Abstract
Background
The involvement of complement system in brain injury has been scarcely investigated. Here we document the pivotal role of mannose binding lectin (MBL), one of the recognition molecules of the lectin complement pathway, in brain ischemic injury.
Methods and Results
Focal cerebral ischemia was induced in mice (by permanent or transient middle cerebral artery occlusion) and rats (by 3-vessels occlusion). We first observed that MBL is deposited on ischemic vessels up to 48h after injury and that functional MBL/MASP2 complexes are increased. Next we demonstrated that: 1) MBL−/− mice are protected from both transient and permanent ischemic injury; 2) Polyman2, the newly synthesized mannosylated molecule selected for its binding to MBL, improves neurological deficits and infarct volume when given up to 24h after ischemia in mice; 3) anti-MBL-A antibody improves neurological deficits and infarct volume when given up to 18h after ischemia, as assessed following 28d in rats.
Conclusions
Our data show an important role for MBL in the pathogenesis of brain ischemic injury and provide a strong support to the concept that MBL inhibition may be a relevant therapeutic target in humans, one with a wide therapeutic window of application.
Keywords: cerebral ischemia, endothelium, inflammation, stroke, complement system
Stroke is a leading cause of death and permanent disability worldwide. Despite recent substantial progress in prevention and management, stroke still remains a large unmet medical need. Ischemic stroke, that includes 87% of all strokes and is caused by thrombotic or embolic occlusions, occurs when the blood supply to part of the brain is restricted, leading to energy deficit. In areas with the lowest residual flow, cells die rapidly, while in those with less severe ischemia that surround the lesion core, cells may remain viable for hours/days and represent possible therapeutic targets1. Presently, the only treatment available for stroke is thrombolysis with tissue plasminogen activator (tPA). The narrow 3h therapeutic window imposed by safety concerns (only recently extended to 4.5h2), allows less than 5% of patients eligible for tPA treatment3,4. Thus the inadequacy of available therapeutic strategies demands the identification of novel ameliorative treatments endowed with a wide therapeutic window.
Several pathogenetic mechanisms contribute to ischemic injury. Among them, the inflammatory response, after being rapidly triggered, progresses over hours to days, making it an attractive target for therapeutic intervention. The complement system is a powerful arm of inflammation. It becomes rapidly activated after injury and acts as a trigger for several aspects of the inflammatory response. Complement is a proteolytic cascade of several circulating and cell-associated proteins that contribute to the evolution of injury by several potential mechanisms including inflammatory molecule synthesis, recruitment of cells to the site of injury, activation of phagocytosis, induction of endothelial damage and increased vascular permeability, and by directly inducing cell death. Depending on the trigger, complement activation may proceed through three separate pathways, namely the classical, lectin and alternative pathways5. Both animal models and clinical observations indicate that the complement system is one of the mechanisms contributing to ischemic injury6–8.
C1-INH, an endogenous inhibitor of the complement system endowed with several anti-inflammatory effects, acts as a potent neuroprotective agent against acute brain injury9–13. We showed previously that recombinant C1-INH markedly reduced cerebral damage when administered up to 18h after ischemia in mice12. Our data further suggested that the remarkable protective action was due to its binding to mannose binding lectin (MBL), one of the recognition molecules responsible for the first step of complement activation of the lectin pathway14. MBL is a serum protein that acts as a circulating pattern recognition receptor. MBL targets include pathogen-associated molecular patterns (PAMPs), i.e. carbohydrates exposed on the surface of a pathogen but also “altered self” or damage-associated molecular patterns (DAMPs), i.e. dying or damaged cells through recognition of changes in glycosylation pattern on the cell surface. Binding of MBL to its targets leads to complement activation14.
Our previous data prompted us to hypothesize that MBL plays a pathogenetic role in the ischemic injury and that compounds that bind and inhibit MBL may represent a promising therapeutic modality for stroke. Here we document the pivotal role of MBL in cerebral ischemic injury by using different experimental models of focal ischemia. Since the degree of reperfusion is variable in the clinical setting1,15, we used both transient and permanent middle cerebral artery occlusion (tMCAo and pMCAo, respectively) in mice9,10,12. Additionally, an inhibitory monoclonal antibody against rat MBL-A12,16 was also used in a reversible 3-vessel occlusion model (3-vo) in rats17.
Methods
Animals
Male C57Bl/6 (26–28 g, Harlan, Italy, and Taconic Farms for the complement hemolytic assay) and C57Bl/6 with target mutation of both MBL-A and MBL-C genes (MBL−/−, 26–28 g, Jackson Laboratories,) mice, Crl:CD (SD)BR (250–280 g, 7–8 weeks, Charles River, Calco, Italy) rats were used. Additional information in Supplemental Methods.
Drugs
Anti-rat MBL-A monoclonal antibodies (clones P2D5 and 14C, 1 mg/kg) were diluted in PBS and injected intravenously (iv)16,18.
Polyman2 was dissolved in PBS at a concentration of 450 (or 900) μM. One hundred microliters of this solution were injected iv in order to obtain circulating levels corresponding to 30 (or 60) μM, corresponding to 142 (or 284) μg/mouse, which represented the best binding concentration to MBL in SPR experiments. Additional information on Polyman2 toxicity in Supplemental Methods.
Focal ischemia in mice: transient and permanent middle cerebral artery occlusion
Surgery
Transient ischemia was achieved by middle cerebral artery occlusion (tMCAo) by means of a siliconized filament (7-0, Doccol Corporation) introduced into the internal carotid artery and advanced to block the MCA for 30 min as described previously9,10,19. Surgery-associated mortality rate was 8%. For permanent ischemia (pMCAo) MCA was permanently occluded by electrocoagulation20,21. Mortality rate for this model was 8.5%. In both ischemia models, sham-operated (sham) mice received identical anaesthesia and surgery without artery occlusion.
Neurological deficits
Forty-eight hours after tMCAo each mouse was rated on neurologic function scales unique to the mouse. Scores range from 0 (healthy) to 56 (the worst performance in all categories) and represent the sum of the results of general and focal deficits (13 categories). Results are expressed as composite neurological score.
Quantification of infarct size and edema
Infarct volumes were calculated on twenty-micron coronal brain cryosections stained with cresyl violet by the integration of infarcted areas after correction for the percentage of brain swelling due to edema. Edema was determined by subtracting the area of the ipsilateral from that of the contralateral hemisphere.
Additional information on surgery, neurological deficits and quantification of infarct size and edema in Supplemental Methods.
Focal ischemia in rats: three vessel occlusion (3-vo)
Surgery was performed as described previously17,22. The right common carotid artery (CCA) and the MCA were occluded. Subsequently, to produce a lesion in the MCA region, the contralateral CCA was occluded for 1h using traction with fine forceps. This procedure induces a blood flow drop in the MCA territory. Reperfusion was induced by forceps release. Sham rats were operated the same way but the MCA and CCAs were not occluded. Surgery-associated mortality rate was 7%.
Neurological deficits
Postural reflex was assessed by Bederson test, sensorimotor integration by De Ryck’s limb-placing test, ability to integrate motor responses by the foot fault test as previously described17.
Quantification of infarct size
Injury was quantified on 14 serial 1-mm thick sections stained with triphenyltetrazolium chloride (TTC, Sigma-Aldrich)17. Alternatively, 4 weeks after ischemia, ischemic volume was evaluated by structural Magnetic Resonance Imaging (MRI) analysis (see below).
Additional information on surgery, neurological deficits and quantification of infarct size in Supplemental Methods.
MRI measurements
Imaging was performed on a 7T small bore animal Scanner (Bruker Biospec). The morphological images were obtained with a RARE T2-weighted sequence which covered the whole rat brain volume.
Volumetric measurements
The volume measurements of structural MRI images were obtained manually using custom made software as previously described23. For each animal, the regions of interest (ROIs) were manually chosen and drawn on the images for volumetric assessments. We computed the lesion volume including the whole infarcted tissue (T2w hyperintense tissue indicative of edema24) across different brain areas. This measurement did not include the ventricle volume. Measurements of brain atrophy in cortex, hippocampus and striatum (G. Paxinos and C. Watson, The rat brain in stereotaxic coordinates, 5th ed., Academic Press, New York, 2005) included residual T2w hypointense tissue. Data from each animal were obtained by the integration of averaged ROI area for slice thickness. Additional information in Supplemental Methods.
Immunofluorescence and confocal analysis
Twenty-micron thick coronal sections were incubated with the primary monoclonal antibodies anti-mouse MBL-A or MBL-C (1:100, Hycult Biotechnology). Alexa546 fluoro-conjugated goat-anti-rat IgG (1:500, Molecular Probes) was used as a secondary antibody. Brain vessels and nuclei were stained with Alexa488 fluoro-conjugated isolectin (IB4, 1:200, Invitrogen) and 4′-6-diamidino-2-phenylindole (DAPI, 1μg/ml, Invitrogen) respectively. Images were acquired by confocal microscopy as described previously21. Three-dimensional images were acquired over a 10–12 μm z-axis with a 0.23 μm step size and processed using Imaris software (Bitplane) and Photoshop CS2 (Adobe Systems Europe Ltd).
Semiquantitative evaluation of MBL deposition on blood vessels was performed at each time point12. Additional information in Supplemental Methods.
Functional MBL/MASP-2 assay
Blood samples were collected from the vena cava in 10mM EDTA and 0.125% polybrene (Sigma-Aldrich). Functional MBL/MASP-2 complexes were measured in plasma by ELISA (Hycult Biotechnology)12.
Western blot
Equal amounts of plasma proteins (10 μg/sample) were electrophoresed on a 5% SDS polyacrylamide gel and transferred to PVDF membranes. Rabbit anti-C3 polyclonal antibody (1:100) followed by rabbit peroxidase-conjugated antibody (1:2500; both Santa Cruz Biotechnology) were used. Results were standardized by using the total protein loaded. Additional information in Supplemental Methods.
Surface plasmon resonance (SPR)
SPR binding studies were carried out using the ProteOn XPR36 system (Bio-Rad), as previously described12. Briefly, polymannosylated dendrimers were flowed onto rhMBL covalently immobilized on the sensor chip. The resulting sensorgrams were fitted by the simplest 1:1 interaction model to obtain the binding constants. Additional information in Supplemental Methods.
Complement hemolytic assay
Mice were divided in the following groups (n=3/group): 1) WT + saline (vehicle control) and 2) WT + Polyman2. Under isoflurane anesthesia, 50 μl of normal saline (control) or 50 μl saline containing 142 μg Polyman2 was injected intravenously. After 1h, blood was collected via heparinized syringes and serum prepared. The CH50 assay was performed as we have previously described but with modifications25. Additional information in Supplemental Methods.
Blinding and Exclusion Criteria
In each and every experiment, animals (including C57Bl/6 and MBL−/− mice and Crl:CD(SD)BR rats) were allocated at random to surgery and treatment group taking care of distributing them equally across experimental days and batches to avoid systematic errors. All the experimental procedures including surgery, drug treatment, behavioural tests, immunohistochemistry, quantification of infarct size, MRI analysis and biochemical assays were performed by investigators blinded to the experimental conditions. Animals, in which the intravenous administration of drug or vehicle was not successful, were excluded from the study (mice: 13/86 and rats: 2/77).
Statistics
Data are expressed as mean+standard deviation (SD) or as scatter dot plots and mean (bars). GraphPad Prism 5 software was used for statistical analysis. All the data have been checked for normal distribution by Kolmogorov-Smirnov test and for the constancy of variances by Bartlett test, in case of more than two groups, or by F test, in case of two groups. Kruskal-Wallis test has been used when data were not distributed normally. T-test with Welch’s correction has been used for normally distributed data with unequal variances. Additional information on the tests used are reported in legend to figures and in Supplemental Methods.
Results
The lectin pathway in brain ischemia
We first investigated whether MBL could be detected in the ischemic brain. We assessed the presence of MBL-A and -C, the two isoforms present in mice26, at 30 min and 6, 12, 24, 48 h following tMCAo or pMCAo (Fig. 1). MBL was detected in tMCAo mice starting from 6h, and in pMCAo mice from 30 min. In both models the strongest signal was observed at 12–24h, and persisted up to 48h after ischemia (Fig. 1e,f). Both MBL-A and -C were present on ipsilateral vessels of ischemic mice, and deposited on the luminal side of the vessels after tMCAo (Fig. 1a,c) or pMCAo (Fig. 1b,d), as evidenced by single plane (Fig. 1a′-d′) and three-dimensional images (Fig. 1a″-d″). Coronal sections of vessels were obtained by clipping the three-dimensional renderings (Fig. 1a‴-d‴) and further confirmed the intraluminal deposition of both MBL isoforms. Sham-operated mice showed only occasional, transient deposition of MBL 30 min after sham injury (supplemental Fig. 1). No MBL signal was detected at longer time points in sham mice nor in the contralateral side of the ischemic animals (data not shown).
Figure 1.
MBL is deposited on ischemic vessels after tMCAo and pMCAo. Representative images of staining for MBL-A or MBL-C (red), vessels (IB4, green) and nuclei (Hoechst, blue) in the ipsilateral cortex 12h after tMCAo (a,c) or pMCAo (b,d). After tMCAo, both MBL-A (a) and MBL-C (c) show deposition on ischemic vessels. Single xy plane views with z projections of the white boxes in a and c confirm that both isoforms were located in the luminal space of vessels (a′, c′). 3D renderings (a″, c″) and clipped volumes (a‴, c‴: clipping in correspondence of the yellow planes) further demonstrate intraluminal deposition of MBL-A and MBL-C. After pMCAo MBL-A and MBL-C show similar intraluminal deposition by fluorescence (b, b′, d, d′), 3D renderings (b″, d″) and clipped volumes (b‴, d‴). Confocal analysis, scale bar 20μm (a, b, c, d) and 5μm (a′, b′, c′, d′). Semiquantitative evaluation of MBL-A and MBL-C staining in ipsilateral cortices at different time points after tMCAo (e) or pMCAo (f), n=3. Scores were assigned blindly as follows: − = no positivity, + = low positivity, ++ = intermediate positivity, +++ = high positivity12.
We next assessed the presence of circulating MBL/MASP-2 functional complexes, as index of lectin pathway activation. We previously demonstrated that tMCAo induced a significant increase in MBL/MASP-2 complexes compared to sham-operated mice12. In the present study, we found that both 30 min and 24h after pMCAo, ischemic mice had a significant increase (up to 26.6% and 31.9% respectively) in circulating functional MBL/MASP-2 complexes compared to sham-operated mice (Fig. 2a,b), indicating an early and persistent activation of the lectin complement pathway following pMCAo. Notably, 24h after pMCAo, ischemic mice had a significant increase in circulating C3 fragments compared to sham-operated mice (up to 64.5%; Fig. 2c) indicating a long lasting activation of the complement system.
Figure 2.
The lectin pathway is involved in pMCAo injury. Functional MBL/MASP-2 complexes in mice plasma samples collected 30min (a) or 24h (b) after sham surgery or pMCAo. C3 complement activation fragments in plasma samples collected 24h after sham surgery or pMCAo (c). Infarct volume assessed 48h after pMCAo in WT and MBL−/− mice (d). Data are reported as scatter dot plots and mean (bars), n=8–10 mice per group, unpaired t-test; *P<0.05,**P<0.01,***P<0.001.
Susceptibility of MBL−/− mice to MCAo injury
To further establish the relevance of MBL after ischemic injury, we then assessed the susceptibility of MBL−/− mice to ischemia. Firstly we assessed cerebral blood flow perfusion rates by laser Doppler flowmetry (Perimed Italy) and found that in MBL−/− they were not different from wild type mice (supplemental Fig. 2). Similarly to what was observed after tMCAo (27,28 and supplemental Fig. 2), MBL−/− mice subjected to pMCAo had smaller lesions compared to wild type mice, corresponding to a 48% decrease of infarct volume (Fig. 2d).
Surface Plasmon Resonance analysis of mannosylated molecules
We then sought to identify a synthetic MBL ligand directed against the mannose recognition binding site (carbohydrate recognition domain, CRD) to be used as a possible therapeutic tool in ischemic injury. Different polymannosylated dendrimers, including Polyman1 and Polyman229, as well as the new molecules Polyman6, Polyman5, Polyman8, Polyman17, Polyman12, Polyman14 (Ribeiro R., Varga N., Sutkeviciute I., Fieschi F., Bernardi A., Rojo J., unpublished data), were compared for their ability to bind MBL by surface plasmon resonance (SPR) assay (Fig. 3a and supplemental Fig. 3). These studies highlighted the importance of both the number of pseudo-mannosides present on the dendrimeric scaffold (supplemental Fig. 3) and the structure of the scaffold (e.g. Polyman2 vs Polyman17 in Fig. 3a), and indicated Polyman2 as the best MBL ligand, with an affinity of 6 μM (Fig. 3b).
Figure 3.
Binding of mannosylated dendrimers to MBL evaluated by SPR. MBL was immobilized on the surface of sensor chip whereas the dendrimers were injected for 3 min at a flow rate of 100 μl/min. Left panel (a) shows the maximal binding (in Resonance Units, RU) obtained when injecting 100 μM of dendrimers with different structures. The data shown are from a single experimental session but the same rank order was obtained in independent injections, using different concentrations or different flow rates. No binding was observed on a parallel surface immobilizing BSA. Right panel (b) shows the sensorgrams obtained injecting four different concentrations of Polyman2 (black lines), together with the corresponding fittings (white lines). The analysis of these sensorgrams allowed estimation of the binding constants shown below.
Effect of the mannosylated molecule Polyman2 in MCAo injury
Polyman2 was therefore selected for the in vivo studies, and administered iv to tMCAo mice, at doses that reached plasma concentrations of 30 or 60μM (142 or 284 μg/mouse, respectively) to mimic the concentrations that were functionally active in SPR experiments. Both doses, given at reperfusion (30 min from the beginning of ischemia), induced a marked reduction in ischemic volume by 33.2% and 34.2% compared to vehicle-treated mice (supplemental Fig. 4). Thus all subsequent experiments were performed using 30μM Polyman2.
First we demonstrated that Polyman2 did not induce complement depletion in vivo. Plasma from vehicle or Polyman2 treated mice restored C5 activity to human C5-depleted sera to the same extent over a wide range of plasma concentrations (Fig. 4a). Next, to explore the therapeutic window against ischemic injury, Polyman2 or vehicle was infused iv once at different time points following tMCAo (3, 6, 12, 18, 24 or 30h, from the beginning of ischemia). Neurological deficits, determined 48h after tMCAo, indicated that Polyman2 significantly reduced ischemic functional deficits when given up to 30h after the ischemic insult compared to vehicle-treated mice (Fig. 4b) and reduced the ischemic volume by 46.4% when given 24h after ischemia (Fig. 4c). Notably, in absence of any treatment, the ischemic lesion increases by 55% from 24 to 48h (36.8±13.2 vs 56.2±16.2, n=12). The strongest protective effect (68.3% reduction) was observed when Polyman2 was given 6h following tMCAo (Fig. 4c). At this time point the maximal effect of Polyman2 on reduction of brain edema was also observed (supplemental Fig. 5).
Figure 4.
Polyman2 protects ischemic mice from tMCAo injury with a wide therapeutic window. Haemolytic activity of Polyman2 assayed 1h after iv administration to non ischemic mice. Data are the mean of 3 mice per group and each plasma sample was an average of 3 determinations (a). Composite neurological score (b) and infarct volume (c) assessed 48h from tMCAo in mice receiving a single iv administration of vehicle (n=17, administered at each time point and gathered in a single control group, details in supplemental Fig. 7a) or Polyman2, given 3, 6, 12, 18, 24 or 30h from injury, (n=7, 6, 8, 8, 8 and 5, respectively). Representative images of MBL-A staining in the ipsilateral cortex 24h after tMCAo in a typical vehicle (left panel) or Polyman2 (post-ischemia treatment time: 6h, right) treated mouse. Nuclei, vessels and MBL stainings in blue, green and red, respectively, n=3 (d). C3 complement activation fragments in plasma samples assessed 48h from surgery in sham or tMCAo mice (e, n=8–10 per group) or in tMCAo mice treated with vehicle, Polyman2, 3 or 6h after injury (f, n=8–10 per group). Composite neurological score (g) and infarct volume (h) assessed 48h from tMCAo in MBL−/− mice receiving vehicle or Poyman2, given 6h from injury, (n=7 per group). Data are expressed as mean+SD (b,c) and as scatter dot plots and mean (bars) (e, f, g, h). One-way ANOVA followed by Dunnett post-hoc test; *P<0.05,**P<0.01,***P<0.001 versus vehicle (b, c, f) and unpaired t-test ****P<0.0001 (e).
In Polyman2-treated mice the ischemia-induced MBL deposition on brain endothelium was effectively dampened at 24h (Fig. 4d) and circulating C3 fragments were decreased 48h after injury (Fig. 4e and f). Importantly, Polyman2 given 6h after injury to MBL−/− mice was not able to affect either the functional or the anatomical damage showing the specificity of the protective effect (Fig. 4g and h).
Mice receiving Polyman2 treatment 3 or 6h after pMCAo did not show a significant decrease of the lesion size nor reduction of MBL deposition or of C3 fragmentation (supplemental Fig. 6).
Effect of the anti-MBL-A antibody administration
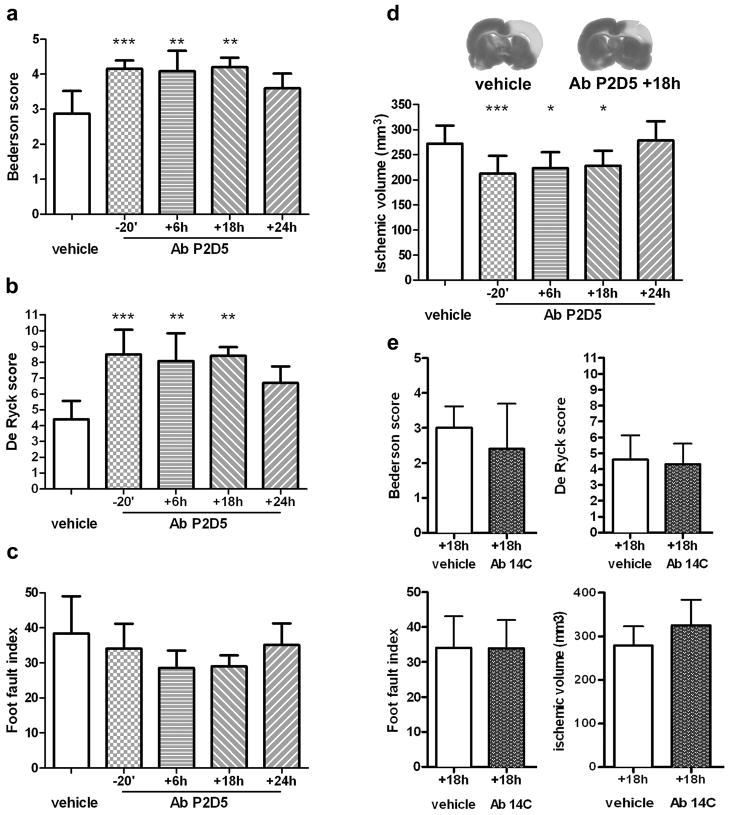
Since no functionally inhibitory antibody against mouse MBL is available, we then assessed the effect of an anti-MBL monoclonal antibody directed against rat MBL-A (mAb P2D5 clone16), the most abundant MBL isoform in rats26. P2D5 has potent neutralizing properties against MBL-A, but does not recognize MBL-C16. This clone (1mg/Kg16) or the vehicle were administered iv 20 min before ischemia or 6, 18 or 24h in rats subjected to reversible 3-vo22. Neurological deficits and ischemic volume were determined 48h after injury. Both postural-reflex deficits (Bederson’s test, Fig. 5a) and sensorimotor deficits (De Ryck’s test, Fig. 5b) were significantly improved in all mAb-treated rats, even those receiving the antibody 18h after injury. The ability to integrate motor responses (foot-fault test, Fig. 5c) was also improved in rats treated up to 18h after injury, but it did not reach statistical significance. Evaluation of anatomical injury demonstrated that rats treated with the mAb up to 18h after ischemia had a significant reduction in ischemic volume (post-treatment at 18h: 19% reduction, Fig. 5d).
Figure 5.
Anti-MBL-A antibody selectively protects against functional and histopathological consequences of ischemia with a wide therapeutic window. Effect of anti-MBL-A mAb treatment (clone P2D5, 1 mg/Kg, iv) on neurological deficits and infarct volume assessed 48h after injury in rats receiving a single administration of vehicle (n=12, administered at each time point and gathered in a single control group, details in supplemental Fig. 7b) or mAb 20 min before, or 6, 18, 24h (n= 10, 6, 6 and 6, respectively) after 3-vo: postural-reflex deficit, (a, Bederson’s test, normal score =5); sensorimotor deficit (b, De Ryck’s test, normal score=16); integration of motor responses (c, foot-fault test, normal score=2); anatomical damage (d, infarct volume). The administration of an isotype control mAb (clone 14C, 1 mg/Kg iv) did not affect any of these parameters (e, n=10–11 per group). Data are expressed as mean+SD. Kruskal-Wallis test followed by Dunn’s test (a,b,c), one-way ANOVA followed by Dunnett post-hoc test (d) and unpaired t-test (e);*P<0.05,**P<0.01,***P<0.001 versus vehicle.
We also assessed the specificity of the protective effect elicited by P2D5 by administering mAb 14C that binds rat MBL-A but is not functionally inhibitory 18 (isotype control antibody, Fig. 5e). When injected 18h after 3vo, mAb 14C did not ameliorate any of the functional deficits or anatomical damage compared to vehicle-treated rats (Fig. 5e).
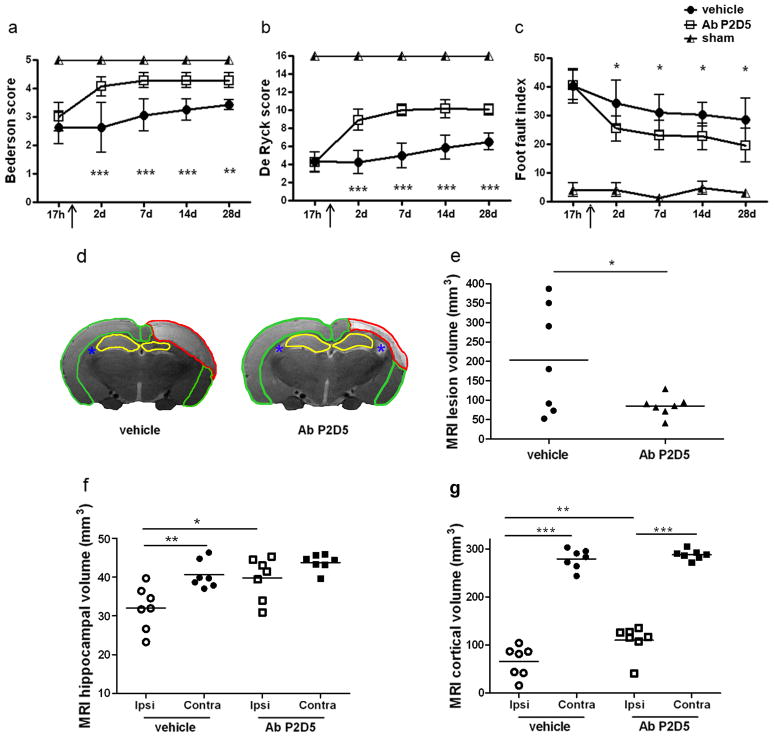
In a subsequent experiment, rats were treated with P2D5 (1 mg/Kg) 18h after ischemia and were analyzed serially out to 28d, to assess whether the antibody’s protective effect was long lasting. Postural and sensorimotor deficits, and the ability to integrate motor responses were significantly improved in mAb-treated rats up to 28d (Fig. 6a,b,c). Since it is not practical to assess classical histological injury at this late time point, we analyzed the ischemic rat brains by MRI, evaluating the extension of the lesion and tissue atrophy. We found that antibody-treated rats had a significant reduction in the overall ischemic volume (58.2%, Fig. 6e) as well as less atrophy in the hippocampus (Fig. 6f) and cortex (Fig. 6g) but not in striatum (not shown) compared to vehicle-treated rats.
Figure 6.
Anti-MBL-A antibody protection form ischemic injury is long lasting. Anti-MBL-A mAb (P2D5 clone, 1 mg/kg, iv) was given 18h after 3-vo (at arrow). Postural-reflex deficit (a, Bederson’s test; normal score=5), sensorimotor deficit (b, De Ryck’s test; normal score=16) and integration of motor responses (c, foot-fault test; normal score=2), were assessed 17h (pre-treatment time), 2, 7, 14 and 28d after 3-vo in vehicle or anti-MBL-A antibody treated rats or in sham-operated rats. Data are reported as mean±SD, n=7 per group. Two-way ANOVA for repeated measures followed by Bonferroni post-hoc test (a,b,c): *P<0.05, **P<0.01,***P<0.001, Ab P2D5 versus vehicle-treated rats at each given time. Structural MRI data analysis of brain volumes (T2w) in ischemic rats treated with vehicle or with anti-MBL-A antibody: total lesion volume (e: measured as the whole T2w hyperintense tissue across brain areas, red ROI in d) and atrophy (measured as residual T2w hypointense tissue) in hippocampus (f, yellow ROI in d) and cortex (g, green ROI in d) for ipsi- and contra- lateral areas. Asterisks (blue) identify ventricles. Data are reported as scatter dot plots and mean (bar), n=7 per group. Two-way ANOVA for mached values (ipsi and contralateral side) followed by Bonferroni post-hoc test (f,g) and unpaired t-test with Welch’s correction (e); *P<0.05,**P<0.01,***P<0.001, comparisons as indicated by horizontal lines.
Discussion
This study establishes a pivotal role for MBL in the pathogenesis of brain ischemic injury and demonstrates in multiple models and using two structurally different inhibitors that MBL’s inhibition leads to protection with a surprisingly wide therapeutic window. We have shown that MBL is deposited on the ischemic endothelium after injury (with and without reperfusion) and that functional MBL/MASP2 complexes are increased following pMCAo similarly to what observed after tMCAo12, indicating lectin pathway activation by the ischemic injury. Further, by using three independent lines of evidence, we demonstrated that MBL inhibition is protective by observing the following: 1) the susceptibility of MBL−/− mice to tMCAo and pMCAo is decreased compared to wild type mice; 2) Polyman2, a new dendrimeric molecule binding MBL with high affinity, induces a significant reduction of neurological deficits and ischemic volume when given up to 24h after injury induction; 3) the anti-MBL mAb induces a significant and long lasting reduction of neurological deficits and ischemic volume when given up to 18h after ischemic injury induction in rats.
The most relevant finding of this study is the surprisingly wide therapeutic window that can be obtained by administering either Polyman2 or the anti-MBL mAb. Interestingly, in two different models of ischemia/reperfusion injury (tMCAo in mice and 3-vo in rats), the extent of the protection and the effective therapeutic window were very similar. We do not know if the deposition of MBL on the ischemic vessels has a direct pathogenetic effect, however the observation that it decreases in tMCAo mice treated with Polyman2 (and not in pMCAo mice in which Polyman2 is not effective) suggests that this may be the case. Thus the intraluminal deposition of MBL may be the important event implying that the drugs (Polyman2 or the antibody) do not need to cross the BBB to be effective.
Notably, MBL deposition is triggered early after ischemia and lasts up to 48h. Thus, we hypothesize that the wide therapeutic window may be a result of targeting such long lasting event.
Recent data implicate MBL and/or the lectin pathway in reperfusion injury in several organs30–35. Here we demonstrate that MBL−/− mice are protected even in the absence of reperfusion. MBL−/− mice subjected to pMCAo show a dramatic reduction of the lesion volume compared to WT mice. Similar to observations in tMCAo (present results and27,28), pMCAo mice deposit MBL on ischemic vessels, increase levels of functional MBL/MASP2 complexes and increase circulating C3 fragments, thus implicating that activation of the lectin pathway following brain ischemia does not need reperfusion. However, Polyman2 was ineffective in reducing the ischemic lesion when administered to pMCAo mice, possibly because of its inability to reach the vessels in the ischemic area due to the permanent vessel occlusion. Alternatively, we cannot exclude that higher doses could be needed for Polyman2 to reach the areas where MBL is deposited on the ischemic endothelium following pMCAo. However, since in the clinical setting areas lacking reperfusion are mingled with reperfused areas1,15, this limitation would not in principle prevent a possible efficacy in human stroke.
MBL circulates in complexes with serine proteases (MASPs) and other non-enzymatic proteins36. Upon MBL binding to its carbohydrate target, MASPs are activated and cleave C4 and C2 or form the C3 convertase, which may lead to activation of the entire complement system (e.g., through to formation of C5b-9). Circulating MBL recognizes highly mannosylated glycoproteins expressed on the surface of pathogens or of “altered” self-cells through its C-terminal CRD14,37. Functional assembly into oligomeric structures allows formation of multiple CRD domains (clusters of 2–6 CRDs) which leads to an efficient binding complex with multivalent properties. Although the binding affinity of each individual interaction between the CRD and the mannosylated glycoproteins is relatively low, the self-assembly into high order oligomers provides a way for MBL to recognize repetitive arrays of its carbohydrate targets with high avidity. Thus, to effectively interfere with these multivalent interactions, multivalent inhibitors have to be designed. Polyman2 (previously referred to as Dendron 1229) is a tetravalent pseudo-trimannoside dendron. It is formed by a tetravalent polyester scaffold, decorated with a mimic of linear 1,2-1,6-trimannoside. Polyman2 was selected as the most active MBL binding complex by SPR analysis from a small group of congeners which varied in structure of the dendron scaffold and/or the nature of the mannose-based ligand. Polyman2 has a high solubility in physiological media, negligible cytotoxicity and a stability of 6–12h at pH 7.4 in aqueous solution29. In vivo, upon administration to approximately 60 ischemic mice we could not detect any apparent sign of toxicity. Importantly, Polyman2 does not activate and deplete complement.
Due to the presence in vivo of several structures potentially capable of recognizing mannosides38, Polyman2 could also act on other CRD targets. The inability to exert an additional protective action in MBL −/− mice suggests that the protective effect of Polyman2 involves its interaction with MBL.
MBL is widely known as the first step of activation of the lectin complement pathway. We cannot exclude that MBL may have a direct toxic effect, independent from its ability to activate complement. Recent data obtained in a renal ischemia injury model show that MBL-mediated tubular injury is independent of complement activation and that MBL-mediated cell death precedes complement activation39. Thus future studies are needed to fully elucidate this aspect in brain injury.
In humans MBL is encoded by a single gene while in rodents it is encoded by two different genes, MBL-A and MBL-C. Both rodent MBLs form higher oligomeric structures and activate complement. They display slightly different ligand specificities and may be differently expressed in different tissues, however whether they also possess other common or divergent biological functions is not known yet26,40.
Detailed analysis of the MBL gene in humans has revealed that a surprisingly high percentage of individuals (15–30% depending on the population considered) carries a genetic deficiency in MBL which leads to low circulating levels of serum MBL41. These subjects have not been found to be prone to severe infections in prospective studies, rather the high diffusion of “MBL-low” individuals suggests that a relative lack of MBL may be beneficial to the host under certain circumstances42. Notably, two human studies have recently investigated the role of MBL in stroke and found that a genetic deficiency in MBL is associated with a better outcome after acute stroke in humans27,43 thus further strengthening the hypothesis that inhibition of MBL in the clinical setting of stroke may have significant and beneficial outcomes.
As recently highlighted44, MBL has been up to now a somewhat forgotten molecule. However, recent data are strongly suggesting an important role for MBL and/or the lectin pathway in the pathogenesis of brain injury12,27,43,45. Our data provide strong support to the concept that MBL inhibition may be a relevant therapeutic target in humans, one with a wide therapeutic window of application. Thus we propose MBL as a novel therapeutic target for stroke.
Supplementary Material
Acknowledgments
Funding Sources: This study was partially supported by Italian Ministry of Health, Young Investigators Award 2009 (to Elisa R. Zanier) and by Cariplo 2009-2630 - Innovative Materials. F.O. was funded by a fellowship in memory of Amalia Ghezzi. G.L.S. and V.I.P. were funded by National Institutes of Health grants AI089781, HL056086 and HL099130.
The authors would like to thank Carlotta Ceriani and Daiana De Blasio for skilful technical assistance and Mauro Tettamanti for statistical advice.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 2.Lees JS, Mishra NK, Saini M, Lyden PD, Shuaib A. Low body temperature does not compromise the treatment effect of alteplase. Stroke. 2011;42:2618–2621. doi: 10.1161/STROKEAHA.110.611210. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 4.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mocco J, Mack WJ, Ducruet AF, Sosunov SA, Sughrue ME, Hassid BG, Nair MN, Laufer I, Komotar RJ, Claire M, Holland H, Pinsky DJ, Connolly ES., Jr Complement component C3 mediates inflammatory injury following focal cerebral ischemia. Circ Res. 2006;99:209–217. doi: 10.1161/01.RES.0000232544.90675.42. [DOI] [PubMed] [Google Scholar]
- 7.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanamadala V, Friedlander RM. Complement in neuroprotection and neurodegeneration. Trends Mol Med. 2010;16:69–76. doi: 10.1016/j.molmed.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Simoni MG, Rossi E, Storini C, Pizzimenti S, Echart C, Bergamaschini L. The powerful neuroprotective action of C1-inhibitor on brain ischemia-reperfusion injury does not require C1q. Am J Pathol. 2004;164:1857–1863. doi: 10.1016/S0002-9440(10)63744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storini C, Rossi E, Marrella V, Distaso M, Veerhuis R, Vergani C, Bergamaschini L, De Simoni MG. C1 inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation. Neurobiol Disease. 2005;19:10–17. doi: 10.1016/j.nbd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Longhi L, Perego C, Ortolano F, Zanier ER, Bianchi P, Stocchetti N, McIntosh TK, De Simoni MG. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit Care Med. 2009;37:659–65. doi: 10.1097/CCM.0b013e318195998a. [DOI] [PubMed] [Google Scholar]
- 12.Gesuete R, Storini C, Fantin A, Stravalaci M, Zanier ER, Orsini F, Vietsch H, Mannesse ML, Ziere B, Gobbi M, De Simoni MG. Recombinant C1 inhibitor in brain ischemic injury. Ann Neurol. 2009;66:332–342. doi: 10.1002/ana.21740. [DOI] [PubMed] [Google Scholar]
- 13.Heydenreich N, Nolte MW, Gob E, Langhauser F, Hofmeister M, Kraft P, Albert-Weissenberger C, Brede M, Varallyay C, Gobel K, Meuth SG, Nieswandt B, Dickneite G, Stoll G, Kleinschnitz C. C1-Inhibitor Protects From Brain Ischemia-Reperfusion Injury by Combined Antiinflammatory and Antithrombotic Mechanisms. Stroke. 2012:43. doi: 10.1161/STROKEAHA.112.660340. [DOI] [PubMed] [Google Scholar]
- 14.Ip WK, Takahashi K, Ezekowitz RA, Stuart LM. Mannose-binding lectin and innate immunity. Immunol Rev. 2009;230:9–21. doi: 10.1111/j.1600-065X.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 15.Caplan LR, Arenillas J, Cramer SC, Joutel A, Lo EH, Meschia J, Savitz S, Tournier-Lasserve E. Stroke-related translational research. Arch Neurol. 2011;68:1110–1123. doi: 10.1001/archneurol.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Bonte LR, Dokken B, Davis-Gorman G, Stahl GL, McDonagh PF. The mannose-binding lectin pathway is a significant contributor to reperfusion injury in the type 2 diabetic heart. Diab Vasc Dis Res. 2009;6:172–180. doi: 10.1177/1479164109336051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, Brines M, Leist M, Ghezzi P, Torup L. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JE, Montalto MC, Stahl GL. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation. 2001;104:1413–1418. doi: 10.1161/hc3601.095578. [DOI] [PubMed] [Google Scholar]
- 19.De Simoni MG, Storini C, Barba M, Catapano L, Arabia AM, Rossi E, Bergamaschini L. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J Cereb Blood Flow Met. 2003;23:232–239. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- 20.Storini C, Bergamaschini L, Gesuete R, Rossi E, Maiocchi D, De Simoni MG. Selective inhibition of plasma kallikrein protects brain from reperfusion injury. J Pharmacol Exp Ther. 2006;318:849–854. doi: 10.1124/jpet.106.105064. [DOI] [PubMed] [Google Scholar]
- 21.Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- 23.Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi F, Limatola C. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42:2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- 25.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–2267. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 26.Hansen S, Thiel S, Willis A, Holmskov U, Jensenius JC. Purification and characterization of two mannan-binding lectins from mouse serum. J Immunol. 2000;164:2610–2618. doi: 10.4049/jimmunol.164.5.2610. [DOI] [PubMed] [Google Scholar]
- 27.Cervera A, Planas AM, Justicia C, Urra X, Jensenius JC, Torres F, Lozano F, Chamorro A. Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke. PLoS One. 2010;5:e8433. doi: 10.1371/journal.pone.0008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducruet AF, Sosunov SA, Zacharia BE, Gorski J, Yeh ML, Derosa P, Cohen G, Gigante PR, Connolly ES., Jr The Neuroprotective Effect of Genetic Mannose-binding Lectin Deficiency is not Sustained in the Sub-acute Phase of Stroke. Transl Stroke Res. 2011;2:588–599. doi: 10.1007/s12975-011-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattin S, Daghetti A, Thepaut M, Berzi A, Sanchez-Navarro M, Tabarani G, Rojo J, Fieschi F, Clerici M, Bernardi A. Inhibition of DC-SIGN-mediated HIV infection by a linear trimannoside mimic in a tetravalent presentation. ACS Chem Biol. 2010;5:301–312. doi: 10.1021/cb900216e. [DOI] [PubMed] [Google Scholar]
- 30.Walsh MC, Bourcier T, Takahashi K, Shi L, Busche MN, Rother RP, Solomon SD, Ezekowitz RA, Stahl GL. Mannose-binding lectin is a regulator of inflammation that accompanies myocardial ischemia and reperfusion injury. J Immunol. 2005;175:541–546. doi: 10.4049/jimmunol.175.1.541. [DOI] [PubMed] [Google Scholar]
- 31.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol. 2005;174:6373–6380. doi: 10.4049/jimmunol.174.10.6373. [DOI] [PubMed] [Google Scholar]
- 32.Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, Divella C, Battaglia M, Lucarelli G, Annunziata G, Palazzo S, Selvaggi FP, Staffieri F, Crovace A, Daha MR, Mannesse M, van Wetering S, Paolo Schena F, Grandaliano G. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648–1659. doi: 10.2353/ajpath.2010.090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trendelenburg M, Theroux P, Stebbins A, Granger C, Armstrong P, Pfisterer M. Influence of functional deficiency of complement mannose-binding lectin on outcome of patients with acute ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2010;31:1181–1187. doi: 10.1093/eurheartj/ehp597. [DOI] [PubMed] [Google Scholar]
- 34.Matthijsen RA, Derikx JP, Steffensen R, van Dam RM, Dejong CH, Buurman WA. Mannose-binding lectin null alleles are associated with preserved epithelial cell integrity following intestinal ischemia reperfusion in man. Mol Immunol. 2009;46:2244–2248. doi: 10.1016/j.molimm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, Dudler T, Parent B, Lhotta K, Wallis R, Farrar CA, Sacks S, Lee H, Zhang M, Iwaki D, Takahashi M, Fujita T, Tedford CE, Stover CM. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2011;108:7523–7528. doi: 10.1073/pnas.1101748108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yongqing T, Drentin N, Duncan RC, Wijeyewickrema LC, Pike RN. Mannose-binding lectin serine proteases and associated proteins of the lectin pathway of complement: Two genes, five proteins and many functions? Biochim Biophys Acta. 2011;1824:253–262. doi: 10.1016/j.bbapap.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 37.Holmskov U, Thiel S, Jensenius JC. Collections and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 39.van der Pol P, Schlagwein N, van Gijlswijk DJ, Berger SP, Roos A, Bajema IM, de Boer HC, de Fijter JW, Stahl GL, Daha MR, van Kooten C. Mannan-Binding Lectin Mediates Renal Ischemia/Reperfusion Injury Independent of Complement Activation. Am J Transplant. 2012;12:877–87. doi: 10.1111/j.1600-6143.2011.03887.x. [DOI] [PubMed] [Google Scholar]
- 40.Wagner S, Lynch NJ, Walter W, Schwaeble WJ, Loos M. Differential expression of the murine mannose-binding lectins A and C in lymphoid and nonlymphoid organs and tissues. J Immunol. 2003;170:1462–1465. doi: 10.4049/jimmunol.170.3.1462. [DOI] [PubMed] [Google Scholar]
- 41.Worthley DL, Bardy PG, Mullighan CG. Mannose-binding lectin: biology and clinical implications. Intern Med J. 2005;35:548–555. doi: 10.1111/j.1445-5994.2005.00908.x. [DOI] [PubMed] [Google Scholar]
- 42.Seyfarth J, Garred P, Madsen HO. The ‘involution’ of mannose-binding lectin. Hum Mol Genet. 2005;14:2859–2869. doi: 10.1093/hmg/ddi318. [DOI] [PubMed] [Google Scholar]
- 43.Osthoff M, Katan M, Fluri F, Schuetz P, Bingisser R, Kappos L, Steck AJ, Engelter ST, Mueller B, Christ-Crain M, Trendelenburg M. Mannose-binding lectin deficiency is associated with smaller infarction size and favorable outcome in ischemic stroke patients. PLoS One. 2011;6:e21338. doi: 10.1371/journal.pone.0021338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osthoff M, Trendelenburg G, Eisen DP, Trendelenburg M. Mannose-binding lectin-the forgotten molecule? Nat Med. 2011;17:1547–1548. doi: 10.1038/nm.2588. [DOI] [PubMed] [Google Scholar]
- 45.Fust G, Munthe-Fog L, Illes Z, Szeplaki G, Molnar T, Pusch G, Hirschberg K, Szegedi R, Szeplaki Z, Prohaszka Z, Skjoedt MO, Garred P. Low ficolin-3 levels in early follow-up serum samples are associated with the severity and unfavorable outcome of acute ischemic stroke. J Neuroinflammation. 2011;8:185. doi: 10.1186/1742-2094-8-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.