Abstract
Comparative effectiveness research fundamentally reorients how clinical evidence is generated and used with the goal of providing actionable information to decision-makers. To achieve this, it is vital that decision-makers and the research enterprise are engaged from research inception, to evidence generation and translation. Practice-based research networks are affiliated clinicians in diverse communities with the goal of conducting research to improve care. Practice-based research networks have the potential to advance all phases of the comparative effectiveness research cycle. The aim of this paper is to explore current and potential roles of practice-based research networks in conducting comparative effectiveness research.
Keywords: community networks, comparative effectiveness research, patient-centered care, practice-based research, translational research
Clinicians, patients and policymakers confront medical decisions on a daily basis with an evidence base that often falls short of providing the critical information needed about comparative harms and benefits of interventions [1]. Initially documented over 30 years ago, unexplained variation in medical practice is a major symptom of this chasm in our evidence base [2]. Spurred by the growing recognition that unexplained practice variation contributes to unsustainable healthcare spending with limited association to improved outcomes, the USA has made new and substantial investments in comparative effectiveness research (CER) [3]. The primary intent of CER is to guide the generation and synthesis of a stronger evidence base to inform everyday healthcare decisions. Unlike the dominant paradigm for developing new medical treatments using placebo-controlled comparisons and surrogate measures of efficacy in select populations, CER is focused on comparing patient-centered benefits and harms of relevant and competing treatment strategies in diverse real-world populations. Put simply, CER is intended to directly inform the decisional dilemmas and comparisons patients and clinicians face according to the priorities and outcomes that are most meaningful to them. Practice-based research networks (PBRNs) are networks of mainly primary care clinics that conduct research to inform and improve practice-relevant questions and may provide unique and important opportunities for CER. The goal of this paper is to explore current and potential roles of PBRNs to perform and advance the goals of CER.
CER in the USA
CER arose from recognition that there is a gap between the research that is conducted and the decision dilemmas of patients, clinicians and other healthcare decision-makers. The mismatch between the products of our current research enterprise, dictated largely by the regulatory environment (e.g., approval for marketing by the US FDA), and the needs of decision-makers is a central theme behind many CER-related legislative initiatives of the past 10 years. The USA laid the foundation for a coordinated CER effort with the creation of the Effective Health Care Program within the Agency for Healthcare Research and Quality (AHRQ) through the Medicare Modernization Act in 2003. The most recent and largest investments have been achieved through the American Recovery and Reinvestment Act (ARRA) in 2009 and the establishment of the Patient-Centered Outcomes Research Institute (PCORI) through the Patient Protection and Affordable Care Act (PPACA) in 2010. The guiding principles for these investments are summarized in two landmark reports from the Federal Coordinating Council (FCC) for CER and the Institute of Medicine (IOM) Initial National Priorities for CER. Both the IOM and FCC reports clearly identify the need for innovation and methods advancement with respect to existing and new data resources [4,5]. Specifically, the IOM and FCC reports highlight community and practice-based research as potential assets to leverage for future CER as follows:
“Successfully examining and evaluating a range of interventions that are effective for priority populations will require a broad range of potential data sources and infrastructure investments. In addition to traditional patient registries and systematic reviews, the inclusion of distributed data networks that utilize community-based infrastructure, such as Federally Qualified Health Centers, will be an important asset in broadening the tools to evaluate effectiveness in various priority populations” – 2009 FCC Report to the President and Congress, page 23 [4];
“Practice-based research networks are designed for research on clinical practice and quality improvement activities. These networks generate both primary and specialty care data, often using data gathered prospectively for the purpose of research (in contrast to most existing data from practice, which document routine clinical care and may have important limitations for research purposes). These data may thus provide detailed clinical information from settings not captured in large integrated systems” – 2010 IOM Report: Initial National Priorities for CER, page 151 [5].
It is clear from these guiding documents that a robust CER program must embrace a diversity of methodological approaches and settings in order to fulfill the promise of CER for decision-makers. Because PBRNs straddle the divide between research and practice, they may be uniquely suited to confront the challenge that CER is a direct response to, namely, generating a more clinically useful evidence base. However, work is necessary to ensure that methodological standards advance to confer acceptable validity and efficiency in this endeavor.
Practice-based research networks
PBRNs have a long history of addressing research questions informed by, and of importance to, practicing clinicians. PBRNs are defined by the AHRQ as: “a group of ambulatory practices devoted principally to the primary care of patients. Typically, PBRNs draw on the experience and insight of practicing clinicians to identify and frame research questions whose answers can improve the practice of primary care. By linking these questions with rigorous research methods, the PBRN can produce research findings that are immediately relevant to the clinician and, in theory, more easily assimilated into everyday practice” [101].
Over the past 30 years, the PBRN landscape has grown in number and diversity, evolving from individual clinicians and their recorded observations about clinical encounters, to networks of well-supported, but distinct, clinical care sites [6]. Many consider the Ambulatory Sentinel Practice Network (ASPN), established in 1981, to be the first US PBRN [6,7]. The early work of ASPN clinicians addressed clinical uncertainties regarding treatment and management of headache, pelvic inflammatory disease and miscarriage. These seminal studies were critical in demonstrating that community-based physicians could generate important research findings within the context of their clinical practices [6].
Currently there are 152 PBRNs (135 primary care and 17 affiliates [dental, pharmacy, international]) registered with the AHRQ PBRN Resource Center [102]. These networks care for 53 million people in 16,900 practices and average 4.9 studies conducted in the last year. Two-thirds (66%) of the practices use electronic health records (EHRs). Clinicians are drawn to participate in PBRNs in order to answer questions directly relevant to their practice with the goals of improving the quality of practice and the health of their community [8]. While originally focused on care delivery in primary care, PBRNs have emerged in a diversity of settings, regions, sizes and research capacities. PBRNs also represent a diversity of research foci such as rural health, uninsured and underinsured, disparities, and women’s health. This variety reflects the heterogeneity of clinical practices and populations and the changing landscape of primary care delivery through the patient-centered medical home and other innovative models. PBRNs therefore have the potential to represent ‘real-world’ laboratories for exploring the effectiveness of healthcare interventions and the fidelity of how they are translated. Additionally, PBRNs are uniquely suited to understand contextual factors and evaluate system-level changes in the delivery of healthcare. Because the ultimate goal of CER is to produce relevant and actionable evidence that is informative for decision-makers such as physicians and patients, PBRNs may be uniquely positioned to move CER forward with respect to primary evidence generation, comparative evidence translation and future research prioritization. Understanding which features of PBRNs are suited to which types of CER research is important. In the sections that follow we present CER areas that may be well suited to PBRNs.
The role of PBRNs in pragmatic clinical trials
For every 113 patient visits to a primary care physician there is less than one (0.7%) admission to an academic hospital, yet most clinical research is conducted in academic and tertiary care centers [9].
Improving the efficiency and utility of clinical trials conducted in real-world settings is a high priority for CER [10]. The diverse patient populations of PBRNs enables them to serve as valuable platforms for conducting pragmatic clinical trials that will generate evidence broadly generalizable to real-world conditions. While clinical trials require extensive additional resources for data collection, protocol-mandated follow-up and intervention facilitation (e.g., randomization and allocation concealment if specified), making trials challenging for community-based clinics, PBRNs have been successful in conducting clinical trials [11–13]. Inclusion of busy primary care clinics within a clinical trial necessitates that the trials be minimally intrusive on the delivery of patient care. Given the competing priorities of detailed data entry and other trial processes with priorities of efficiency and reduced interference in usual care, practices and networks that have robust EHRs capable of automating certain types of data collection might be better suited to participate in clinical trials and other CER research [14]. An example of this potential is the Distributed Ambulatory Research in Therapeutics Network (DARTNet). DARTNet is a federated network of over 200 primary care clinics using different, but connected, EHRs to create a standardized clinically rich data source for conducting clinical research [13]. The DARTNet system collects over 120 standardized data elements from EHR sources such as laboratory, imaging, pharmacy and other billing systems among nonintegrated primary care practice sites. Additionally, DARTNet includes bidirection functionality that enables point-of-care data collection of specific, nonautomated data elements. Pilot data generated to date suggest the feasibility of collecting specific data quickly for pressing comparative effectiveness and harms questions [15]. Through this capacity, DARTNet has positioned itself to be similar to the UK General Practice Research Database (GPRD) and is a promising development for future observational and prospective CER.
PBRNs may be particularly well suited in generating evidence about the comparative benefits and harms of different ways of delivering care. Notably, nearly 25% of the IOM initial priorities for CER deal with questions about healthcare delivery systems. Topics are diverse, but many, such as the use of decision support tools, hospital discharge care coordination and effectiveness of the medical home, are of vital importance to primary care. Many PBRNs have demonstrated the capacity to perform cluster-randomized trials of system-level changes such as those suggested in the IOM report [16,17]. For instance, Gill and colleagues performed a randomized trial of an EHR-based clinical decision support tool reflecting guidelines on the use of NSAIDs in patients at high risk for gastrointestinal bleeding [16]. In this study, 27 clinics associated with the Centricity Healthcare User Research Network, a PBRN focused on improving quality by using a common EHR, were randomized to either receive the decision support tool delivered through their EHR or usual care. While the intervention produced modest improvements in adherence to guidelines, survey data accompanying the rollout also suggested that the intervention did not fit well within clinicians’ workflow, possibly contributing to the disappointing results. This research demonstrates the utility of PBRN research by highlighting not only the primary treatment effect of interest (guideline adherence), but also contextual factors that clinicians must grapple with in order to effectively translate evidence-based interventions into clinical practice.
Finally, while patients with complex chronic disease account for a majority of healthcare spending, they often are not well represented in clinical research [18]. Because CER is defined by patient-centeredness, quantifying the average treatment effect of a highly selected study sample is no longer acceptable. PBRNs may have the unique ability to recruit priority populations that are under-represented in research occurring within an academic medical center but are encountered routinely by community-based primary care clinicians. For example, Safety Net West, a PBRN that consists of primarily Federally Qualified Health Centers, is particularly interested in health disparities research and outcomes in underserved populations [19]. More broadly, PBRNs exist that focus on a number of under-represented populations such as rural communities, children’s health, urban communities and women’s health.
The role of PBRNs in patient-centered decision-making research
A central tenet of the US CER investment is to reorient the way new medical knowledge is generated and used. Specifically, evidence should be generated for the express purpose of informing decision-makers – including patients. However, many decisions that patients and providers face require complex assessments weighing patient-specific preferences for multiple outcomes. Preference-sensitive care includes decisions where two or more options exist, no one option is clearly superior, and patient values and preferences for outcomes should be used to guide decisions [14]. However, a large literature suggests that decisions made around preference-sensitive care are often more aligned with their clinicians’ practice norms and culture rather than the patients’ actual preference for treatment [20]. The recognition that significant unwanted variation exists in the delivery of healthcare, especially preference-sensitive care, highlights the critical need for a greater role for informed decision-making [21]. As a result, both the ARRA and PPACA contain significant language focused on improving the use of informed and shared decision-making.
Patient decision aids, which are evidence-based tools designed to help patients make decisions about healthcare choices, hold great promise for improving decision quality and reducing unwanted variation in the use of preference-sensitive services [22]. The advent of internet-based decision aids has broadened the potential for uptake in practice. Yet, integration of decision aids into mainstream clinical care has not achieved widespread implementation. Supported by the Foundation for Informed Medical Decision Making, several PBRNs are integrating DVD- and pamphlet-based decision aids into primary care visits and studying the barriers, facilitators and processes associated with using these tools. As one of the participating PBRNs, The Oregon Rural Practice-based Research Network is studying how decision aids for selected preference-sensitive conditions are integrated into practice in six rural family medicine clinics. For this project, each clinic selected which decision aid topics were most relevant to their clinic population and have developed and refined processes to deliver the decision aids using plan–do–study–act cycles that build on existing staffing, infrastructure and general patient care logistics. A mixed-methods analytic approach takes into account the complexity issues that occur at the practice, patient and community level. The ultimate goal of this work is to develop a toolkit that will guide decision-aid implementation in other family medicine sites. In general, providers in these practices have been receptive to using decision aids and patient survey data indicate decision aids are valuable to participating patients for clarifying values and preparing for discussions with their provider. During the process, network-associated practice facilitators, who work within PBRNs to assist practices with research and quality improvement activities, were recognized as important components for implementation. Decision aids represent an important tool for translating comparative options for patients and their care providers. PBRNs are a logical environment for studying the extent to which decision aids can efficiently be incorporated in practice.
At a more basic level, PBRNs are also positioned to gather rich data on patient-centered outcomes in diverse populations, practice settings and the contextual factors that drive decision-making. Horn and Gassaway provide a conceptual framework describing how practice-based evidence fills voids left by traditional efficacy research by embracing patient, treatment and outcome heterogeneity [23]. By exploiting the collection of comprehensive data describing how interventions are delivered, to whom, under what circumstances and the resultant patient-reported outcomes, evidence can be made more clearly translatable to practices as well as relevant to patients.
The role of PBRNs in setting research priorities
While PBRNs have been described as real-world laboratories or platforms for translational activities, this is not their only role. In 2007 the AHRQ developed a PBRN Master Contract Program involving ten productive PBRNs. During this 4-year program, 21 task orders were assigned to these contractors addressing a range of topics, including practice redesign, quality measurement methods, patient safety, primary care linkages with community resources and clinical topics including the management of methicillin-resistant Staphylococcus aureus and sleep apnea [24]. Another important component in the US CER effort is engaging decision-makers (e.g., patients, clinicians, policymakers and so on) and other stakeholders throughout the research process, but particularly at the priority setting stage and topic selection. The USA has made significant steps ensuring that research dollars flow to answer priority questions using the most appropriate methodological approaches, most notably through the recent establishment of the PCORI. Uncertainties arise every day in clinical practice that are major drivers of unexplained practice variation and the high cost of healthcare. PBRNs have the ability to direct research that fills the gaps that are most relevant for member clinicians. Westfall and colleagues envision PBRNs as a “two-way interface between the basic science laboratory and clinical practice” [25]. PBRNs can help reframe existing priority areas by drawing on the collective wisdom of community clinicians and/or patients. For example, an early ASPN study confronted the NIH Consensus Guidelines recommending computed tomo graphy scanning for new-onset headaches, which at the time recommended imaging in patients whose headaches were severe, constant, unusual or associated with neurological symptoms [26,27]. Because presentation of severe headache in a primary care setting is not uncommon, the implications of adhering to NIH guidelines would be onerous and costly. To reconcile this, ASPN conducted several surveys to assess current practice and outcomes for clinicians treating patients with headache. This study found that use of computed tomography imaging in patients with a new headache was very low, and diagnosis of severe intracranial disease not strongly associated with symptoms that the guidelines would prompt for imaging. These observations were important in confirming the practice approaches used by many family practice clinicians within ASPN. It is through observations such as this that future research questions can be framed appropriately with comparisons, outcomes and contextual limitations.
Synergy between PBRNs & clinical & translational science awards
Nearly a decade ago, the NIH released a report ‘Reengineering the Clinical Research Enterprise’ or the ‘Roadmap’ initiative, which introduced Clinical and Translational Science Awards (CTSAs). The goals of CTSAs are to accelerate the conduct and translation of biomedical research, reduce the time to bring innovation from ‘bench to bedside’, and bridge the chasm between discovery and translation into routine clinical practice [7,25,28]. It is widely recognized that even when new technology and evidence is developed, a lag of diffusion into clinical practice is often present. Balas and Boren estimate it takes 17 years to translate 14% of original research into behavior changes that benefit patients [29]. CTSAs bring renewed energy to moving new drugs, devices and other techno logies into the clinical research sphere. While a major focus of CTSAs is to stimulate basic science results into human trials (T1 research), an equally important, and often overlooked, translational aspect occurs when practicing clinicians implement new medical knowledge into routine clinical practice (T2) or what is sometimes termed ‘bench to behavior’ [30–33]. Westfall et al. further dissected clinical translation into a third component (T3) that refers to practice-based research and is primarily focused on determining how and why medical knowledge is translated [25]. PBRNs are often described as the proving grounds for new therapeutic and diagnostic approaches [34]. In their study evaluating clinician approaches to managing laboratory test results, Mold et al. demonstrate how PBRN-generated evidence is often quickly adopted [35]. In this study, surveys were used to identify how clinicians manage several specific steps related to laboratory results. After describing a unified approach for tracking results, notifying patients, documenting notification and ensuring appropriate follow-up, many participating clinicians were found to have adopted the recommended management strategy. Examples such as this highlight the importance of practice-based evidence to the translation of evidence-based practice and the role of PBRNs as learning environments for the adoption of best practices. While both CTSAs and PBRNs offer promising and complimentary avenues to generate and translate research into practice, their roles and relationships with each other are evolving and have yet to reach full capacity [36].
PBRN director survey
In an effort to better understand how the PBRN community views their role in conducting CER, we developed a short survey for PBRN directors through the AHRQ PBRN Resource Center Listserv of primary care networks. The web-based survey was sent via email to 118 directors using SurveyMonkey™ and consisted of six questions that explored directors’ knowledge, interest and perception of CER within their PBRN. Additionally, we queried directors about the nature and extent of CTSA relationships. The survey was conducted over 3 weeks between 26 August 2011 and 12 September 2011. The survey tool is available in Supplementary Material 1 (see online at www.futuremedicine.com/doi/suppl/10.2217/CER.11.7). The Oregon Health & Science University institutional review board approved the study (IRB# 7631).
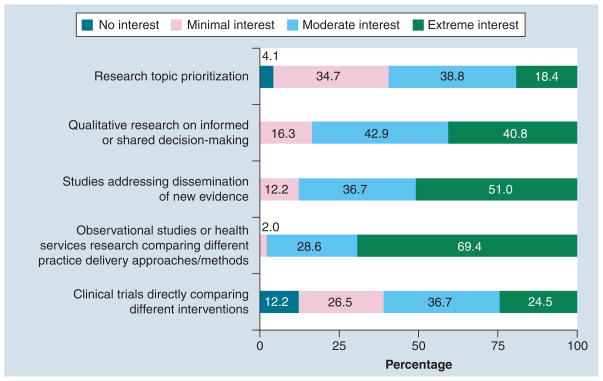
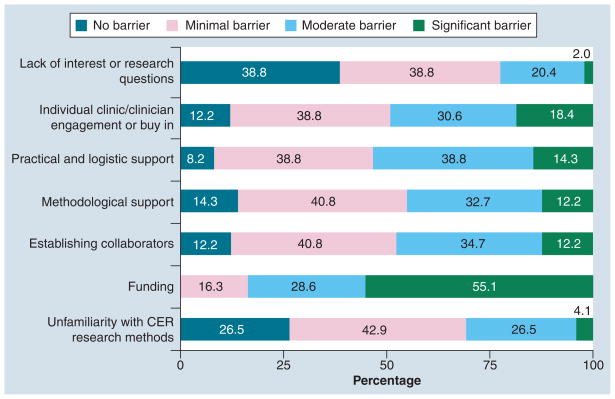
We received 49 responses to our survey (49 of 118 = 41.5% response rate). Table 1 shows that over half (53%) of responding PBRNs report some engagement with an affiliated CTSA. Table 2 presents responses of PBRN directors regarding CER activity by CTSA engagement (minimal, moderate, extensive versus none) and overall to understand how CTSA affiliation may facilitate CER interest or capacity within the PBRN. Generally, PBRN directors were uniformly aware of the US CER effort. Over 25% reported that their PBRN was currently or planning on conducting CER and over 90% of directors felt CER would be somewhat or very important to their PBRN in the future. PBRN directors were most strongly interested in observational research or health services research comparing different practice approaches (98% of respondents reporting moderate or extreme interest), followed by dissemination studies (88%), and research on informed or shared decision-making (84%). Approximately 61 and 57% of PBRN directors reported moderate or extreme interest in clinical trials research and topic prioritization, respectively. PBRN experience with clinical trials is currently limited and directors’ enthusiasm may be tempered by the infrastructure and personnel required for execution [11]. Topic prioritization as a PBRN research activity may not resonate with directors because methods are relatively new or under development. Interest in clinical trials was higher among PBRNs affiliated with a CTSA (73 vs 48%). Figure 1 shows that more than 80% of directors expressed moderate or extreme interest for observational studies, dissemination and translation work, and qualitative research on informed decision-making. When asked about barriers, funding was identified as the most significant barrier to conducting CER in PBRNs, with 84% of directors reporting it as a moderate or significant barrier. Figure 2 describes other barriers reported by PBRN directors. Access to collaborators, methodological support, practical and logistic support, and clinician engagement were reported to be a moderate or significant barrier by approximately 50% of respondents. PBRNs with CTSA engagement were significantly more likely to be interested in research addressing dissemination of new evidence compared with non-CTSA PBRNs. CTSA-engaged PBRNs were less likely to report methodologic support or collaborative opportunity as being a moderate or significant barrier relative to non-CTSA-engaged PBRNs, although the differences were not statistically significant. While this survey may be informative for gauging current activities and aspirations of PBRNs in CER, limitations exist. Most notably, the response rate was 40%, leaving open the possibility that our findings may not reflect all PBRNs. Because the survey was anonymous, we are unable to compare responders to non-responders with respect to PBRN characteristics. Additionally, because of the short time frame and the brevity of the survey it was not possible to capture more informative data on specific topic and methods preferences.
Table 1.
Practice-based research network engagement with Clinical and Translational Science Award centers.
| Level of engagement | Count | % |
|---|---|---|
| None – no affiliated CTSA at academic health center | 17 | 34.7 |
| None – CTSA-affiliated investigators have not engaged in PBRN-based research | 6 | 12.2 |
| Minimal – CTSA-affiliated investigators have proposed or conducted small PBRN-based research projects | 6 | 12.2 |
| Moderate – CTSA-affiliated investigators are consistent collaborators in PBRN research | 12 | 24.5 |
| Extensive – CTSA- affiliated investigators consistently collaborate and provide leadership in PBRN-based research | 8 | 16.3 |
CTSA: Clinical and Translational Science Award; PBRN: Practice-based research network.
Table 2.
Practice-based research network director responses by Clinical and Translational Science Award engagement (none versus minimal, extensive or moderate collaboration).
| Cumulative (n = 49)
|
CTSA engagement (n = 26)
|
No CTSA engagement (n = 23)
|
||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | Count | % | |
| Describe your current level of awareness of CER | ||||||
|
| ||||||
| No awareness | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||
| I’ve heard of CER but don’t know much about it | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||
| I understand the purpose of CER but am not familiar with specific ongoing research | 16 | 32.7 | 7 | 26. | 9 | 39.1 |
|
| ||||||
| I understand the purpose of CER and am familiar with specific CER initiatives in the USA | 20 | 40.8 | 12 | 46.2 | 8 | 34.8 |
|
| ||||||
| My PBRN is planning to or currently conducting CER | 13 | 26.5 | 7 | 26.9 | 6 | 26.1 |
|
| ||||||
| How important do you anticipate CER will be within your PBRN | ||||||
|
| ||||||
| Very | 21 | 42.9 | 13 | 50.0 | 8 | 34.8 |
|
| ||||||
| Somewhat | 24 | 49.0 | 12 | 46.2 | 12 | 52.2 |
|
| ||||||
| Minimal | 4 | 8.2 | 1 | 3.8 | 3 | 13.0 |
|
| ||||||
| No role | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
|
| ||||||
| CER activities rated as moderate or extreme interest | ||||||
|
| ||||||
| Clinical trials directly comparing different clinical interventions | 30 | 61.2 | 19 | 73.1 | 11 | 47.8 |
|
| ||||||
| Observational studies or health services research comparing different practice delivery approaches | 48 | 98.0 | 26 | 100.0 | 22 | 95.7 |
|
| ||||||
| Studies addressing dissemination of new evidence* | 43 | 87.8 | 26 | 100.0 | 17 | 73.9 |
|
| ||||||
| Qualitative research on informed or shared decision-making | 41 | 83.7 | 23 | 88.5 | 18 | 78.3 |
|
| ||||||
| Research topic prioritization | 28 | 57.1 | 16 | 61.5 | 12 | 52.2 |
|
| ||||||
| Potential barriers rated as a moderate or significant barrier | ||||||
|
| ||||||
| Unfamiliarity with CER research methods | 15 | 30.6 | 9 | 34.6 | 6 | 26.1 |
|
| ||||||
| Funding | 41 | 83.7 | 21 | 80.8 | 20 | 87.0 |
|
| ||||||
| Establishing collaborators | 23 | 46.9 | 10 | 38.5 | 13 | 56.5 |
|
| ||||||
| Methodological support | 22 | 44.9 | 10 | 38.5 | 12 | 52.2 |
|
| ||||||
| Practical and logistic support | 26 | 53.1 | 13 | 50.0 | 13 | 56.5 |
|
| ||||||
| Individual clinic/clinician engagement or buy in | 24 | 49.0 | 11 | 42.3 | 13 | 56.5 |
|
| ||||||
| Lack of interest or research questions | 11 | 22.4 | 6 | 23.1 | 5 | 21.7 |
p < 0.05 between CTSA-engaged and non-CTSA-engaged PBRNs.
CER: Comparative effectiveness research; CTSA: Clinical and Translational Science Award; PBRN: Practice-based research network.
Figure 1. Practice-based research networks’ interests in comparative effectiveness research.
Directors were asked to rate their practice-based research network’s current interest in specified areas of comparative effectiveness research according to the following scale: significant barrier, moderate barrier, minimal barrier or no barrier.
Figure 2. Practice-based research networks’ perceived barriers to comparative effectiveness research.
Directors were asked to rate specified barriers to conducting CER at their practice-based research network according to the following scale: significant barrier, moderate barrier, minimal barrier or no barrier.
CER: Comparative effectiveness research.
Conclusion
In this paper, we presented potential roles for PBRNs in conducting CER, highlighting three potential applications. First, PBRNs are staffed with clinicians practicing medicine under typical conditions in diverse communities and therefore may be the ideal setting for pragmatic clinical trials. The capacity for conduct of such resource-intensive research likely varies and needs further investigation. Responding directors were less enthusiastic about clinical trials research compared with other types of CER. Second, as the CER investment pays off in terms of patient-centered evidence, translating information will be vital. Tools that facilitate informed decision-making will be important in translating CER benefit and harms for patients and providers. The use of decision aids, while promising, may also require reformulating how care is delivered and decisions are made. PBRNs are well positioned to understand how decision aids can be best incorporated into clinical practice in ways that facilitate eventual adoption. Finally, a central tenet of CER is a sustained and meaningful dialog with decision-makers to both translate findings and inform the research priorities. PBRN research has traditionally been informed by the needs and concerns of practicing clinicians. Therefore, PBRNs, functioning as a bidirectional conduit, have the potential to assist in future priority setting activities for CER. While intuitively appealing, PBRN experience with this activity is limited.
While PBRNs seem poised to make meaningful contributions to the CER landscape, it is important to acknowledge limitations and barriers that they may confront. First, PBRN-mediated research is predicated on clinician participation. Research that interferes with clinic operations and practice flow is likely not going to be appealing. PBRN research is done best when it operates on the fringe of clinical practice or is directly aligned with a clinic’s current priorities for organizational change. Research that is not predominately conducted by clinic personnel and requires extensive onsite visits by study staff may not optimally reflect real-world conditions and will be expensive. Finally, while PBRNs present opportunities to study common diseases and risk factors, they may be less well suited to studying rare disease.
Future perspective
We have highlighted several characteristics that may facilitate the conduct of CER activities within PBRNs including uniform use of EHR, external practice facilitators to enable practice flexibility and support of a CTSA. While PBRNs seem well suited to assist in the prioritization of research because of their proximity to important decision-makers, what is less clear is the mechanism required to systematically elicit those priorities. Future resources should be directed towards understanding how the PBRN community can contribute to this dialog. Our informal survey of directors suggests that PBRNs are interested, and many report current engagement, in CER. In addition to generating new evidence and serving as laboratories for evidence translation, PBRNs can provide a robust link from communities and practices to academic centers and funders to inform the research agenda. While the US CER investment begins to bear fruit in terms of evidence generation, infrastructure and work-force development, much work remains to advance the methodology required to meet its full potential. Through methods advancement and providing assistance in overcoming barriers, CTSAs may play a vital role in enhancing the potential for CER within PBRNs.
Supplementary Material
Executive summary.
Background
Despite unprecedented amounts of medical information, patients and clinicians are making clinical decisions without sufficient, relevant scientific evidence.
Comparative effectiveness research (CER) seeks to bridge the evidence gaps faced by patients, clinicians and policymakers by fundamentally altering how research priorities are set and research is conducted.
CER in the USA
Centralized federal involvement in CER has evolved and grown over time from initial modest investments in the Agency for Healthcare Research and Quality Effective Health Care Program in 2003 to substantial funding through the American Recovery and Reinvestment Act of 2009 and the Patient Protection and Affordable Care Act of 2010.
Federal and Institute of Medicine reports consider practice-based research networks (PBRNs) as playing an important role in CER.
Practice-based research networks
PBRNs are collections of ambulatory practices involved in research with the goal of answering practice-relevant questions that improve care.
PBRNs are uniquely positioned to conduct research involving diverse patient populations, explore the translation of new knowledge under real-world conditions, identify logistic and practical issues critical for implementing patient-centered decision-making, and serve as vital linkages between the community and academia in framing future research needs.
The role of PBRNs in pragmatic clinical trials
Pragmatic clinical trials are those conducted under conditions that more closely parallel actual clinical practice and as such are important to CER.
PBRNs are well positioned to participate in the conduct of pragmatic clinical trials.
Because of the resource-intensive nature of clinical trials, PBRNs likely vary in their ability to undertake this activity.
The role of PBRNs in patient-centered decision-making research
A primary focus of CER is promoting truly informed and shared decision-making between patients and providers.
The use of decision aids to frame complex preference-sensitive decisions for patients and clinicians has gained momentum for promoting informed decision-making but has yet to become integrated in routine care.
PBRNs are ideally suited to study contextual factors that facilitate or impede use of decision aids under real-world clinic conditions.
The role of PBRNs in setting research priorities
Reflecting community priorities and practice, PBRNs can serve as a two-way conduit for both translation of new knowledge and prioritizing and informing future research directions.
Synergy between PBRNs & Clinical & Translational Science Award centers
Clinical and Translational Science Award (CTSA) centers were established by the NIH to promote translation of research into practice. Although the approach and intent differ from PBRNs, they may work together synergistically.
While CTSAs and PBRNs have proven track records in generating and translating new evidence into practice, continued engagement will be vital in refining methodology and infrastructure so PBRNs can reach their full potential to contribute to the
US CER program.
PBRN director survey
PBRNs’ directors are enthused about the potential of CER, with many conducting or planning to conduct future CER.
While funding remains a key barrier, directors are interested in a great diversity of areas in the CER portfolio.
Responses from PBRN directors confirm a synergistic potential between PBRNs and CTSAs regarding CER.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Institute of Medicine of the National Academies. Learning What Works Best: the Nation’s Need for Evidence on Comparative Effectiveness in Healthcare. Institute of Medicine of the National Academies; Washington, DC, USA: 2007. [Google Scholar]
- 2.Wennberg J, Gittelsohn J. Small area variations in health care delivery. Science. 1973;182(117):1102–1108. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 3.Congressional Budget Office. Research on the Comparative Effectiveness of Medical Treatments. Congressional Budget Office; Washington, DC, USA: 2007. [Google Scholar]
- 4.Federal Coordinating Council for Comparative Effectiveness Research. Report to the President and Congress. US Department of Health and Human Services; Washington, DC, USA: 2009. [Google Scholar]
- 5.Institute of Medicine of the National Academies. Initial National Priorities for Comparative Effectiveness Research. National Academies Press; Washington, DC, USA: 2009. [Google Scholar]
- 6.Green LA, Hickner J. A short history of primary care practice-based research networks: from concept to essential research laboratories. J Am Board Fam Med. 2006;19(1):1–10. doi: 10.3122/jabfm.19.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Lindbloom EJ, Ewigman BG, Hickner JM. Practice-based research networks: the laboratories of primary care research. Med Care. 2004;42(Suppl 4):III45–III49. [PubMed] [Google Scholar]
- 8.Fagnan LJ, Handley MA, Rollins N, Mold J. Voices from left of the dial: reflections of practice-based researchers. J Am Board Fam Med. 2010;23(4):442–451. doi: 10.3122/jabfm.2010.04.090189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green LA, Fryer GE, Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344(26):2021–2025. doi: 10.1056/NEJM200106283442611. [DOI] [PubMed] [Google Scholar]
- 10.Helfand M, Tunis S, Whitlock EP, et al. A CTSA agenda to advance methods for comparative effectiveness research. Clin Transl Sci. 2011;4(3):188–198. doi: 10.1111/j.1752-8062.2011.00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloane PD, Dolor RJ, Halladay J. Increasing the role of practice networks in medical research. J Am Board Fam Med. 2009;22(4):348–351. doi: 10.3122/jabfm.2009.04.090107. [DOI] [PubMed] [Google Scholar]
- 12.Nutting PA, Beasley JW, Werner JJ. Practice-based research networks answer primary care questions. JAMA. 1999;281(8):686–688. doi: 10.1001/jama.281.8.686. [DOI] [PubMed] [Google Scholar]
- 13.Pace WD, Cifuentes M, Valuck RJ, Staton EW, Brandt EC, West DR. An electronic practice-based network for observational comparative effectiveness research. Ann Intern Med. 2009;151(5):338–340. doi: 10.7326/0003-4819-151-5-200909010-00140. [DOI] [PubMed] [Google Scholar]
- 14.Wennberg JE, Fisher ES, Skinner JS. Geography and the debate over Medicare reform. Health Aff (Millwood) 2002:W96–W114. doi: 10.1377/hlthaff.w2.96. Suppl Web Exclusives. [DOI] [PubMed] [Google Scholar]
- 15.Libby AM, Pace W, Bryan C, et al. Comparative effectiveness research in DARTNet primary care practices: point of care data collection on hypoglycemia and over-the-counter and herbal use among patients diagnosed with diabetes. Med Care. 2010;48(6):S39–S44. doi: 10.1097/MLR.0b013e3181ddc7b0. [DOI] [PubMed] [Google Scholar]
- 16.Gill JM, Mainous AG, 3rd, Koopman RJ, et al. Impact of EHR-based clinical decision support on adherence to guidelines for patients on NSAIDs: a randomized controlled trial. Ann Fam Med. 2011;9(1):22–30. doi: 10.1370/afm.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas SJ, Grant RW, Lester WT, et al. A cluster-randomized trial of a primary care informatics-based system for breast cancer screening. J Gen Intern Med. 2011;26(2):154–161. doi: 10.1007/s11606-010-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinetti ME, Studenski SA. Comparative effectiveness research and patients with multiple chronic conditions. N Engl J Med. 2011;364(26):2478–2481. doi: 10.1056/NEJMp1100535. [DOI] [PubMed] [Google Scholar]
- 19.Gold R, DeVoe J, Shah A, Chauvie S. Insurance continuity and receipt of diabetes preventive care in a network of federally qualified health centers. Med Care. 2009;47(4):431–439. doi: 10.1097/mlr.0b013e318190ccac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dartmouth Atlas of Health Care. Preference-Sensitive Care. A Dartmouth Atlas Project Topic Brief. Dartmouth Center for Evaluative Clinical Services; Lebanon, NH, USA: 2007. [PubMed] [Google Scholar]
- 21.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision-making. annals of internal medicine. Ann Intern Med. 2005;143(4):293–300. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor AM, Wennberg JE, Legare F, et al. Toward the tipping point: decision aids and informed patient choice. Health Aff. 2007;26(3):716–725. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 23.Horn SD, Gassaway J. Practice based evidence: incorporating clinical heterogeneity and patient-reported outcomes for comparative effectiveness research. Med Care. 2010;48(Suppl 6):S17–S22. doi: 10.1097/MLR.0b013e3181d57473. [DOI] [PubMed] [Google Scholar]
- 24.Pace WD, Fagnan LJ, West DR. The Agency for Healthcare Research and Quality (AHRQ) Practice-Based Research Network (PBRN) relationship: delivering on an opportunity, challenges, and future directions. J Am Board Fam Med. 2011;24(5):489–492. doi: 10.3122/jabfm.2011.05.110080. [DOI] [PubMed] [Google Scholar]
- 25.Westfall JM, Mold J, Fagnan L. Practice-based research: blue highways on the NIH roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 26.Green LA, Hames CG, Sr, Nutting PA. Potential of practice-based research networks: experiences from Ambulatory Sentinel Practice Network (ASPN) J Fam Pract. 1994;38(4):400–406. [PubMed] [Google Scholar]
- 27.Taylor RB. Family Medicine: Principles and Practice. Springer-Verlag; New York, NY, USA: 2003. [Google Scholar]
- 28.Tapp H, Dulin M. The science of primary health-care improvement: potential and use of community-based participatory research by practice-based research networks for translation of research into practice. Exp Biol Med (Maywood) 2010;235(3):290–299. doi: 10.1258/ebm.2009.009265. [DOI] [PubMed] [Google Scholar]
- 29.Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement. Schattauer; Germany: 2000. Yearbook of medical informatics. [PubMed] [Google Scholar]
- 30.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 31.Avorn J, Fischer M. ‘Bench to behavior’: translating comparative effectiveness research into improved clinical practice. Health Aff. 2010;29(10):1891–1900. doi: 10.1377/hlthaff.2010.0696. [DOI] [PubMed] [Google Scholar]
- 32.Dorsey ER, de Roulet J, Thompson JP, et al. Funding of US biomedical research, 2003–2008. JAMA. 2010;303(2):137–143. doi: 10.1001/jama.2009.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerner JF. Knowledge translation versus knowledge integration: a ‘funder’s’ perspective. J Contin Educ Health Prof. 2006;26(1):72–80. doi: 10.1002/chp.53. [DOI] [PubMed] [Google Scholar]
- 34.Mold JW, Peterson KA. Primary care practice-based research networks: working at the interface between research and quality improvement. Ann Fam Med. 2005;3(Suppl 1):S12–S20. doi: 10.1370/afm.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mold JW, Cacy DS, Dalbir DK. Management of laboratory test results in family practice. An OKPRN study Oklahoma Physicians Resource/Research Network. J Fam Pract. 2000;49(8):709–715. [PubMed] [Google Scholar]
- 36.Fagnan LJ, Davis M, Deyo RA, Werner JJ, Stange KC. Linking practice-based research networks and Clinical and Translational Science Awards: new opportunities for community engagement by academic health centers. Acad Med. 2010;85(3):476–483. doi: 10.1097/ACM.0b013e3181cd2ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101. [Accessed 5 August 2011];Agency for Healthcare Research and Quality: primary care practice-based research network FAQ. http://pbrn.ahrq.gov/portal/server.pt/community/practice_based_research_networks_%28pbrn%29__frequently_asked_questions_%28faq%29/860.
- 102.PBRN Resource Center. http://portal.pbrn.ahrq.gov/AnnualConference/2011/PBRN Resource Center Update.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.