Abstract
Superoxide (SO, O2·−) and its reaction product peroxynitrite (PN, ONOO−) have been shown to be important in the development of pain of several etiologies. While significant progress has been made in teasing out the relative contribution of SO and PN peripherally, spinally, and supraspinally during the development and maintenance of central sensitization and pain, there is still a considerable void in our understanding. Further research is required in order to develop improved therapeutic strategies for selectively eliminating SO and/or PN. Furthermore, it may be that PN is a more attractive target, in that unlike SO it has no currently known beneficial role. Our group has been at the forefront of research concerning the role of SO and PN in pain, and our current findings have led to the development of two new classes of orally active catalysts which are selective for PN decomposition while sparing SO.
Keywords: superoxide, peroxynitrite, pain, nociception, central sensitization
1. Introduction
Pain is a global affliction that affects people of every age, gender, and ethnicity. In the United States alone, the American Pain Society estimates that about 105 million people suffer from chronic pain [1]. Global pain market sales in 2009 exceeded $50 billion [1]. This does not take into account the indirect costs such as loss of productive work time and decreased quality of life. Most current strategies of pain management involve traditional non-steroidal anti-inflammatory drugs (NSAIDs) and opioid narcotics. NSAIDs have limited efficacy and while opioids are highly efficacious in the treatment of pain, their use is severely limited by debilitating side effects, the risk of addiction, the development of tolerance[2], and potentially morphine-induced hyperalgesia [3]. Selective cyclooxygenase (COX)-2 inhibitors have been shown to be effective in certain types of chronic pain, however concerns over their side-effects including increased risks of heart attack and stroke [4] have led to a decline in their use. Thus it is clear that there is a desperate need for new analgesic agents that would maintain efficacy over long-term treatment without the risk of tolerance, addiction, or intolerable side effects.
There is significant evidence linking both superoxide (SO, O2·−) and peroxynitrite (PN, ONOO−) to pain of several etiologies. Because SO and PN contribute to the development and maintenance of both peripheral and central sensitization, they are attractive targets for pain management strategies as will be discussed further in later sections. Indeed, current studies have shown that therapeutic strategies targeting SO and PN are able to both prevent and reverse the pathologies associated with pain of various etiologies.
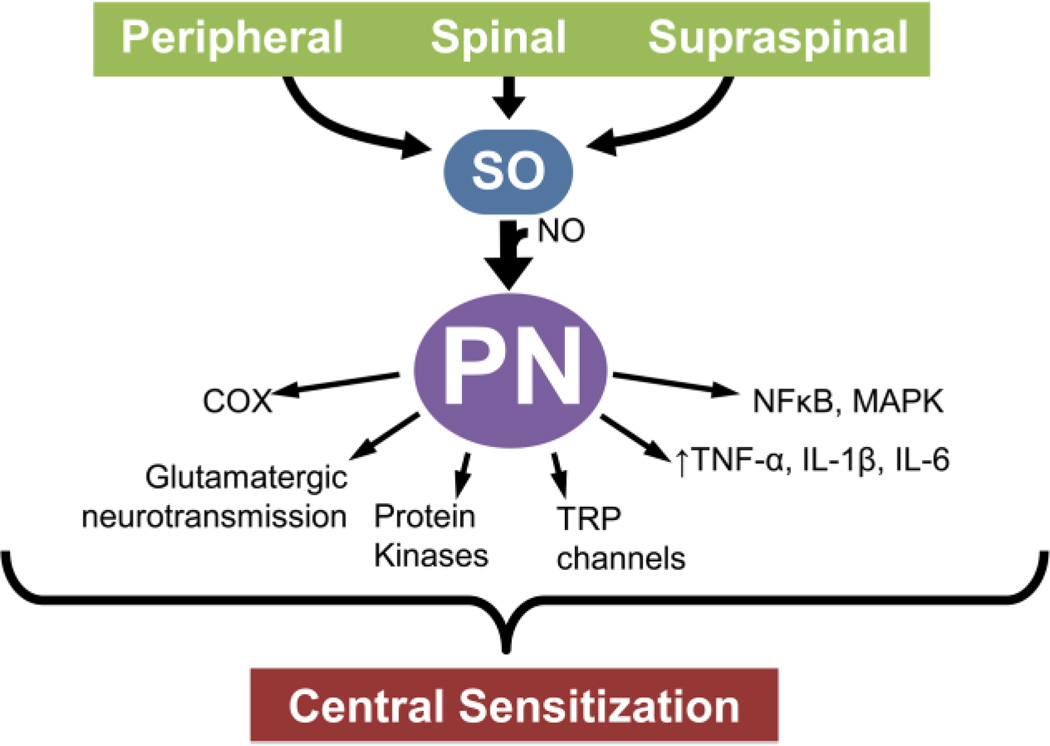
Modulation of protein kinases, alterations in glutamatergic neurotransmission, neuroinflammation, and modulation of ion channels are some of the hallmarks of the development of central sensitization and can occur in the periphery, spinal cord, or supraspinally. Both SO and PN have been shown to be involved in these signaling pathways (Fig. 1). In this review, we will briefly discuss the contributions of SO and PN in pain as well as current therapeutics and future directions for the targeting of these important species.
Fig. 1. Peroxynitrite modulates central sensitization through a variety of mechanisms.
Peroxynitrite (PN) formed within the periphery, the spinal cord, and in supraspinal areas modulates central sensitization associated with pain of several etiologies. Pathways known to be potentially impacted as a result of overt formation of PN are depicted.
2. Existing therapeutic strategies targeting SO and PN
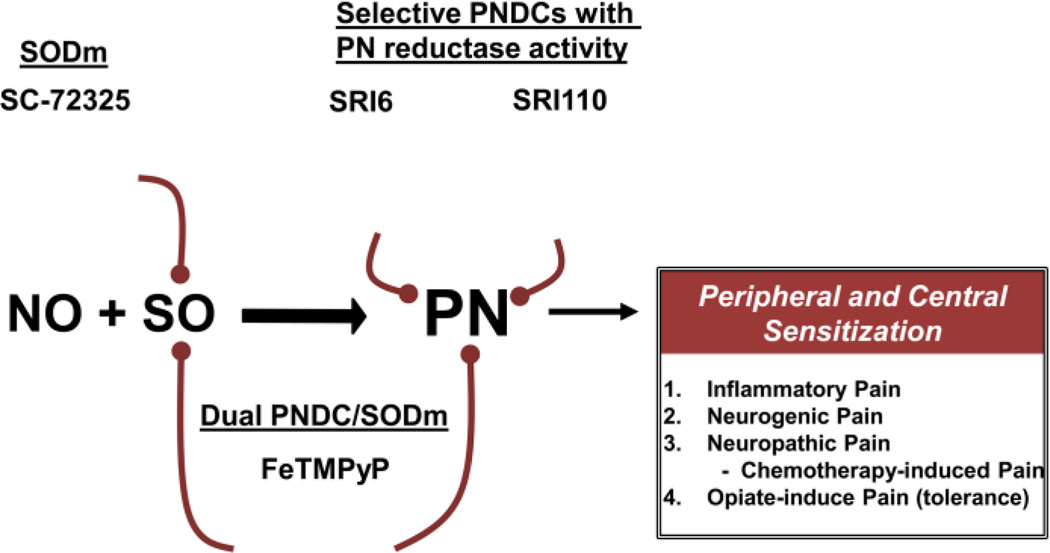
Our improved understanding of SO and PN in pain, has led to the development of therapeutic strategies based on their elimination (Fig. 2). There are two major strategies pursued in the effort to target SO and PN: preventing the formation of PN and decomposing the PN that already exists [5]. Several metal-based catalysts and non-metal scavenger systems [6] have been reported to effectively prevent the formation of PN through the dismutation of SO (superoxide dismutase mimetics, SODms) or to decompose PN once it is formed (PNdecomposition catalysts, PNDCs) [7, 8]. The two most promising classes of therapeutic agents are synthetic porphyrins (e.g. MnTE-2-PyP5+, FeTM-4-PyP5+), and functionalized manganese (II) polyazamacrocycles (e.g. SC-72325) [6]. While both of these classes will act as SODms, the metalloporphyrins also act as PNDCs [6, 9] making them excellent candidates for potential therapeutic agents. An orally available PNDC, Fe(III)tetrakis-2-(N-triethylene glycol monomethyl ether) pyridyl porphyrin, has been shown to prevent the development of diabetic neuropathic pain [10, 11]. We have recently described the synthesis and evaluation of a new lipid soluble manganese(III) porphyrin, SRI6 [12]. SRI6 is able to shield the charged metal center from the membrane during passive transport by employing beta-fused cyclohexenyl substituents. It has therefore been shown to be orally active in a number of animal models of inflammatory and neuropathic pain. Because of the removal of charged electron-withdrawing meso-functionality used for PNDCs (such as FeTMPyP5+), the metal-centered reduction potential for SRI6 is out of the useful range for SOD activity [13] but well within the oxygen atom transfer reactivity manifold of PN [14]. Thus, our design for oral activity runs in parallel with selectivity for decomposing PN while sparing SO. We have also extended this design to a new class of 2-electron PN reducing catalysts, exemplified by SRI110 [15, 16]. SRI110 and similar analogues have been shown to react with PN to produce the manganese(V)O intermediate with concomitant reduction of PN to nitrite. The manganese(V)O species are rapidly reduced to the manganese(III) resting form of the catalyst by endogenous reductants completing a reductase type cycle. Neither SRI6 or SRI110 has appreciable reactivity toward SO as determined by the xanthine/xanthine oxidase assay [17] and both are effective in blocking acute and chronic neuropathic thus supporting the key role of peroxynitrite in these settings pain [15, 16].
Fig. 2. Therapeutic strategies targeting peroxynitrite.
Targeting peroxynitrite (PN) with superoxide dismutase mimetics (SODm), peroxynitrite decomposition catalysts (PNDCs) which also have SODm activity, or with SO-sparing PNDCs (e.g. SRI6, SRI110) may provide a promising novel therapy for pain management.
3. Roles of SO and PN in pain
Superoxide itself a powerful reactive oxygen pro-nociceptive species, will combine with nitric oxide (NO) to form PN which is also a potent pro-nociceptive nitroxidative species [18]. These discoveries were made possible by the use of SODms (e.g. SC-72325) and PNDCs (e.g. FeTM-4-PyP5+ and MnTE-2-PyP5+) as tools to dissect out the signaling pathways that these species are involved in. The direct contribution of SO and PN to the development of pain has since been demonstrated by intraplantar injection, which led to the development of hyperalgesia [19, 20]. Additionally, increased formation of PN/SO has been shown to be important to the development of thermal hyperalgesia associated with acute and chronic inflammation [20], in the development of orofacial pain [21], and in the development of opiate-induced hyperalgesia and antinociceptive tolerance [22]. Furthermore, a role for SO and PN in pain has been supported using a variety of non-selective agents such as phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL) which have shown efficacy in various pain states including inflammatory pain [23], neurogenic pain [24], neuropathic pain [24–27], and chemotherapy-induced pain [28, 29] Recent studies have shown that treatment with PBN was able to prevent the development or inhibit established paclitaxel-induced mechanical hypersensitivity [28, 29] while TEMPOL treatment was able to only prevent its development [29] leading them to believe that reactive oxygen species but not superoxide alone play a causative role in paclitaxel-induced neuropathic pain. As emphasized in several review articles [13, 30–32] non-selective agents such as TEMPOL or PBN cannot be used to delineate the contribution of a specific nitroxidative species (i.e. SO vs. PN), since these agents will remove many reactive oxygen and nitrogen species including but not limited to NO, SO, PN and hydroxyl radicals [30–32]. When using these non-selective probes it is important to keep this in mind and discuss results more generally and in terms of “nitroxidative stress and nitroxidative species for instance” rather than implicating/excluding a specific species such as SO or PN. This may otherwise lead to inappropriate conclusions and importantly misinterpretation of results.
4. Pathways leading to SO and PN formation
Understanding the enzymatic pathways that lead to the formation of SO and PN is a critical step in targeting it. When SO and NO production is increased, PN is preferentially formed as SO has a greater reactivity with NO than with mitochondrial manganese superoxide dismutase (MnSOD) [33] which normally keeps the levels of SO under control [34]. Furthermore, PN can inactivate MnSOD by nitrating Tyr-34 in a manganese-catalyzed process [35]. Thus in a feed-forward mechanism, PN-driven inactivation of MnSOD results in the overproduction SO leading to increased levels of PN [36]. Our group has shown that spinal tyrosine nitration and thus inactivation of MnSOD results in the accumulation of SO and PN during the development and maintenance of central sensitization associated with pain of several etiologies and in the development of opiate induced hyperalgesia and antinociceptive tolerance [37]. Another important source of these species is from the mitochondrial electron transport chain. Mitochondria consume about 85% of the oxygen utilized by the cell and are a major source of SO production [38]. PN indirectly promotes the reduction of oxygen to SO by increasing protein kinase C (PKC) activity (which will be further discussed in section 5.1) leading to increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity. NADPH oxidase produces SO upon activation eventually resulting in the formation of PN [39]. We have therefore proposed that post-translational nitration and inactivation of MnSOD and activation of NADPH oxidase represent two pathways that together contribute to the maintenance of central sensitization [32].
5. SO and PN in nociceptive signaling
There are many factors involved in the development of central sensitization associated with acute and chronic inflammatory and non-inflammatory neuropathic pain (Fig. 1). This includes but is not limited to modulation of protein kinases, alterations in glutamatergic neurotransmission, neuroinflammation, and modulation of ion channels. These changes may occur in the periphery, in the spinal cord, and at supraspinal sites. Although a thorough understanding of the role of SO and PN in central sensitization is not yet understood, it is apparent that all of these signaling pathways involved in central sensitization can be influenced by them. Due to limits on space, we will briefly describe these signaling pathways and refer to review articles whenever possible.
5.1 Interaction of SO and PN with protein kinases
Protein kinases play a vital role in many of the signal transduction pathways involved in the development of peripheral and central sensitization. PKC activation has been shown to be required for the development of peripheral [40] and central sensitization [41] in several pain states. The influence of PKC on the development of pain can be seen in several pathways. In the periphery, activation of PKC has been shown to depolarize unmyelinated afferent neurons, sensitize afferent neurons, enhance currents in afferent neurons, and when inhibited can block sensitization in afferent neurons [42]. In the central nervous system (CNS), PKC can be activated both in the neurons and astrocytes. Upon such stimulation as peptide neurotransmitters or glutamatergic receptor stimulation [41], PKC can be activated leading to enhanced synaptic plasticity by increasing excitability, shortening the latency to first spike, increasing spike frequency, and increasing action potential amplitude [42]. Protein Kinase A (PKA) activity has been shown to be important in the phosphorylation and activation receptors such as the N-methyl-D-aspartate receptor (NMDAR) as well as increasing COX-2 protein expression [43]. Likewise, calcium/calmodulin-dependent protein kinase II (CaMKII) activity has been shown to increase synaptic strength via modulation of receptors [43].
The modulation of these protein kinases therefore is extremely important to peripheral and central sensitization. Both SO and PN are capable of activating PKC [44–46]. PN does this either by direct nitration [45] or by stimulating proteolytic activation of PKC [46]. The evidence for SO and PN’s involvement with PKA and CAMKII is less clear-cut; however there is some indirect evidence of it. PBN, an antioxidant, reduced the levels of PKA-specific NMDA receptor phosphorylation and attenuated capsaicin-induced hyperalgesia [24]. Additionally, PN decomposition prevented prostaglandin (PG)E2-mediated thermal hyperalgesia and potential downstream PKA activity following intraplantar SO administration [20].
5.2 Effect of SO and PN on COX enzymes
Another way by which SO and PN may influence central sensitization is through modulation of the COX enzymes since it is well known that spinal prostaglandins play key roles in pain and in particular inflammatory pain [47]. The COX enzymes (COX-1 and COX-2) have long been known as targets of NO [48]. Since then it has been shown that PN plays a role in the activation of COX enzymes by modulating key amino acids residues in the polypeptide backbone [49]. Consequently, PN can increase COX-2 protein levels and stabilize its mRNA which can ultimately result in an increase in the release of prostaglandins [50]. This activation of COX enzymes by NO and PN and the ensuing release of proinflammatory and pronociceptive prostaglandins contributes to the development of peripheral sensitization during inflammation [20]. This interaction of NO and PN with the COX enzymes is more fully described in Mollace et. al. 2005 [51].
5.3 Impact of SO and PN on glutamatergic neurotransmission
One of the most important roles of PN in the development of central sensitization may be its ability to affect glutamatergic signaling via nitration of vital proteins involved in glutamatergic neurotransmission. Glutamate is a critical neurotransmitter involved in the development of inflammatory pain [52], neurogenic pain [53], neuropathic pain [54] and in the development of opioid hyperalgesia/ tolerance [55]. Glutamate is the primary endogenous ligand for NMDAR. PN can irreversibly nitrate the tyrosine residues present on NR1, a subunit of NMDAR leading to a constant activation of the receptor ultimately resulting in excitotoxicity [56]. PN can also indirectly affect NMDARs through activation of PKC leading to enhanced NR1 phosphorylation [57]. Increased neuronal activity has been linked to the development of central sensitization associated with pain of several etiologies and the inhibition of NMDAR activation has been shown to reduce neuronal activity [58].
In order to maintain proper neuronal activity, the extracellular glutamate concentration must be highly regulated to control receptor activation and to protect the neuron from excitotoxicity [59]. Glutamate transporters (GTs) located within the plasma membranes of both the neuron and the glia are responsible for removing glutamate from the synapse and extrasynaptic regions [60]. Of the five membrane subtypes that have been cloned, three in the spinal cord are considered essential to maintain proper glutamate levels: GLAST (EAAT1), GLT-1 (EAAT2), and EAAC1 [61]. The first two are found on glial cells, while the last is found on neurons. The GTs in glia are responsible for more than 90% of total glutamate transport [62]. If these transporters are nitrated, their inactivation results in increased glutamate concentration and altered synaptic transmission [63]. These transporters also play a role in the reuptake of cysteine, an important part in the biosynthesis of glutathione (GSH). GSH protects the cell from oxidative stress and depletion of it results in enhanced oxidative stress leading to neuronal degeneration [64]. Additionally, glutamate synthetase (GS), which catalyzes the conversion of glutamate and ammonia to glutamine, can be inactivated via nitration by PN [65]. Inactivation of GS has been linked to pain of various etiologies [22, 66]. Therefore, PN has the ability to alter glutamatergic signaling by various means ultimately resulting in central sensitization.
5.4 Influence of SO and PN on neuroinflammation
Neuroinflammation has been shown to be instrumental in the development and maintenance of central sensitization [67]. Glial cells (astrocytes and microglia) once activated via various substances including neurotransmitters and proinflammatory mediators [68, 69] will respond in a wide variety of changes including but not limited to alterations in morphology, increased expression of specific cell surface markers, increased secretion of certain proteins, and increased glial cell proliferation [70]. Spinal glial cells in an enhanced response state have been found to be influential in the development of pathological pain associated with a number of pain syndromes [69]. In an enhanced response state, glial cells can release pro-inflammatory cytokines (e.g. TNFα, IL-1β, and IL-6) and nitroxidative species (e.g. NO, SO, and PN), which can then sensitize the neurons in the dorsal horn leading to pain [68, 69]. These glial-derived proinflammatory mediators act not only on neurons, but also on glial cells leading to an amplification loop [71].
The proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β contribute to central sensitization by initiating several pathways including the inhibitor of κBα (IκBα), c-Jun N-terminal kinase (JNK), and p38 pathways which in turn promote proinflammatory cytokine transcription through nuclear factor κB (NFκB) and activator protein (AP-1) [72, 73].
PN has been shown to contribute to central sensitization in inflammation and chronic morphine administration by increasing the formation of glial-derived cytokines within the spinal cord [18, 22]. PN is able to activate NFκB and several mitogen-activated protein kinases (MAPKs) (e.g. p38 and ERK1/2) which are responsible for the regulation of many proinflammatory mediators and cytokines [74–76]. Additionally, PN can initiate poly (ADP-ribose) polymerase (PARP) activation[77] whose activity has been linked to MAPK activation[78] and the production of proinflammatory cytokines[79]. Administration of a SODm was able to prevent the increased formation of pro-inflammatory cytokines in spinal cord and attenuate the development of hyperalgesia [19, 22, 37]. Thus PN can contribute to central sensitization via neuroinflammation and the subsequent release of glial-derived pro-inflammatory cytokines.
5.5 Interaction of SO and PN with TRP channels
Transient receptor potential cation channel, subfamily V, member 1 (TRPV1) has been shown to be activated both by endogenous and exogenous pain stimuli [80]. These receptors have been found in the periphery and the CNS and have been shown to be required during inflammatory thermal hyperalgesia [81]. TRPV1 has been associated with increased glutamatergic signaling [82] and the facilitation of long-term potentiation [83], which are both important to central sensitization. TRPV1 activation can be achieved via phosphorylation by PKA, PKC or CAMKII [84, 85]. Modification of cysteine residues on TRPV1 results in the resistance to desensitization, the sensitization of normal receptors, and the reactivation of desensitized TRPV1 [86]. Ultimately, this may lead to persistent pain signaling in nociceptive neurons [86]. Therefore SO and PN may indirectly influence TRPV1. Furthermore, it was recently shown that activation of TRPV1 in retinal explants enhanced a biomarker of PN-mediated protein nitration, 3-nitrotyrosine [87].
5.6 Involvement of SO and PN with GABA
γ-aminobutyric acid (GABA) is involved in modulating the inhibitory synaptic transmission within the spinal cord [88]. It is well established that a disruption in the spinal GABAergic system can contribute to the development of neuropathic pain. The mechanisms behind this loss of inhibition are still not clearly understood. Interestingly a recent study demonstrated that PBN administered either systemically or intrathecally could temporarily reverse mechanical hyperalgesia by reducing spinal GABA release while not interfering with glycine transmission in a model of spinal nerve ligation [89]. This led the authors to conclude that excessive levels of reactive oxygen species within the spinal cord may then play a role in the induction of pain by interfering with spinal inhibitory transmission via the inhibition of GABA release. Additional work to tease out this relationship is therefore warranted.
5.7 Role of SO and PN in supraspinal nociceptive modulation
Supraspinal nociceptive modulation is essential to the development and maintenance of pain states [90]. The role of SO and PN in this process is unclear, however it has recently been shown that PN-mediated activity occurs in the brain during morphine antinociceptive tolerance and is prevented with PNDCs (MnTnHex-2-PyP5+ and MnTE-2-PyP5+) [91]. Additionally, intracerebroventricular injections of free radical scavengers (e.g. PBN) are able to attenuate inflammation and nerve injury-induced hypersensitivity to both noxious and non-noxious stimuli [92, 93].
The rostral ventromedial medulla (RVM) is an important region for supraspinal nociceptive modulation where nitroxidative species may functionally contribute [31]. The RVM is responsible for instigating spinal mechanisms that result in both responsiveness and non-responsiveness to neuropathic pain [94]. It contributes to the development and maintenance of central sensitization by engaging descending facilitation. Descending facilitation is a result of numerous alterations to the local microenvironment and depends upon NO (the precursor of PN) [95]. Many of the changes that occur within the RVM during descending facilitation are similar to those seen within the spinal cord during central sensitization [96–99]. These changes include protein kinase activation, enhanced glutamatergic neurotransmission, and neuroimmune activation which are all influenced by SO and PN in the spinal cord. This led us to our hypothesis and current line of study which suggests that nitroxidative species within the RVM contribute to central sensitization [100, 101]. Additionally, support exists for a specific supraspinal locus that modulates nociception during oxidative stress in that reactive oxygen species in the amygdala activate the type 1 metabotropic glutamate receptor to enhance nociception [102, 103].
6. Future directions
We are currently limited by the lack of compounds that can selectively target SO or PN. To further our understanding of the contribution of SO and PN in pain, it is necessary that better pharmacological tools be made available. The metalloporphyrin-based PN scavengers are very promising candidates in strategies that target PN and SO in a standalone mode or that work in combination with other analgesics (Fig. 2). Since metalloporphyrin systems have evolved in nature to be encased in protein (e.g. the cytochromes), small molecule porphyrin-based PNDCs will require peripheral synthetic modification to impart human pharmaceutical properties (e.g. membrane solubility, reduced charge, reduced non-target binding, reduced toxicity, optimal pharmacokinetics, etc). As the metal center in these systems is the site for antioxidant action, the periphery of the porphyrin macrocycle and other catalyst ligand systems are wide-open for synthetic manipulations aimed to control in vivo performance without negative perturbation of the catalytic apparatus. As we have seen with SRI6 and SRI110, not only can we enhance drug-like properties through a medicinal chemistry approach, but we can also engineer unique modes of catalytic activity (e.g. selectivity for PN removal over SO). We believe that through further combinations of medicinal chemistry and catalysis chemistry explorations of the ligand periphery, true drug candidates with tuned selectivities can be engineered. We have also recently explored the role of PN in chemotherapy-induced peripheral neuropathy (CIPN). Peripheral neuropathy is the most common treatment-limiting complication in cancer patients receiving several first-line chemotherapeutics including paclitaxel, oxaliplatin, and bortezomib [104]. CIPN severely limits the usefulness of these drugs and seriously hampers the ability to treat cancer effectively. We have recently observed that treatment with a PNDC is able to both prevent and reverse the development of CIPN regardless of the mechanism of action of the chemotherapeutic without interfering with its antitumor effects [105, 106]. These findings could potentially save countless lives, as it would allow for chemotherapeutics to be used at more efficacious dosages.
7. Conclusions
It is eminently clear that there is a void in current analgesic therapeutic treatments. As more of the roles of SO and PN in the development and maintenance of pain are unveiled, targeted therapeutic strategies (e.g. the use of a selective PNDC to reduce PN levels) will become more attractive for the long-term treatment of pain. It is also of interest to note that PNDCs are able to synergize with other analgesics including non-selective COX-1/COX-2 inhibitors, selective COX-2 inhibitors [20], and opiates (Salvemini, unpublished observations). This would allow for these drugs to be used at lower dosages, increasing their efficacy and reducing the risk of intolerable side effects. Thus whether used alone or in combination with other analgesics, the potential impact these agents could have both to individuals and society would be indescribable.
Highlights.
A clear need exists for new, efficacious analgesics that lack risky side effects.
Superoxide and peroxynitrite are key players in the development/maintenance of pain.
Strategies targeting these species provide a promising therapy for pain management.
Acknowledgements
Supported by NIH/NIDA R01 DA024074 and NIH/NIAMS RC1 AR05823.
Abbreviations
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
- AP-1
Activator protein 1
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- JNK
c-Jun N-terminal kinase
- CNS
Central Nervous System
- CIPN
Chemotherapy-induced peripheral neuropathy
- COX
Cyclooxygenase
- EAAC
Excitatory amino acid channel
- EAAT
Excitatory amino acid transporter
- ERK
Extracellular signal-regulated kinases
- FeTM-4-PyP5+
Fe(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachlorideporphyrin
- GABA
γ-Aminobutyric acid
- GT
Glutamate transporters
- GLAST
Glutamate-aspartate transporter
- GLT-1
Glutamate transporter 1
- GS
Glutamine synthetase
- GSH
Glutathione
- IκBα
Inhibitor of κBα
- IL
Interleukin
- MnSOD
Manganese Superoxide Dismutase
- MAPK
Mitogen-activated protein kinase
- MnTE-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- NO
Nitric Oxide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NMDAR
N-methyl-D-aspartate receptor
- NSAIDs
Non-steroidal anti-inflammatory drugs
- NFκB
Nuclear factor κB
- PN, ONOO−
Peroxynitrite
- PNDCs
Peroxynitrite-decomposition catalysts
- PBN
Phenyl N-tert-butylnitrone
- PARP
Poly (ADP-ribose) polymerase
- PG
Prostaglandin
- PKA
Protein kinase A
- PKC
Protein kinase C
- RVM
Rostral ventromedial medulla
- SO, O2·−
Superoxide
- SODms
Superoxide dismutase mimetics
- TRPV1
Transient receptor potential cation channel, subfamily V, member 1
- TNF
Tumor necrosis factor
- Tyr
Tyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Melnikova I. Pain market. Nat Rev Drug Discov. 2010;9:589–590. doi: 10.1038/nrd3226. [DOI] [PubMed] [Google Scholar]
- 2.Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- 4.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. The Journal of clinical investigation. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 6.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 7.Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci U S A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11:204–214. [PubMed] [Google Scholar]
- 9.Batinic-Haberle I, Reboucas JS, Spasojevich I. Superoxide Dismutase Mimics: Chemistry, Pharmacology and Therapeutic Potential. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. American Journal of Physiology - Endocrinology And Metabolism. 2007;293:E1645–E1655. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 11.Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrew SK Rausaria, Salvemini Daniela, Neumann William L. Metal-charge-shielded manganese porphyrins are potent orally active peroxynitrite decomposition catalysts. Abstracts of papers, 241st ACS National Meeting & Exposition; March 27–31,2011; Anaheim, CA. 2011. United States, MEDI-53. [Google Scholar]
- 13.Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13:877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 15.Rausaria S, Ghaffari MME, Kamadulski A, Rodgers K, Bryant L, Chen Z, Doyle T, Shaw MJ, Salvemini D, Neumann WL. Retooling Manganese(III) Porphyrin-Based Peroxynitrite Decomposition Catalysts for Selectivity and Oral Activity: A Potential New Strategy for Treating Chronic Pain. Journal of Medicinal Chemistry. 2011 doi: 10.1021/jm201233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rausaria S, Kamadulski A, Rath NP, Bryant L, Chen Z, Salvemini D, Neumann WL. Manganese(III) complexes of bis(hydroxyphenyl)dipyrromethenes are potent orally active peroxynitrite scavengers. J Am Chem Soc. 2011;133:4200–4203. doi: 10.1021/ja110427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radi RA, Rubbo H, Prodanov E. Comparison of the effects of superoxide dismutase and cytochrome c on luminol chemiluminescence produced by xanthine oxidase-catalyzed reactions. Biochim Biophys Acta. 1989;994:89–93. doi: 10.1016/0167-4838(89)90066-6. [DOI] [PubMed] [Google Scholar]
- 18.Salvemini D, Neumann W. Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management. Life Sci. 2010;86:604–614. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 20.Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- 21.Yeo JF, Ling SF, Tang N, Ong WY. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Experimental brain research. Experimentelle Hirnforschung. 2008;184:435–438. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]
- 22.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. The Journal of clinical investigation. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 26.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. NeuroReport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kim HK, Zhang YP, Gwak YS, Abdi S. Phenyl N-tert-butylnitrone, a Free Radical Scavenger, Reduces Mechanical Allodynia in Chemotherapy-induced Neuropathic Pain in Rats. Anesthesiology. 2010;112:432–439. doi: 10.1097/ALN.0b013e3181ca31bd. 410.1097/ALN.1090b1013e3181ca1031bd. [DOI] [PubMed] [Google Scholar]
- 29.Fidanboylu M, Griffiths LA, Flatters SJL. Global Inhibition of Reactive Oxygen Species (ROS) Inhibits Paclitaxel-Induced Painful Peripheral Neuropathy. PLoS One. 2011;6:e25212. doi: 10.1371/journal.pone.0025212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids. 2010 doi: 10.1007/s00726-010-0633-0. [DOI] [PubMed] [Google Scholar]
- 32.Salvemini D, Little JW, Doyle T, Neumann WL. Roles of reactive oxygen and nitrogen species in pain. Free Radical Biology and Medicine. 2011;51:951–966. doi: 10.1016/j.freeradbiomed.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 34.Huie RE, Padmaja S. The Reaction of no With Superoxide. Free Radical Research. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 35.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 37.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 38.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 39.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 40.Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Velazquez KT, Mohammad H, Sweitzer SM. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol Res. 2007;55:578–589. doi: 10.1016/j.phrs.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hongpaisan J, Winters CA, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J Neurosci. 2004;24:10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon-RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- 46.Chakraborti T, Das S, Chakraborti S. Proteolytic activation of protein kinase Calpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein. Biochemistry. 2005;44:5246–5257. doi: 10.1021/bi0477889. [DOI] [PubMed] [Google Scholar]
- 47.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney international. 2007;71:290–297. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- 48.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markey CM, Alward A, Weller PE, Marnett LJ. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987;262:6266–6279. [PubMed] [Google Scholar]
- 50.Trostchansky A, O'Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R, Rubbo H. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free radical biology & medicine. 2007;42:1029–1038. doi: 10.1016/j.freeradbiomed.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 51.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Wang P, Zou X, Li D, Fang L, Gong K, Lin Q. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol. 2010;222:93–107. doi: 10.1016/j.expneurol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain. 1994;56:69–75. doi: 10.1016/0304-3959(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 56.Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 57.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 58.Le Guen S, Catheline G, Besson JM. Effects of NMDA receptor antagonists on morphine tolerance: a c-Fos study in the lumbar spinal cord of the rat. Eur J Pharmacol. 1999;373:1–11. doi: 10.1016/s0014-2999(99)00272-1. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 60.Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol. 2004;189:222–230. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 61.Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 63.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochemical pharmacology. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 65.Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain. 2010;149:100–106. doi: 10.1016/j.pain.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 68.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature reviews. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradesi S, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Effect of ovarian hormones on intestinal mast cell reactivity to substance P. Life Sci. 2001;68:1047–1056. doi: 10.1016/s0024-3205(00)01008-0. [DOI] [PubMed] [Google Scholar]
- 72.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends in pharmacological sciences. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. Journal of Neurochemistry. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 75.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the "bad guys": implications for improving clinical pain control and the clinical utility of opioids. Brain, behavior, and immunity. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masini E, Bani D, Vannacci A, Pierpaoli S, Mannaioni PF, Comhair SA, Xu W, Muscoli C, Erzurum SC, Salvemini D. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free radical biology & medicine. 2005;39:520–531. doi: 10.1016/j.freeradbiomed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Pacher P, Szabo C. Role of the Peroxynitrite-Poly(ADP-Ribose) Polymerase Pathway in Human Disease. The American journal of pathology. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veres B, Radnai B, Gallyas F, Varbiro G, Berente Z, Osz E, Sumegi B. Regulation of Kinase Cascades and Transcription Factors by a Poly(ADP-Ribose) Polymerase-1 Inhibitor, 4-Hydroxyquinazoline, in Lipopolysaccharide-Induced Inflammation in Mice. Journal of Pharmacology and Experimental Therapeutics. 2004;310:247–255. doi: 10.1124/jpet.104.065151. [DOI] [PubMed] [Google Scholar]
- 79.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proceedings of the National Academy of Sciences. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 81.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 82.Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, Maione S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- 83.Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64:286–292. doi: 10.1016/j.biopsych.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 84.Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 85.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 86.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leonelli M, Martins DO, Britto LR. TRPV1 receptors are involved in protein nitration and Muller cell reaction in the acutely axotomized rat retina. Exp Eye Res. 2010;91:755–768. doi: 10.1016/j.exer.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 88.Yoshimura M, Nishi S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurones in the rat spinal cord in vitro. Journal of Physiology. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–852. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 91.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 94.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011 doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 96.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 98.Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 99.Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–241. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Little Z JWC, Doyle T, Salvemini D. Supraspinal descending facilitation of central sensitization depends on peroxynitrite regulation of opioid signaling. Journal of Pain. 2011;12(S2):P36. [Google Scholar]
- 101.Little Z JWC, Doyle T, Salvemini D. Superoxide derived peroxynitrite is essential to supraspinal descending facilitation of nociception. FASEB J. 2011;25:674.672. [Google Scholar]
- 102.Li Z, Ji G, Neugebauer V. Mitochondrial Reactive Oxygen Species Are Activated by mGluR5 through IP3 and Activate ERK and PKA to Increase Excitability of Amygdala Neurons and Pain Behavior. The Journal of Neuroscience. 2011;31:1114–1127. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ji G, Neugebauer V. Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons. J Neurophysiol. 2010;104:218–229. doi: 10.1152/jn.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bennett GJ. Pathophysiology and Animal Models of Cancer-Related Painful Peripheral Neuropathy. The Oncologist. 2010;15:9–12. doi: 10.1634/theoncologist.2009-S503. [DOI] [PubMed] [Google Scholar]
- 105.Leesa KB Janes, Salvemini Daniela. Neuroscience. Washington DC: United States; 2011. [November 12–16, 2011]. Scavenging peroxynitrite blocks the development of paclitaxel, oxaliplatin and bortezomib-induced neuropathic pain, Abstract. [Google Scholar]
- 106.Doyle Z TC, Salvemini D. Peroxynitrite decomposition catalysts block paclitaxel-induced neuropathic pain: microarray analysis of spinal cord gene expression. Journal of Pain. 2011;12(S2):37. [Google Scholar]