptor for HGF/UVA melanosis | 0.208 | −0.080 | −0.202 | 0.04751 | 0.04457 | |||
| FGFRIHC | receptor for bFGF/melanocyte growth | 0.154 | −0.127 | −0.151 | 0.50786 | 0.19625 | |||
| NGF-R | receptor for NGF | −0.155 | −0.156 | −0.047 | 0.39043 | 0.16614 | |||
| GSF2RA | receptor for GM-CSF | −0.867 | −0.071 | −1.020 | 0.00000 | 0.00007 | X | X | |
validated by Immunohistochemistry
Table 4. Changes in the expression of cloned pigment genes after UVA and/or UVB exposure.
Heat maps and “Discriminatory for” columns were generated using the same procedure as in Table 2.
| median (log2 ratio) | ANOVA | Discriminatory for | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Development | SSR | UVA | UVB | p-value | p-value (FDR) | SSR vs O | UVA vs O | UVB vs O | |
| MCOLN3 | cation channel ion homeostasis | 1.184 | 0.000 | 0.451 | 0.00231 | 0.00231 | X | ||
| LMX1A | transcription factor | 0.173 | 0.221 | 0.768 | 0.00004 | 0.00041 | X | ||
| MREG/DSU | melanosome transport | 0.164 | 0.259 | 0.010 | 0.23354 | 0.12067 | |||
| KRT2A | keratin | 0.107 | −0.114 | 0.035 | 0.42014 | 0.17366 | |||
| SNAI2 | Waardenberg sundrome 2 | 0.058 | −0.402 | −0.200 | 0.00333 | 0.00786 | |||
| IKBKG | NFkB signaling | 0.046 | 0.089 | 0.408 | 0.01641 | 0.02247 | X | ||
| SOX18 | transcription factor | −0.037 | 0.296 | 0.570 | 0.00656 | 0.01231 | X | ||
| ED1 | sweat gland, tooth, hair morphogenesis | −0.069 | 0.517 | −0.669 | 0.24194 | 0.12335 | |||
| KRT1 | limits melanization | −0.075 | 0.199 | 0.266 | 0.00702 | 0.01292 | X | ||
| BRCA1 | development of various organs | −0.320 | 0.334 | −0.336 | 0.04077 | 0.04053 | X | ||
| FGFR2 | Crouzon, Apert, Pfeiffer syndromes | −0.360 | −0.301 | −0.297 | 0.17011 | 0.09899 | |||
| GNAQ | limits melanocyte proliferation | −0.519 | −0.654 | −0.947 | 0.27587 | 0.13371 | |||
| WNT1 | WNT signaling pathway | −1.613 | −0.661 | −1.047 | 0.00481 | 0.01002 | X | ||
|
| |||||||||
| Component of melanosomes and their precursors | SSR | UVA | UVB | ||||||
|
| |||||||||
| RAB38 | targeting of Tyrp1 to melanosome | 0.117 | −0.248 | −0.089 | 0.32982 | 0.14957 | |||
|
| |||||||||
| Melanosome construction/protein routing | SSR | UVA | UVB | ||||||
|
| |||||||||
| LEF1 | WNT signaling pathway | 0.652 | 0.350 | 0.621 | 0.01237 | 0.01874 | X | ||
| LYST | Chediak Higashi syndrome | 0.285 | 0.092 | 0.055 | 0.51243 | 0.19738 | |||
| HPS1 | Hermansky Pudlak syndrome type 1 | 0.193 | 0.421 | 0.108 | 0.16421 | 0.09706 | |||
| PLDN | vesicle docking and fusion | −0.283 | −0.271 | −0.826 | 0.00002 | 0.00028 | X | ||
|
| |||||||||
| Melanosome transport | SSR | UVA | UVB | ||||||
|
| |||||||||
| MYO7A | Usher syndrome type 1B | 0.293 | 0.111 | 0.449 | 0.34022 | 0.15254 | |||
|
| |||||||||
| Eumelanin and pheomelanin | SSR | UVA | UVB | ||||||
|
| |||||||||
| ASIP | Eumelanin/pheomelanin switch | 0.411 | −0.049 | 0.814 | 0.00000 | 0.00004 | X | ||
| GGT1 | pheomelanin synthesis | 0.247 | 0.095 | 0.601 | 0.05191 | 0.04723 | X | ||
| OSTM1 | pheomelanin and osteoclast function | 0.199 | 0.221 | −0.080 | 0.25748 | 0.12805 | |||
| ATRN | Eumelanin/pheomelanin switch | −0.079 | −0.096 | −0.296 | 0.20371 | 0.11072 | |||
|
| |||||||||
| Systemic effects | SSR | UVA | UVB | ||||||
|
| |||||||||
| ERCC2 | XP group D, NER | 0.176 | 0.201 | 0.524 | 0.00006 | 0.00057 | X | ||
| ATP7B | Wilson disease, copper transport | 0.053 | −0.377 | −0.083 | 0.02321 | 0.02807 | |||
| BCL2 | apoptosis inhibitor | 0.024 | 0.137 | 0.538 | 0.03835 | 0.03884 | X | ||
| RPL24 | eye, coat, skeletal | 0.011 | 0.539 | 0.133 | 0.00293 | 0.00722 | |||
Changes in expression of melanocyte-specific proteins after UVA and/or UVB exposure
Many of the significantly differentially expressed genes following UV exposure were known pigment-related genes [current list maintained at http://www.espcr.org/micemut/]. Thus we next validated those changes in gene expression by characterizing the levels of various known melanocyte-specific proteins that are directly involved in pigmentation (Table 2). Overall, many genes in that group were significantly differentially expressed with an adjusted p-value <0.05. The expression patterns of those genes were increased after SSR or, to a lesser extent, UVB although there were a few exceptions as discussed below. Eleven genes, all of them known pigment-related loci, had a ≥2-fold increased expression after SSR or UVB exposure, and of those, the 6 genes encoding melanosomal proteins (Pmel17, TYRP1, TYR, MART1, OA1 and DCT), showed more than a 3-fold increased expression after SSR or UVB. Only 1 melanocyte-specific gene, GSF2RA (the receptor for GM-CSF) was significantly down-regulated by SSR and by UVB. UVA alone had no significant effect on the expression of any of these genes encoding melanocyte-specific proteins, including GSF2RA.
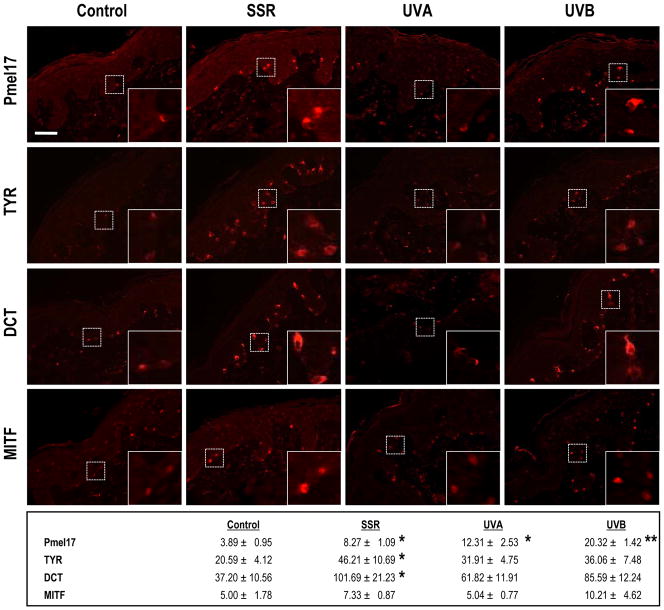
We used immunohistochemistry to validate many of these changes in expression at the protein level and confirmed that SSR or UVB exposure significantly increased the expression of many of those melanocyte-specific proteins (Pmel17, TYR, DCT and MITF are shown in Fig. 2). As expected from the microarray analysis, UVA did not significantly increase the expression of MITF above the control, but did increase TYR, DCT and Pmel17 to some extent.
Figure 2.
Immunohistochemistry of melanocyte-specific proteins encoded by UV-regulated genes. Pmel17, TYR, DCT and MITF were identified by staining with α PEP13h, α PEP7h, α PEP8 and Ab3, respectively, using Texas red. All specimens were from subject 2 and all are at the same magnification (200X); insets show regions depicted by the dashed boxes at 600X magnification. Numbers represent quantitation (mean ± SEM) of staining density in 6 different images each from 1 biopsy from each of the 4 areas on each of the 6 subjects (n=6 specimens, 36 images for each area). * = p <0.05. Bar = 50 μm.
Altered expression of paracrine factors and their receptors after UVA and/or UVB exposure
Since melanocyte differentiation is closely regulated by other cells in the skin (Yamaguchi et al., 2004; 2007), we next examined the expression levels of various known secreted factors that influence melanocyte function (Table 3). Surprisingly, only HGF showed more than a 1.5-fold change after UVB exposure, and none of the rest of the genes encoding known paracrine melanogenic factors showed a significant increase after any type of UV exposure, although some factors, such as IL-1A, IL-1B and GM-CSF, showed > 2-fold decreases after UVB exposure. Note that several genes encoding receptors expressed by melanocytes that are activated by paracrine melanogenic factors showed increased expression after SSR or UVB exposure, e.g. KIT (the receptor for SCF), EDNRB (the receptor for ET1), and MC1R (the receptor for α MSH).
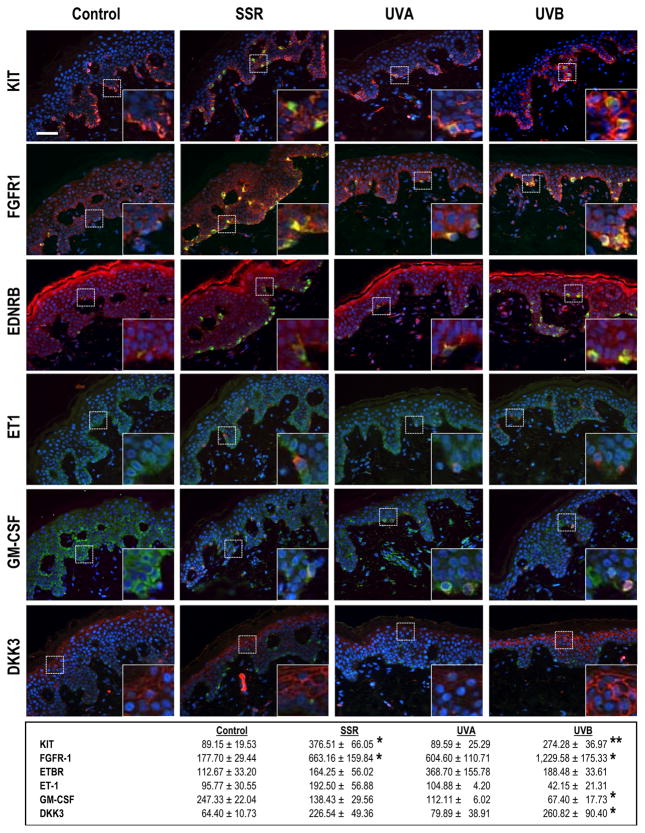
We confirmed many of these effects on melanogenic receptors and their ligands using immunohistochemistry (Fig. 3). SSR or UVB increased protein expression of KIT throughout the basal layer of the epidermis. Increased expression of FGFR1 (the receptor for bFGF) by melanocytes as well as by keratinocytes throughout the basal layer of the epidermis was also remarkable, especially after SSR or UVB exposure. The decreased expression of GM-CSF as well as the unchanged expression of ET1 after all types of UV exposure was also confirmed by immunohistochemistry. DKK3, which is expressed in non-palmoplantar (trunk) skin, in contrast to the high expression of DKK1 in palmoplantar skin (Yamaguchi et al., 2004), was also increased after SSR or UVB exposure, especially in keratinocytes of the upper epidermis.
Figure 3.
Immunohistochemistry of paracrine melanogenic factors and their receptors in human skin exposed to SSR, UVA or UVB. KIT, FGFR1, EDNRB, ET1, GM-CSF and DKK3 were identified by staining with specific antibodies as listed in SI Materials and Methods and Texas red as the chromogen. Melanocytes are identified in the sections by staining with MART1 (green) and nuclei are stained with DAPI (blue). All specimens were from subject 2, and all are at the same magnification (200X); insets show regions depicted by the dashed boxes at 600X magnification. Numbers represent quantitation (mean ± SEM) of staining density in 6 different images each from 1 biopsy from each of the 4 areas on each of the 6 subjects (n=6 specimens, 36 images for each area). * = p <0.05; ** = p <0.01. Bar = 50 μm.
UV-regulated expression of cloned pigment genes
We also examined the expression levels of all known cloned pigment-related genes (other than the melanocyte-specific ones discussed above) to determine if any of them responded to any type of UV (Table 4). Interestingly, only UVB exposure induced a signficant response and only in some genes, including WNT1 that showed a significant decrease in expression and MCOLN3 and ASIP that showed significant increases in expression.
Identification of UV-regulated genes
Data mining of the gene expression patterns should provide important clues to factors involved in UV responses of the skin that have not been previously identified. We generated a list of genes with gene expression up-regulated after UVA and/or UVB exposure (Storey adjusted p-value <0.05 and fold change >3.0) that had not been previously reported (partial list shown in Table 5). Antibodies are available for several of those and we used immunohistochemistry to characterize their protein expression levels (Fig. 4). Melanocytes were identified using a MART1 antibody (green), nuclei were localized using DAPI (blue), and the specific markers were localized by appropriate antibodies (red). The increase in melanocyte density elicited by SSR or UVB is readily seen in the basal layer of the epidermis, but no such increase was elicited by UVA.
Table 5. UV-regulated targets in the skin.
Heat maps and “Discriminatory for” columns were generated using the same procedure as in Table 2.
| median (log2 ratio) | ANOVA | Discriminatory for | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Target | Full Name/Function | SSR | UVA | UVB | p-value | p-value (FDR) | SSR vs O | UVA vs O | UVB vs O |
| CCL18 | macrophage inflammatory protein 4 | 2.890 | 0.562 | 2.297 | 0.00000 | 0.00010 | X | X | |
| TRIM63 (MURF1) IHC | tripartite motif-containing 63 | 2.702 | 1.022 | 2.678 | 0.00031 | 0.00167 | X | ||
| GMPR | guanosine monophosphate reductase | 2.300 | 0.770 | 1.743 | 0.00001 | 0.00014 | X | X | |
| TRPM1 | Melastatin, MLSN, MLSN1 | 2.258 | 0.497 | 1.680 | 0.00271 | 0.00682 | X | X | |
| IGFBP7 IHC | insulin-like growth factor binding protein 7 | 2.209 | 0.657 | 1.944 | 0.00000 | 0.00008 | X | X | |
| MPLP1 IHC | Myelin proteolipid protein (CNS) | 2.190 | 0.369 | 1.532 | 0.00043 | 0.00207 | X | X | |
| PCSK2 | Proprotein convertase 2 | 2.119 | 0.210 | 1.925 | 0.00001 | 0.00014 | X | X | |
| LRRN6A (LINGO-1) IHC | nervous system specific | 2.069 | 1.003 | 1.883 | 0.00000 | 0.00005 | X | X | |
| FEZ1 | fasciculation and elongation protein ζ 1 | 2.015 | 0.808 | 1.414 | 0.00002 | 0.00031 | X | X | |
| ARMC9 | Melanocyte specific protein KU-MEL-1 | 1.651 | 0.825 | 1.442 | 0.00004 | 0.00042 | X | X | |
validated by Immunohistochemistry
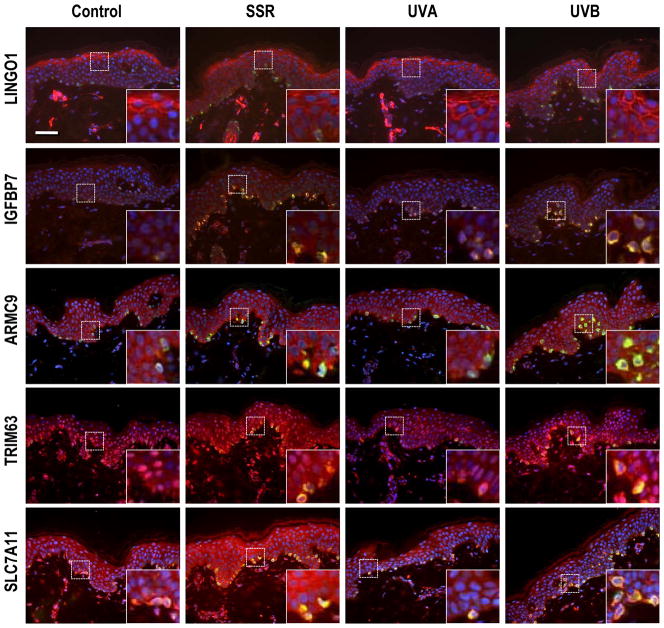
Figure 4.
Immunohistochemistry of factors encoded by UV-regulated genes. Localization of IGFBP7, TRIM63, SLC7A11 and MPLP using specific antibodies as listed in SI Materials and Methods and Texas red as the chromogen. Melanocytes were identified in the sections by co-staining with MART1 (green) and nuclei are stained with DAPI (blue). All specimens were from subject 8, and all are at the same magnification (200X); insets show regions depicted by the dashed boxes at 600X magnification. Bar = 50 μm.
LRRN6A (also known as LINGO-1) had a strong membrane-localized expression in the upper epidermis that was dramatically increased by SSR or UVB (but not UVA) and correlated closely with the microarray data. IGFBP7 had a weak but specific expression in melanocytes in unirradiated or UVA-exposed skin, and its expression was strongly increased by SSR or UVB, which also correlated closely with the microarray data. ARMC9 (also known as KU-MEL-1) and TRIM63 (also known as MURF1) was expressed in melanocytes and in keratinocytes in the basal layer of unexposed or UVA-exposed skin, but was strongly increased following exposure to SSR or UVB, again correlating well with the microarray data.
SLC7A11 is an interesting solute carrier that has been associated with the regulation of pigmentation, and somehow modulates the production of eumelanin versus pheomelanin (Chintala et al., 2005), but its regulation by UV has not been previously reported. The microarray results (Table 2) show that expression of SLC7A11 was significantly decreased (about 2-fold) by UVB, a pattern distinct from most other pigment-related genes. We used 3 different antibodies to stain SLC7A11, and each of them gave distinct staining patterns, none of them consistent with the microarray data. SLC7A11 antibody #39040 was reactive in the mid-epidermis, but not in melanocytes, and there was no change following exposure to UVA and/or UVB (not shown). SLC7A11 antibody #55574 (Fig. 4) stained positively over the entire epidermis, including melanocytes, and that staining was increased in all epidermal cells following exposure to UVA and/or UVB, which contrasts with its down-regulated expression at the transcriptional level. The localization of SLC7A11 in the skin and its responses to UV exposure remain unclear at this time, and further investigation will be needed to elucidate the role of SLC7A11 in response to UV.
DISCUSSION
The skin of most individuals generates a significant tanning response when repetitively exposed to UV (Parrish et al., 1981; Miller et al., 2008). It was previously reported that different wavelengths of UV induce different levels of modulation of melanocyte-specific markers, presumably via different mechanisms (Schlenz et al., 2005). Recently, we reported that pheomelanin and eumelanin levels were much higher after UVB or SSR exposure than after UVA exposure, even though all 3 types of UV resulted in comparable tanning responses (Wolber et al., 2008). In the same study, we also found that SSR was more effective in eliciting those effects than was UVB alone. Various other studies have shown that in the case of mixtures of UVA and UVB, UVA-rich sources are more effective in producing a tan than are those from UVB-rich sources (Bech-Thomsen et al., 1994; Ravnbak and Wulf, 2007). Therefore, there seems to be a synergistic effect on melanogenesis when UVA and UVB are combined. It is well-known that UVB is a strong stimulator of the expression of various pigment-related genes, such as TYR, TYRP1 and DCT, as well as the transcription factor MITF, and therefore the tanning response elicited by UVB is thought to be via nascent melanin synthesis due to the increased function of those factors, a mechanism supported by the results of this study. In contrast, UVA seems to elicit no similar increase in any melanogenesis-related factors. IPD and PPD have been known for some time to reflect the relatively rapid oxidative effects of UVA on preexisting melanin and melanin intermediates in the skin (as discussed in (Brenner et al., 2009; Coelho et al., 2009)). IPD and PPD typically appear relatively quickly and then disappear within a short time thereafter. Our study suggests that the longer term tans elicited by repetitive UVA exposure are similar in mechanism to IPD and PPD and reflect changes (probably oxidative in nature) in preexisting melanin and/or melanogenic intermediates. It would be interesting to determine if higher physical doses of UVA would stimulate expression of genes that were up-regulated by UVB, but such analysis must await future studies.
One important consideration to keep in mind is that genes identified in this study are not those that respond quickly to UV exposure, but rather those that remain increased after ~2–3 weeks of repetitive exposure. The majority of genes were regulated similarly after SSR or UVB exposure, and also in many cases, the fold increase of gene expression was greater following SSR exposure than with UVB alone, which is consistent with the studies mentioned above. As expected, SSR or UVB increased the expression of many known melanocyte-specific genes, such as TYR, DCT and MART-1, and this effect was confirmed at the protein level by immunohistochemistry. However, UVA did not induce such up-regulation of pigment cell specific genes, which indicates that UVA elicits the tanning of skin via a distinct mechanism. It is remarkable that the long-term changes in gene expression noted were highly reproducible in the 6 different individuals and were relatively limited, representing <5% of the entire genome. Further, most of the UV-responsive genes identified are expressed by melanocytes, a relatively minor population of cells in the skin but one which has a critical function in providing protection from UV damage. The use of suction blister biopsies as the tissue source no doubt maximized the number of epidermal cells and minimized the number of dermal cells subjected to the microarray analysis. Nevertheless, the vast majority of cells in those blister roofs are keratinocytes and melanocytes represent only a very small subpopulation. Despite that, genes expressed by melanocytes and fibroblasts were the most significantly modulated by UV in this study (Suppl. Table 1).
An interesting consideration is the wide range of responses to UV in functional groups of genes (shown in Suppl. Fig. 1) involved in different biological processes. As noted above, the most dramatically up-regulated genes were those involved in the pigmentation pathway, but genes involved in cell communication, adhesion, motility, morphogenesis, development, and immune responses (among other things) were also significantly regulated. Given the vast literature on the effects of UV to affect those processes (e.g. decreasing the immune functions of the skin) this microarray database should provide a wealth of resources to other groups interested in the responses of those other genes to UV exposure in the skin.
Melanocyte function is regulated by various autocrine and paracrine factors produced by different types of cells in the skin, including melanocytes, keratinocytes and fibroblasts (Yamaguchi et al., 2007). Therefore, we hypothesized that the different responses of human skin pigmentation to different wavelengths of UV may be due to the different paracrine factors involved in those processes. Surprisingly however, levels of most of the well-known paracrine melanogenic factors were not significantly changed in human skin in situ after repetitive exposure to SSR, UVA or UVB, either at the gene expression level or at the protein level. Several previous studies reported the increased expression of several paracrine factors (such as bFGF, ET-1 and SCF) after UV exposure, and demonstrated the effects of those cytokines on melanocyte differentiation (Halaban et al., 1988; Imokawa et al., 1995; 1996; Grichnik et al., 1998; Hachiya et al., 2001). We did not find increased expression of any of those paracrine factors in our study. However, those earlier studies employed a single acute irradiation of 2 MED UVB, whereas we used repetitive suberythemal doses of UV, which are more physiological and produce much lower levels of inflammation and vasodilation of local blood vessels. We found that in human skin in situ, SSR or UVB strongly increased the expression of the receptors for those 3 ligands, EDNRB, KIT and FGFR. The sum of these results suggests that the effects of the secreted paracrine factors are regulated more by their receptor levels on melanocytes after repetitive suberythemal doses of UV.
In an effort to identify factors involved with responses of human skin to UV, we validated the expression patterns of proteins encoded by several genes identified in our study that were regulated by UV that had not been previously reported to be UV-responsive or related to skin tanning. LINGO1 has been associated with the function/survival of neurons although its specific function(s) has not yet been characterized (Mi et al., 2005). Melanocytes are closely related to neurons, being derived from the neural crest, and LINGO1 is not only expressed in melanocytes in unexposed skin but is greatly stimulated by UVB and SSR. LINGO1 was also expressed in the upper epidermis (by keratinocytes) and that expression was also increased following UVB or SSR exposure. Further characterization of the roles of LINGO1 in the epidermis, particularly with respect to its impact on survival of UV-exposed cells, should prove interesting. IGFBP7 was previously identified to be reduced in expression in psoriatic skin but is up-regulated following UVB phototherapy (Hochberg et al., 2007). IGFBP7 is a secreted protein that inhibits BRAF-MEK-ERK signaling and induces senescence and apoptosis (Wajapeyee et al., 2008) and thus acts as a tumor suppressor. The specific expression of IGFPB7 by melanocytes at the basement membrane, and its dramatic up-regulation by UVB and SSR, suggests that it may be important in preventing the UV-induced transformation of melanocytes to melanoma cells. TRIM63 (MURF1) is a ubiquitin ligase normally associated with muscle function whose expression is regulated by exercise (stress) (Clarke et al., 2007); its role in the skin and response to UV stress is currently unknown and also deserves further study since it may be functional in regulating UV responses at the post-translational level. Even less is known about ARMC9 (KU-MEL-1) which is also expressed by melanocytes and keratinocytes in the basal layer and is markedly up-regulated by UVB or SSR. ARMC9 (KU-MEL1) is primarily known as an antigen that elicits antibody production in 2 distinct pigmentary conditions, vitiligo and melanoma (Kiniwa et al., 2001), thus its expression by melanocytes in normal skin and its UV-responsive characteristic are interesting.
An interesting consideration is what transcription factor(s) drive the longer-term UV responses in the skin. Earlier studies have shown that several key transcription factors, namely p53 (Cui et al., 2007), MITF (Tadokoro et al., 2005) and SOX9 (Passeron et al., 2007) are involved in the rapid up-regulation of the melanogenic pathway after a brief UV exposure. It is clear from our current study that after 2–3 weeks of repetitive UV exposure, the roles of p53 and SOX9 have decreased and that MITF, SOX10 and PAX3 (reviewed in (Busca and Ballotti, 2000)), and perhaps others identified in this study play a more significant role.
In summary, this study provides valuable insights into the regulation of genes in human skin in response to different types of UV, i.e. UVA and/or UVB. Perhaps the single most striking finding is that the majority of significantly UV-responsive genes are those involved in regulating skin pigmentation. Future studies should identify many other regulators of melanogenesis in human skin involved in UV responses based on this microarray database.
MATERIALS AND METHODS
Subjects and UV irradiation protocol
Six volunteers (age 37.3 ± 15.7) with Fitzpatrick skin type II–III were included in this study, which was approved by the Research Involving Human Subjects Committees of Beiersdorf AG. Written informed consent was obtained from each donor and the study was conducted according to the Helsinki guidelines. A solar simulator (Oriel 4 - solar simulator 1600 W, Oriel Instruments, Stratford, CT, USA) was used for UV irradiation. UV wavelengths below 290 nm were removed with an optical filter (WG320, ITOS – Gesellschaft für Technische Optik mbH, Mainz, Germany) for SSR. For UVA and UVB radiation, a ‘BC-Blocker’ filter (cut-off: 320 nm, Oriel Instruments) and a custom-made filter combination (WG 320 + UG11 + bandpass 290–320, Tafelmayer, Rosenheim, Germany) were used, respectively.
The back of each subject was repetitively irradiated using the 3 different sources of UV for 2 weeks (5 times per week, 10 times total) after preliminary determination of their MEDs. For SSR, irradiation doses of 0.4 MED in the first week and 0.5 MED in the second week were used (Schlenz et al., 2005; Miyamura et al., 2007). In order to obtain comparable visual tanning reactions for UVA and UVB, adapted doses of UVA (2.3 times the UVA dose in the SSR irradiation) and UVB (1.1 times the UVB dose in the SSR irradiation) were used (Wolber et al., 2008). The average SSR exposure for 1 MED for these subjects was 0.24 J/cm2 (±0.06, n=6). Suction blister biopsies were taken 3 days after the last irradiation, one half of which was frozen for whole human genome microarray analysis and the other half was fixed in 4% formalin in PBS and embedded in paraffin for immunohistochemistry and for melanin staining. A non-irradiated skin biopsy from a comparable skin region served as the control for each donor.
Whole human genome microarray analysis procedure
A single-color hybridization of human RNAs on Agilent Whole Human Genome Oligo Microarrays and bioinformational analysis were performed for each skin biopsy sample by Miltenyi Biotec GmnH (Bergisch Gladbach, Germany). Briefly, total RNA was prepared from each biopsy using standard RNA extraction protocols (Trizol, Sigma), and were quality-checked using an Agilent 2100 Bioanalyzer platform (Agilent Technologies, Waldbronn, Germany). To produce Cy3-labeled cRNA, the RNA samples were amplified and labeled using the Agilent Low RNA InputLinear Amp Kit (Agilent Technologies) following the manufacturer’s protocol. The hybridization procedure was performed according to the Agilent 60-mer oligo microarray processing protocol using the Agilent Gene Expression Hybridization Kit (Agilent Technologies). Briefly, 1.65 μg Cy3-labeled fragmented cRNA in hybridization buffer was hybridized overnight (17 hr, 65°C) to Agilent Whole Human Genome Oligo Microarrays 4x44K, using Agilent’s recommended hybridization chamber and oven. Fluorescence signals of the hybridized Agilent Microarrays were detected using Agilent’s Microarray Scanner System (Agilent Technologies). The signal intensities from the single-experiment raw data lists were normalized by dividing their median intensity values. The ratio lists of the array of each treatment sample vs. the respective donor-matched control sample were generated using the RosettaResolver™ Software (Rosetta Inpharmatics, Seattle, WA). Further analyses were conducted using probes with a ratio of at least 1.7 (up- or down- regulated) and an associated p-value of 0.001 or better in at least one comparison of a treatment sample with the matched control (21,581 probes were retained after this filtering step).
Immunohistochemistry
Paraffin embedded skin biopsies were examined for expression of various melanosomal proteins and secreted factors/receptors using indirect immunofluorescence. Melanosomal proteins were detected using the following primary antibodies: anti-human MITF mouse monoclonal Ab3 (1:4,000 dilution; NeoMarkers, Fremont, CA) and anti-human MART-1 mouse monoclonal Ab-3 (1:200 dilution; NeoMarkers), polyclonal rabbitα PEP7h for human TYR (1:8,000 dilution), polyclonal rabbit α PEP8h for human DCT (1:7,500 dilution) and polyclonal rabbit α PEP13hfor human Pmel17 (1:4,000 dilution) (Virador et al., 2001). For the secreted paracrine factors and their receptors, anti-human c-kit goat IgG (1 μg/ml; R&D Systems, Minneapolis, MN), anti-FGFR1 rabbit polyclonal antibody (1:400 dilution; Abcam, Cambridge, MA), anti-ETBR rabbit polyclonal antibody (1:100 dilution; Abcam), anti-ET-1 mouse monoclonal antibody (1:200 dilution; Abcam), anti-human GM-CSF mouse monoclonal antibody (25 μg/ml; R&D) and anti human DKK-3 goat IgG (1 μg/ml; R&D) were used. In addition, anti-human LINGO-1 antibody (2 μg/ml; R&D), anti-human IGFBP-7 antibody (2 μg/ml; R&D), anti-ARMC9 (1 μg/ml; Sigma-Aldrich, Inc., St. Louis, MO), anti-MURF1 antibody (0.625 μg/ml, Abcam), anti-SLC7A11 antibody (cat#39050 20 μg/ml, cat#55573 20 μg/ml, both from Novus Biologicals, Littleton, CO) were used. After incubation with the primary antibody in the presence of 5% serum overnight at 4°C, sections were then incubated with appropriate secondary antibodies, Alexa Fluor® 594 goat anti-rabbit IgG (H+L), Alexa Fluor® 488 goat anti-mouse IgG (H+L), Alexa Fluor® 594 donkey anti-goat IgG (H+L), (1:400 dilution; all from Molecular Probes, Inc., Eugene, OR) or with fluorescein horse anti-mouse IgG (1:100 dilution; Vector Laboratories, Inc., Burlingame, CA) with 5% serum for 1 hr at room temperature. Nuclei were counterstained with DAPI (Vector). Fluorescence was observedand photographed using a fluorescence microscope (model DMR B/DMLD; Leica, Wetzlar, Germany), a 3CCD 3-chip color video camera (Dage-MTI, MI City, IN), andimages were processed usingScion Image software (Scion, Frederick, MD).
Melanin staining
Paraffin-embedded tissues were processed with the Fontana-Massonsilver stain to observe the melanin distribution in skin specimens (Bancroft and Stevens, 1982). Stained samples were observed and photographed using the above-mentioned microscope system using visible light and images were analyzed using Scion Image software, as previously detailed (Tadokoro et al., 2003).
Statistical and bioinformatics analyses
All statistical/bioinformatics analyses were conducted using R (version 2.9) and based on log2 ratios generated by RosettaResolver™ software. One-factor ANOVA was used to test for differences among the four groups (UVA, UVB, SSR and control). Student’s paired t-test was used to identify differentially expressed probes for pair-wise comparison. All p-values in differential expression analyses were adjusted using the Storey FDR correction (Storey and Tibshirani, 2003). Gene set analysis was done using the R package GSA (Efron and Tibshirani, 2006). DAVID (Dennis, Jr. et al., 2003) was used for functional analysis (using second-level Gene Ontology terms) and Suppl. Figure 1 was generated using Genesis (Sturn et al., 2002). The R script and its input data are available upon request.
Supplementary Material
Acknowledgments
The authors thank Sergio G. Coelho for helping with the UV spectra graph. This research was supported in part by the Intramural Research Program of the National Cancer Institute at NIH.
Abbreviations
- DT
delayed tanning
- IPD
immediate pigment darkening
- MED
minimal erythema dose
- PPD
persistent pigment darkening
- SSR
solar simulated radiation
- UV
ultraviolet
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002a;15:112–118. doi: 10.1034/j.1600-0749.2002.1o071.x. [DOI] [PubMed] [Google Scholar]
- Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002b;15:119–126. doi: 10.1034/j.1600-0749.2002.1o072.x. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. Churchill Livingstone; New York: 1982. [Google Scholar]
- Bech-Thomsen N, Ravnborg L, Wulf HC. A quantitative study of the melanogenic effect of multiple suberythemal doses of different ultraviolet radiation sources. Photodermatol Photoimmunol Photomed. 1994;10:53–56. [PubMed] [Google Scholar]
- Black HS, de Gruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, De Fabo EC, Ullrich SE, Tyrrell RM. Photocarcinogenesis: an overview. Photochem Photobiol. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Brenner M, Coelho SG, Beer JZ, Miller SA, Wolber R, Smuda C, Hearing VJ. Long-lasting molecular changes in human skin after repetitive in situ UV irradiation. J Invest Dermatol. 2009;129:1002–1011. doi: 10.1038/jid.2008.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busca R, Ballotti R. Cyclic AMP: a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci USA. 2005;102:10964–10969. doi: 10.1073/pnas.0502856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Claverie JM. Computational methods for the identification of differential and coordinated gene expression. Hum Mol Genet. 1999;8:1821–1832. doi: 10.1093/hmg/8.10.1821. [DOI] [PubMed] [Google Scholar]
- Coelho SG, Choi W, Brenner M, Miyamura Y, Yamaguchi Y, Wolber R, Smuda C, Batzer J, Kolbe L, Ito S, Wakamatsu K, Zmudzka BZ, Beer JZ, Miller SA, Hearing VJ. Short- and long-term effects of UV radiation on the pigmentation of human skin. J Invest Dermatol Symp Proc. 2009;14:32–35. doi: 10.1038/jidsymp.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D’Orazio JA, Fung CY, Schanbacher CF, Granter SR, Fisher DE. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- D’Orazio JA, Nobuhisa T, Cui R, Arya M, Spry M, Wakamatsu K, Igras V, Kunisada T, Granter SR, Nishimura EK, Ito S, Fisher DE. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Meth Enzymol. 2000;319:359–366. doi: 10.1016/s0076-6879(00)19035-4. [DOI] [PubMed] [Google Scholar]
- Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Stanford Tech Report. 2006. On testing the significance of sets of genes. [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. New Eng J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Park HY, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Burchette J, Shea CR. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol. 1998;111:233–238. doi: 10.1046/j.1523-1747.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J Invest Dermatol. 2001;116:578–586. doi: 10.1046/j.1523-1747.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott GA, Moellmann GE, McGuire JS. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988;107:1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing VJ. Regulating melanosome transfer: who’s driving the bus? Pigment Cell Res. 2007;20:334–335. doi: 10.1111/j.1600-0749.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Hochberg M, Zeligson S, Amariglio N, Rechavi G, Ingber A, Enk CD. Genomic-scale analysis of psoriatic skin reveals differentially expressed insulin-like growth factor-binding protein-7 after phototherapy. Br J Dermatol. 2007;156:289–300. doi: 10.1111/j.1365-2133.2006.07628.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Miyagishi M, Yada Y. Endothelin-1 as a new melanogen: coordinated expression of its gene and the tyrosinase gene in UVB-exposed human epidermis. J Invest Dermatol. 1995;105:32–37. doi: 10.1111/1523-1747.ep12312500. [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Kimura M, Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for human melanocytes in UVA-induced melanosis. Biochem J. 1996;313 ( Pt 2):625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte versus the sun: who will win the final round? Pigment Cell Res. 2003;16:434–447. doi: 10.1034/j.1600-0749.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- Kiniwa Y, Fujita T, Akada M, Ito K, Shofuda T, Suzuki Y, Yamamoto A, Saida T, Kawakami Y. Tumor antigens isolated from a patient with vitiligo and T-cell-infiltrated melanoma. Cancer Res. 2001;61:7900–7907. [PubMed] [Google Scholar]
- Kushimoto T, Basrur V, Valencia JC, Matsunaga J, Vieira WD, Muller J, Appella E, Hearing VJ. A new model for melanosome biogenesis based on the purification and mapping of early melanosomes. Proc Natl Acad Sci USA. 2001;98:10698–10703. doi: 10.1073/pnas.191184798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Hatao M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J Invest Dermatol. 2004;122:503–509. doi: 10.1046/j.0022-202X.2004.22223.x. [DOI] [PubMed] [Google Scholar]
- Marr DG, Poser I, Shellman YG, Bosserhoff AK, Norris DA. Ultraviolet radiation induces release of MIA: a new mechanism for UVR-induced progression of melanoma. Int J Oncol. 2004;25:105–111. [PubMed] [Google Scholar]
- Mi S, Miller RH, Lee X, Scott ML, Shulag-Morskaya S, Shao Z, Chang J, Thill G, Levesque M, Zhang M, Hession C, Sah D, Trapp B, He Z, Jung V, McCoy JM, Pepinsky RB. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci. 2005;8:745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- Miller SA, Coelho SG, Zmudzka BZ, Bushar HF, Yamaguchi Y, Hearing VJ, Beer JZ. Dynamics of pigmentation induction by repeated UV exposures: dose, dose interval and UV spectrum dependence. Brit J Dermatol. 2008;159:921–930. doi: 10.1111/j.1365-2133.2008.08708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, Ito S, Smuda C, Passeron T, Choi W, Batzer J, Yamaguchi Y, Beer JZ, Hearing VJ. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007;20:2–13. doi: 10.1111/j.1600-0749.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Parrish JA, Zaynoun S, Anderson RR. Cumulative effects of repeated subthreshold doses of ultraviolet radiation. J Invest Dermatol. 1981;76:356–358. doi: 10.1111/1523-1747.ep12520019. [DOI] [PubMed] [Google Scholar]
- Passeron T, Valencia JC, Bertolotto C, Hoashi T, Takahashi K, Le Pape E, Ballotti R, Hearing VJ. SOX9 is a key player in UVB-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci USA. 2007;104:13984–13989. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnbak MH, Wulf HC. Pigmentation after single and multiple UV-exposures depending on UV-spectrum. Arch Dermatol Res. 2007;299:25–32. doi: 10.1007/s00403-006-0728-3. [DOI] [PubMed] [Google Scholar]
- Schlenz K, Smuda C, Batzer J, Stab F, Wenck H, Elsaesser HP, Wolber R. Pigmentation mechanisms induced by different wavelengths of UV light. Pigment Cell Res. 2005;18:S33. [Google Scholar]
- Seline PC, Norris DA, Horikawa T, Fujita M, Middleton MH, Morelli JG. Expression of E and P-cadherin by melanoma cells decreases in progressive melanomas and following ultraviolet radiation. J Invest Dermatol. 1996;106:1320–1324. doi: 10.1111/1523-1747.ep12349048. [DOI] [PubMed] [Google Scholar]
- Shibahara S, Yasumoto K, Amae S, Udono T, Watanabe K, Saito H, Takeda K. Regulation of pigment cell specific gene expression by MITF. Pigment Cell Res. 2000;13:98–102. doi: 10.1034/j.1600-0749.13.s8.18.x. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Im S, Tada A, Scott C, Akcali C, Davis MB, Barsh GS, Hearing VJ, Abdel-Malek ZA. Participation of the melanocortin-1 receptor in the UV control of pigmentation. J Invest Dermatol Symp Proc. 1999;4:29–34. doi: 10.1038/sj.jidsp.5640177. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, Hearing VJ. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 2003;17:1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- Virador V, Matsunaga N, Matsunaga J, Valencia JC, Oldham RJ, Kameyama K, Peck GL, Ferrans VJ, Vieira WD, Abdel-Malek ZA, Hearing VJ. Production of melanocyte-specific antibodies to human melanosomal proteins: Expression patterns in normal human skin and in cutaneous pigmented lesions. Pigment Cell Res. 2001;14:289–297. doi: 10.1034/j.1600-0749.2001.140410.x. [DOI] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikonkal NM, Brash DE. Ultraviolet radiation signature mutations in photocarcinogenesis. J Investig Dermatol Symp Proc. 1999;4:6–10. doi: 10.1038/sj.jidsp.5640173. [DOI] [PubMed] [Google Scholar]
- Wolber R, Schlenz K, Wakamatsu K, Smuda C, Nakanishi Y, Hearing VJ, Ito S. Pigmentation effects of solar simulated radiation as compared with UVA and UVB radiation. Pigment Cell Melanoma Res. 2008;21:487–491. doi: 10.1111/j.1755-148X.2008.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: Pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006;20:1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.