Summary
DNA sequencing is a powerful technique for identifying allelic variation within the natural killer (NK) cell immunoglobulin-like receptor genes. Because of the relatively large size of the KIR genes, each locus is amplified in two or more overlapping segments. Sanger sequencing of each gene from a preparation containing one or two alleles yields a sequence that is used to identify the alleles by comparison with a reference database.
Keywords: natural killer cell, killer immunoglobulin-like receptor, DNA sequencing, alleles
1. Introduction
The human killer cell immunoglobulin-like receptors (KIR) are encoded by 14 genes: KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL4, KIR2DL5, KIR2DS1, KIR2DS2, KIR2DS3, KIR2DS4, KIR2DS5, KIR3DL1, KIR3DL2, KIR3DL3, KIR3DS1 (1). These genes likely arose from gene duplications and unequal crossing over since they share extensive sequence homology. Each gene is divided into 8–9 exons that encode the signal peptide, two or three extracellular domains, stem, transmembrane region, and cytoplasmic tail. The genes are about 9–16 Kb in length. The number of KIR loci present varies among individuals. For example, some individuals might carry only seven of the 14 KIR genes while other individuals might carry 12 of the 14 KIR genes. A clear understanding of the KIR gene system will be important to understand the basis for the strategies described in this chapter and to correctly interpret the sequencing results.
1.1 Overview of Methods
This protocol describes the amplification and sequencing of each KIR gene from genomic DNA. The polymerase chain reaction is used to obtain two or more overlapping amplicons covering all or most of each gene (Figure 1). The nucleotide sequences of the exons carried by each amplicon are determined using Sanger sequencing (2) with primers that anneal in the introns and flank each exon. Both alleles of a locus, if present, are sequenced concurrently and the allele assignments made by comparison to a KIR reference database. Some loci (KIR2DL1, KIR2DL2, KIR2DL3, KIR2DL5, KIR2DS4) require special steps in order to obtain unambiguous sequences as described in Table 1. An initial survey of the KIR genes present or absent in the sample using sequence specific priming will provide the information necessary to determine the additional steps required to obtain allele assignments.
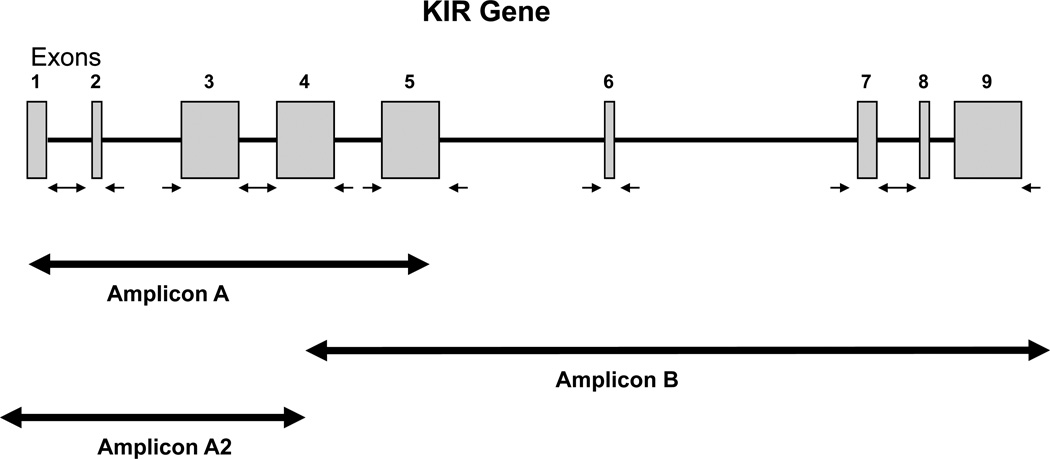
Figure 1.
Amplification of overlapping amplicons covering the KIR2DL1 coding region sequence. KIR genes have eight to nine exons. PCR amplification primers are designed to generate two or more overlapping amplicons. The figure shows the three amplicons, A, B, and A2, that cover the coding sequence of the KIR2DL1 gene. If the sample does not contain KIR2DS1, the laboratory needs only to generate the A and B amplicons for sequencing as described in Table 1. Amplicon A will allow the sequence determination from nucleotide 11 of exon 1 through nucleotide 632 of exon 5; amplicon B will cover nucleotide 332 in exon 4 through the last nucleotide of exon 9. If the sample contains the KIR2DS1 gene, the laboratory will perform instead three amplifications generating amplicons A, B, and A2. The A2 amplicon will contain only KIR2DL1 and will provide the sequence covering nucleotide 1 in exon 1 through nucleotide 330 of exon 4. The A amplicon which contains DNA from both KIR2DL1 and KIR2DS1 genes will provide sequence information covering the region where the A2 antisense and the B sense primers anneal i.e., around nucleotide 331 in exon 4. The small arrows under the exons denote the positions of sequencing primers that anneal in the introns and that provide the sequence of both sense and antisense DNA strands for each exon. Tables 2 and 3 list the amplification and sequencing primers for all the KIR loci and describe their annealing sites.
Table 1.
Summary of Amplification Protocols for 15 KIR Locia
| Locus | Specific Amplification or Allele Isolation Protocol Required |
|---|---|
| KIR2DL1 |
|
| KIR2DL2 |
|
| KIR2DL3 |
|
| KIR2DL4 |
|
| KIR2DL5 |
|
| KIR2DS1 |
|
| KIR2DS2 |
|
| KIR2DS3 |
|
| KIR2DS4 |
|
| KIR2DS5 |
|
| KIR3DL1 |
|
| KIR3DL2 |
|
| KIR3DL3 |
|
| KIR3DS1 |
|
Samples will differ in their requirement for the strategies listed in this table depending on the KIR genes present in each sample. Once the KIR genes present and absent are evaluated by an initial assay (as described in Chapter ???), the laboratory should use this table to select the methods required to obtain DNA for sequencing. For example, to obtain the allele assignments of KIR2DL1: If a cell carries KIR2DL1 and not KIR2DS1, two PCR amplifications are performed to yield KIR2DL1 amplicon A (yielding the sequence of nucleotide 10 through nucleotide 632) and KIR2DL1 amplicon B (nucleotide 332 through the last nucleotide of exon 9). These two overlapping amplicons are subsequently sequenced to identify the KIR2DL1 alleles. However, if the cell carries both KIR2DL1 and KIR2DS1, amplicon A will include both KIR2DL1 and KIR2DS1 which makes it difficult to interpret the sequence data. In this case, it is necessary to perform an additional amplification of KIR2DL1 generating amplicon A2 (nucleotide 1 through nucleotide 330) which does not include KIR2DS1. Because the antisense primer generating amplicon A2 anneals at nucleotide 331 which is the annealing site of the sense primer for amplicon B, the A2 amplicon does not provide a clear assessment of the sequence in the region of nucleotide 331. This information is provided by amplicon A.
1.2. Use of Methods in Clinical Practice
The impact of genetic variation in the KIR gene complex on the functional activity of NK cells is yet to be fully understood. The presence of specific KIR genes has been associated with susceptibility or resistance to infectious and autoimmune diseases and to malignancy (1) (3). In hematopoietic progenitor cell transplantation for acute myelogenous leukemia, a decreased frequency of relapse and infection has been noted in transplants with donors carrying haplotypes with increased numbers of activating KIR genes (4),(5). Less is known about the impact of KIR allelic polymorphism on the immune response. Allelic variation alters the level of protein expression and the affinity of ligand binding as demonstrated for KIR2DL2/KIR2DL3 (6) and KIR3DL1 (7),(8). For example, in HIV infection, allotypic variation of KIR3DL1 influences disease progression and levels of the pathogen in plasma (9). Thus, as we learn more about their impact, identification of KIR alleles may be used to predict the response of an individual to a disease or to therapy and to select optimal stem cell donors for patients with some malignancies.
2. Materials
Use reagent grade water (e.g., UltraPure™ distilled water, Invitrogen, Carlsbad, CA, USA) unless noted. Storage conditions of commercial reagents are indicated by the vendor.
2.1. DNA preparation
Whole blood drawn into a standard blood tube containing the anti-coagulant acid citrate dextrose (ACD) (see Note 1).
QIAampR DNA Blood Mini Kit (QIAGEN, Valencia, CA, USA): The kit contains buffers AL, AW1, AW2, protease and solvent for protease, spin columns, collection tubes and instruction manual. The buffers in the kit, AW1 and AW2, are provided as concentrates. When opening a new bottle, add the appropriate amount of 96–100% ethanol (as written on the label). To reconstitute the protease, add the supplied solvent to the protease powder and invert the bottle several times to mix. Store for 2 months at 4°C after preparation.
96–100% ethanol
Phosphate buffered saline (PBS)
1.5 ml microcentrifuge tubes
Pipettor (5–200 µl) and tips
Heat block or water bath at 56°C
Vortex mixer
Centrifuge capable of holding 1.5 ml tubes with a maximum speed of 20,000 × g (14,000 rpm)
2.2. Polymerase chain reaction
Genomic DNA prepared as described in Section 3.1
Positive and negative control genomic DNA (National Marrow Donor Program Cell Repository, Minneapolis, MN, USA; http://www.cibmtr.org/samples/) (See Note 2)
Taq polymerase and buffer: Platinum Taq DNA Polymerase High Fidelity 5 units/µl with 10X High Fidelity PCR Buffer (Invitrogen, Carlsbad, CA, USA)
50 mM MgSO4 (Invitrogen) according to Table 2
10 mM dNTP mixture (Roche, Mannheim, Germany)
KIR locus PCR primers: 10 µM of each oligonucleotide primer in water, store at −20°C. Table 1 describes the primer sets needed based on the presence or absence of specific KIR genes in the sample. Primers are listed in Table 2 (see Note 3)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St.Louis, MO)
5 M betaine solution (Sigma-Aldrich)
Reagent grade water
1Kb DNA ladder (e.g., Tracklt™1Kb Plus DNA ladder, Invitrogen)(see Note 4)
Agarose (e.g., UltraPure™ Agarose, Invitrogen)
10X TBE buffer (e.g., UltraPure™ 10X TBE buffer, Invitrogen) diluted with deionized water at an operational resistivity of 18.2 MΩ cm-1 at 25°C to 1X
Ethidium bromide solution (10 mg/ml) (Invitrogen) (see Note 5)
5X sucrose cresol (0.04% cresol red in 30% sucrose) gel loading solution
Agencourt AMPure kit (Beckman Coulter, Beverly, MA, USA)
70% ethanol in water (e.g., Warner-Graham Company, Cockeysville, MD, USA)
1.5 ml sterile disposable tubes (Fisher Scientific, Dallas, TX, USA)
Semi-skirted PCR tray (Fisher Scientific, Dallas, TX, USA)
Tape seals (One Lambda, Canoga Park, CA, USA)
Single channel and multi-channel (8 or 12 channel) pipettors (0.5 µl-200 µl) and tips
Thermal cycler (e.g., model 2720, Applied Biosytems, Foster City, CA, USA)
Vortex mixer
Flat bed slab gel unit (tray 11.9 cm (length) × 11.5 cm (width)) and power supply (e.g., RunOne™ Electrophoresis Unit, Embi Tec, San Diego, CA, USA)
UV transilluminator
Gel photography system
Agencourt SPRIPlate 96R magnet plate (Beckman Coulter)
Centrifuge capable of holding 1.5 ml tubes and plates with a maximum speed of 20,000 × g (14,000 rpm) (e.g., model 5424 (for tubes) and model 5804 (for plates with A-2-deep well plate rotor), Eppendorf, Hauppauge, NY, USA)
Table 2.
KIR locus specific polymerase chain reaction amplification primersa and conditions
| KIR Locus |
Amplicon | Sense Primer | Antisense Primer | Annealing Sites-- Sense/Antisenseb |
Amplicon Size (bp) |
PCR Reaction Conditions | PCR Reaction Components (50 µl) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Annealing Temp (° C)—Initial/ Secondary Cycles |
Extension Time (min) |
MgSO4 (µl) |
DMSO/ Betaine (µl) |
Taq | ||||||
| KIR2DL1 | A | TGTAAAACGACGGCCAGTGGCAGCACCATGTCGCTCT | CAAGCAGTGGGTCACTTGAC | 10T/633G | 5605 | 64/61 | 5 | 2.0 | 2.5/− | High Fidelity |
| A2 | ATAACATCCTGTGCGCTGCT | GGGTCACTGGGAGCTGACAC | 5UTR/331G | 3825 | 66/64 | 5 | 1.5 | 3.0/− | High Fidelity | |
| B | ACTCACTCCCCCTATCAGG | TGTTGACTCCCTAGAAGACG | 331G/3UTR | 10282 | 62/59 | 10 | 2.0 | −/− | High Fidelity | |
| KIR2DL2 | A | TCTCAGCACAGACAGCACC | GCCCTGCAGAGAACCTACA | 5UTR/505T | 5382 | 62/58 | 7 | 2.0 | 2.0/− | High Fidelity |
| B | CCATGATGGGGTCTCCAAA | TCAATGCCTGCATCGAAGGTTTCT | 246A/IN6 | 5348 | 60/57 | 5 | 3.5 | −/10 | High Fidelity | |
| C | TCACCCACTGAACCAAGCTCT | TGTTGACTCCCTAGAAGACG | 708T/3UTR | 5228 | 62/58 | 7 | 2.0 | 2.0/− | High Fidelity | |
| D | AATGCCTCTTCTCCTCCAGGTCTA | CTCTCCTCTGGGTCTCTCCTGACCG | 375A/IN5 Nested Ex5 | 568 | 62/57 | 1.5 | - | −/10 | Taq/10X PCR buffer with MgCl2 | |
| KIR2DL3 | A | TGTAAAACGACGGCCAGTGGCAGCACCATGTCGCTCA | GCCCTGCAGAGAACCTACG | 10A/505C | 5385 | 62/58 | 5 | 3.0 | −/10 | High Fidelity |
| B1 | GTTCTGTTACTCACTCCCCCT | CTCTCCTCTGGGTCTCTCCTGACCG | 325T/IN5 | 2131 | 62/58 | 5 | 3.0 | −/10 | High Fidelity | |
| B2 | CGTTCTGCACAGAGAAGGGAAc | 194A/IN5 | 2262 | 62/58 | 5 | 3.0 | −/10 | High Fidelity | ||
| C1 | TCAAGACAGTGGGCGTCACATACA | CTTCGTGAGACTTACTTTTTTTGTTGC | IN6/809G | 3344 | 62/58 | 5 | 3.0 | −/10 | High Fidelity | |
| C2 | ACACCTGCATGTTCTGATTGG | GCAGGAGACAACTTTGGATCA | 746G/1024T | 879 | 62/58 | 5 | 3.0 | −/10 | High Fidelity | |
| D | AGCAAGGGGAAGCCTCACTCATTC | CCAATGACAATGAGAATG | IN2/IN4 Nested--Ex4 | 419 | 62/57 | 1.5 | - | −/10 | Taq/10X PCR buffer with MgCl2 | |
| KIR2DL4 | A | CACCCACGGTCATCATCC | CCCTTTCSCTGTTGGAGTGT | 28C/IN6 | 5378 | 64/57 | 6 | 2.0 | 2.0/− | High Fidelity |
| A2 | TCCTGGCAGCAGAAGCTGCACC | GGAAAGAGCCGAAGCATC | 5UTR/581G | 2564 | 64/57 | 5 | 2.0 | 2.0/− | High Fidelity | |
| B | CATGTTCTAGGAAACCCTTCT | TGGGCTAAGCAAAGGAGTGT | 666T/3UTR | 5420 | 64/57 | 6 | 2.0 | 2.0/− | High Fidelity | |
| KIR2DL5 | A | ATCTTGTGTTCGGGAGGTTG | TCATAGGGTGAGTCATGGAG | 5UTR/589C | 3274 | 64/62 | 5 | 2.0 | 2.0/− | High Fidelity |
| B | GAGGGGAGGGCCCATGAACC | GGAAGAGCGATCCCCTAAGA | 491C/3UTR | 6193 | 64/62 | 7 | 2.0 | 2.0/− | High Fidelity | |
| A*001+ | CTCCCGTGATGTGGTCAACATGTAAA | TCATAGGGTGAGTCATGGAG | 5UTR/589C | 3109 | 64/62 | 5 | 2.0 | 2.0/− | High Fidelity | |
| B*002+ | CTCCCATGATGTAGTCAACATGTAAG | TCATAGGGTGAGTCATGGAG | 5UTR/589C | 3109 | 64/62 | 5 | 2.0 | 2.0/− | High Fidelity | |
| KIR2DS1 | A | GGCAGCACCATGTCGCTCA | GCATCTGTAGGTCCCTCCA | 10A/576T | 5540 | 64/60 | 7 | 1.5 | 1.0/− | High Fidelity |
| B | TCTCCATCAGTCGCATGAR | GGGTGTCTTGGGCCTCTC | 272R/3UTR | 10227 | 64/60 | 10 | 2.0 | 1.0/− | High Fidelity | |
| KIR2DS2 | A | ATCCTGTGCGCTGCTGAGCTGAG | CACGCTCTCTCCTGCCAA | 5UTR/418T | 5239 | 62/58 | 7 | 1.5 | 2.0/− | High Fidelity |
| B | CTTCTGCACAGAGAGGGGAAGTA | TTATGCGTATGACACCTCCTGAT | 197A/893A | 10253 | 62/58 | 10 | 1.5 | 2.0/− | High Fidelity | |
| KIR2DS3 | A | ATCCTGTGCGCTGCTGAGCTGAG | GCATCTGTAGGTTCCTCCT | 5UTR/576A | 5919 | 64/61 | 7 | 2.0 | −/− | High Fidelity |
| B | GACATGTACCATCTATCCAC | TTATGCGTATGACACCTCCTGATGGTCC | 485C/888G | 8427 | 60/57 | 10 | 2.0 | −/− | High Fidelity | |
| KIR2DS4 | A | CATGTCGCTCATGGTCATCAT | ACACTCTCACCTATGATCACC | 20T/360G | 5122 | 64/58 | 7 | 2.0 | −/− | High Fidelity |
| B | ATCCTGCAATGTTGGTCG | TTATGCGTATGACACCTCCTGAT | 153G/893A | 10299 | 64/58 | 10 | 1.5 | −/− | High Fidelity | |
| C | CGCAGTGACCCTCTGGACATGc | GTGACGGAAACAAGCAGTGGA | 360G/642 T Nested Ex 5 | 1875 | 62/57 | 1.5 | - | −/10 | Taq/10X PCR buffer with MgCl2 | |
| KIR2DS5 | A | CCATCATGATCTTTCTTTCCAGC | CCTCCGTGGGTGGCAGGGT | 35C/563A | 4541 | 62/58 | 5 | 2.0 | −/− | High Fidelity |
| B | CATTGATGGGGTCTCCAAGGG | TTATGCGTATGACACCTCCTGATGGTCC | 248G/888G | 10188 | 62/58 | 10 | 2.0 | −/− | High Fidelity | |
| KIR3DL1 | A | TGTCKRCACCGGCAGCACC | TAGGTCCCTGCAAGGGCAA | 5UTR/560T | 3454 | 60/57 | 5 | 1.5 | 2.0/− | High Fidelity |
| B | CCATCGGTCCCATGATGCT | GACAACTTTGGATCTGGGCTY | 560T/1303Y | 10365 | 60/57 | 11 | - | −/− | Expand Long/Buffer 3 | |
| M | CAARCCCTTCCTGTCTGCCT | GAGAGAGAAGGTTTCTCATATG | 100T/659C | 3265 | 60/57 | 5 | 1.5 | 2.0/− | High Fidelity | |
| KIR3DL2 | A | GTCGTCAGCATGGCGTGC | TGCATCCAAGGCTTCCACC | 30C/IN6 | 8706 | 60/57 | 8 | 1.5 | 2.0/− | High Fidelity |
| A2 | TGTCTGCACCGGCAGCACC | GACCACACGCAGGGCAG | 5UTR/898C | 5421 | 60/57 | 5 | 2.0 | −/10 | High Fidelity | |
| B | TCACATCTCTCCTGTCCCG | GGCTGTTGTCTCCCTAGAAA | IN5/1362T | 7693 | 60/57 | 8 | 1.5 | 2.0/− | High Fidelity | |
| KIR3DL3 | A | TTTCCAGGGTTCTTCTTGCTGG | TGACCCTCAGCACYGCAGT | 49G/799A | 4415 | 62/60 | 5 | 3.0 | 2.0/− | High Fidelity |
| A2 | TGTCTGCACCGGCAGCACC | CCGACAACTCATAGGGTA | 5UTR/605T | 3361 | 62/60 | 5 | 3.0 | −/10 | High Fidelity | |
| B | CCCGGAGCTTGTTTGACATT | AGAAGACAACTTTGGATCTGC | 756T/3UTR | 6569 | 58/54 | 7 | 3.0 | −/− | High Fidelity | |
| KIR3DS1 | A | TGTCKRCACCGGCAGCACC | CTGTGACCATGATCACCAT | 5UTR/A337 | 2116 | 60/57 | 3 | 1.0 | 1.5/− | High Fidelity |
| B | GGCAGAATATTCCAGGAGG | AGAGCGATGCCCTAAGATGA | 235G/3UTR | 12324 | 60/57 | 11 | - | −/− | Expand Long/Buffer 3 | |
UTR, untranslated region and/or other 5’ or 3’ noncoding sequences; IN, intron. The designations such as 10T/633G indicates the nucleotide at the annealing site of the 3’ end of the sense/antisense primers. Position 1 is defined as the first nucleotide of the ATG codon in exon 1 according to the IPD/KIR database (http://www.ebi.ac.uk/ipd/kir/). The numbering of KIR2DS4 is based on an allele that does not contain the deletion.
Primer sequence is not identical to KIR gene sequence; a substitution was added to avoid the primer from self annealing.
2.3. Nested PCR for KIR2DL2 amplicon B, KIR2DL3 amplicon A, and KIR2DS4 amplicon B
AMPure-purified amplicons: KIR2DL2 amplicon B, KIR2DL3 amplicon A, and KIR2DS4 amplicon B. Table 1 describes the use of nested PCR to either isolate the product of a specific gene or to clarify the sequence in a specific area.
Taq DNA Polymerase 5 units/ul (Roche, Mannheim, Germany) with 10X PCR Buffer with MgCl2 (Roche)
10 mM dNTP mixture (Roche)
KIR locus PCR primer solutions for nested PCR: 10 µM of each oligonucleotide primer in water. Primers are listed in Table 2.
Reagent grade water
5 M betaine solution (Sigma-Aldrich)
Supplies and equipment described in Section 2.2
2.4. Isolation of KIR2DL2 and KIR2DL3 by HaploPrep
Genomic DNA carrying KIR2DL2 or KIR2DL3. Table 1 describes the use of HaploPrep to isolate a specific gene segment for sequencing in those samples containing a second gene sharing extensive sequence homology with the gene being characterized.
HaploPrep™ Kit (QIAGEN, Valencia, CA, USA) with hybridization buffer H
KIR locus HaploPrep probes 2DL2-999T and 2DL3-1316T, 100 µM of each probe in 1X Tris EDTA (TE) buffer (Invitrogen), stored at −20°C
Reagent grade water
Heating block with heated lid at 95°C (e.g., TruTemp DNA Microheating System, Robbins Scientific, Sunnyvale, CA, USA)(see Note 6)
BioRobot EZ1 (QIAGEN) with HaploPrep card and manual
2.5. Restriction enzyme digestion for the KIR2DL3 locus
Genomic DNA from cells carrying KIR2DL3. Table 1 describes the use of restriction enzyme digestion to eliminate a highly homologous gene when present in the sample.
Restriction endonuclease BclI (15U/µl) and 10X NE Buffer 3 (New England BioLabs, Ipswich, MA, USA)
Reagent grade water
Phenol:chloroform:isoamyl alcohol 25:24:1,V/V/V (e.g., UltraPure™ phenol:chloroform:isoamyl alcohol, Invitrogen) (see Note 7)
3M sodium acetate (Sigma-Aldrich)
70% ethanol in water (Warner-Graham Company) at −20°C
Heating block at 50°C
-20 oC freezer
Supplies and equipment described in Section 2.2
2.6. KIR2DS4 allele isolation by cloning
Nested PCR amplicon of KIR2DS4 from Section 2.3. Table 1 describes the use of cloning to separate alleles in specific KIR2DS4 heterozygous samples.
TOPO TA Cloning Kit (Invitrogen) including SOC medium and instruction manual
LB agar plates containing 50 ug/ml ampicillin
40 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside) in dimethylformamide
100 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) in water
Reagent grade water
Sterile toothpicks
1.5 ml sterile disposable tubes (Fisher Scientific, Dallas, TX, USA)
37°C Shaking and non-shaking bacterial incubators
Centrifuge capable of holding 1.5 ml tubes with a maximum speed of 20,000 × g (14,000 rpm) (e.g., model 5424 (for tubes), Eppendorf, Hauppauge, NY, USA)
Heating block at 42°C and 94°C
2.7. Long template PCR for KIR3DL1/KIR3DS1 amplicon B
Genomic DNA from samples carrying KIR3DL1 or KIR3DS1. Table 1 summarizes the strategies used to obtain amplicons for specific KIR genes.
Expand Long Template PCR System with Taq DNA polymerase and 10X Expand Long Template buffer 3 (Roche, Mannheim, Germany)
10 mM dNTP mixture (Roche, Mannheim, Germany)
KIR locus PCR primer solutions for KIR3DL1 and KIR3DS1 B amplicons: 10 µM of each oligonucleotide primer in water. Primers are listed in Table 2.
Reagent grade water
Supplies and equipment described in Section 2.2
2.8. DNA sequencing
Amplified DNA purified with AMPure from Section 3.2
BigDye Terminator v1.1 diluted 1:1 with 5X sequencing buffer (Applied Biosystems, Foster City, CA, USA)
KIR locus sequencing primers: 1.5 µM of each oligonucleotide primer in water. Store at −20°C (Table 3)(see Note 8).
Dimethyl sulfoxide (DMSO)
Agencourt CleanSEQ kit (Beckman Coulter, Beverly, MA, USA)
Ethanol: 73% solution in water
Reagent grade water
Thermal cycler (e.g., model 2720, Applied Biosytems, Foster City, CA, USA)
3730xl DNA Analyzer with POP7, 1X running buffer with EDTA, and manual (Applied Biosytems)
Centrifuge capable of holding plates with a maximum speed of 20,000 × g (14,000 rpm) (e.g., model 5804 (for plates), Eppendorf, Hauppauge, NY, USA)
Single channel and multi-channel pipettors (0.5 µl-200 µl) and tips
Semi-skirted PCR tray (Fisher Scientific, Dallas, TX, USA)
Tape seals (One Lambda, Canoga Park, CA, USA)
Agencourt SPRIPlate 96R magnet plate (Beckman Coulter)
Table 3.
DNA sequencing primers for KIR loci
| KIR Locus | Primer | Sequence (5'-3') | Strand | Nucleotide Positiona |
Sequence Covers Exonb |
Use with Amplicon |
|---|---|---|---|---|---|---|
| 2DL1 | 2DL1-SEQ-E1R | GGCCCATCACTCCATCTCT | Antisense | 167-185 | Exon 1 | A/A2 |
| 2DL1-SEQ-E2F | CAAGACTCACAGCCCAGTG | Sense | 917-935 | Exon 2 | A/A2 | |
| 2DL1-SEQ-E2R | GGAGGCAAGGTCAGAAATGT | Antisense | 1161-1180 | Exon 2 | A/A2 | |
| 2DL1-SEQ-E4F | GAYGCCTTCTRAACTCACAAC | Sense | 3450-3470 | Exon 4 | A/A2 | |
| 2DL1-SEQ-E4R | AAGTCCTRGATCATTCACTC | Antisense | 3825-3844 | Exon 4 | A | |
| 2DL1-SEQ-E5F | AAGATCCTCCCTGAGGAAAC | Sense | 5277-5296 | Exon 5 | A/B | |
| 2DL1-SEQ-E5R | AGGCTCTAGGATCATAGGACA | Antisense | 5634-5654 | Exon 5 | B | |
| 2DL1-SEQ-E6F | GCCTTTCTTTATGCCAATGT | Sense | 8488-8507 | Exon 6 | B | |
| 2DL1-SEQ-E6R | TGTCAGAGCTGTGAGATGCT | Antisense | 8887-8906 | Exon 6 | B | |
| 2DL1-SEQ-E7F | ATCTGGGTGCTTGTCCTAA | Sense | 12951-12969 | Exon 7 | B | |
| 2DL1-SEQ-E7R | AGGGACCATCCTGTTTGTGA | Antisense | 13252-13271 | Exon 7 | B | |
| 2DL1-SEQ-E89F | AAATGAGGACCCAGAAGTGC | Sense | 13580-13599 | Exons 8, 9 | B | |
| 2DL1-SEQ-E89R | TGTTGACTCCCTAGAAGACG | Antisense | 13987-14006 | Exons 8, 9 | B | |
| 2DL2 | 2DL2-SEQ-E1R | GGCCCATCACTCCATCTCT | Antisense | 129-147 | Exon 1 | A |
| 2DL2-SEQ-E2F | CAAGACTCACAGCCCAGTG | Sense | 861-879 | Exon 2 | A | |
| 2DL2-SEQ-E2R | TTGAGCACCCCAGTCTAACC | Antisense | 1170-1189 | Exon 2 | A | |
| 2DL2-SEQ-E4F | GACACCTTCTAAACTCACAAC | Sense | 3382-3402 | Exon 4 | A | |
| 2DL2-SEQ-E4R | AAGTCGTGGATCATTCACTC | Antisense | 3754-3773 | Exon 4 | A | |
| 2DL2-SEQ-E5F1 | GGTCATAGAGCAGGGGAGTG | Sense | 5136-5155 | Exon 5 | A | |
| 2DL2-SEQ-E5F2 | AATGCCTCTTCTCCTCCAGGTCTA | Sense | 5209-5233 | Exon 5 | D | |
| 2DL2-SEQ-E5R | TCTCTGCATCTGTCCATGCT | Antisense | 5602-5621 | Exon 5 | A/B/D | |
| 2DL2-SEQ-E6F | CCCAGGGCCCAATATTAGAT | Sense | 8681-8700 | Exon 6 | B | |
| 2DL2-SEQ-E6R | TCAATGCCTGCATCGAAGGTTTCT | Antisense | 9193-9217 | Exon 6 | B/C | |
| 2DL2-SEQ-E7F | ATCTGGGTGCTTGTCCTAA | Sense | 12993-13011 | Exon 7 | C | |
| 2DL2-SEQ-E7R | AGGGACCATCCTGTTTGTGA | Antisense | 13294-13313 | Exon 7 | C | |
| 2DL2-SEQ-E89F | AAATGAGGACCCAGAAGTGC | Sense | 13622-13641 | Exons 8, 9 | C | |
| 2DL2-SEQ-E89R | GGAGACAACTTTGGATCTGGA | Antisense | 13976-13996 | Exons 8, 9 | C | |
| 2DL3 | 2DL3-SEQ-E1R | GGCCCATCACTCCATCTCT | Antisense | 129-147 | Exon 1 | A |
| 2DL3-SEQ-E2F | CAAGACTCACAGCCCAGTG | Sense | 861-879 | Exon 2 | A | |
| 2DL3-SEQ-E2R | TTGAGCACCCCAGTCTAACC | Antisense | 1170-1189 | Exon 2 | A | |
| 2DL3-SEQ-E4F | GACACCTTCTAAACTCACAAC | Sense | 3382-3402 | Exon 4 | A/D | |
| 2DL3-SEQ-E4R | CCAATGACAATGAGAATG | Antisense | 3731-3748 | Exon 4 | A/D | |
| 2DL3-SEQ-E4F-218T | TTAAGGACACTTTGCACCTCAT | Sense | 3542-3563 | Exon 4 | A/D | |
| 2DL3-SEQ-E4R-282T | TAGCATCTGTAGGTCCCTGCA | Antisense | 3627-3647 | Exon 4 | A/D | |
| 2DL3-SEQ-E4F-166C | TGGTCAGATGTCAGGTTT C | Sense | 3493-3511 | Exon 4 | A/D | |
| 2DL3-SEQ-E5F1 | GGTCATAGAGCAGGGGAGTG | Sense | 5136-5155 | Exon 5 | A/B1/B2 | |
| 2DL3-SEQ-E5F2 | AATGCCTCTTCTCCTCCAGGTCTA | Sense | 5209-5233 | Exon 5 | A/B1/B2 | |
| 2DL3-SEQ-E5R | TTCTCTCTGCATCTGTCCATG | Antisense | 5608-5628 | Exon 5 | B1/B2 | |
| 2DL3-SEQ-E5R-618A | AGTTTGACCACTCGTAT | Antisense | 5480-5496 | Exon 5 | B1/B2 | |
| 2DL3-SEQ-E6F | TGAACCAACCTCAAAGATTTCC | Sense | 8698-8719 | Exon 6 | C1 | |
| 2DL3-SEQ-E6R | TTCTACCTCCCCAGGTTT C | Antisense | 8860-8878 | Exon 6 | C1 | |
| 2DL2/3-SEQ-E7F | ATCTGGGTGCTTGTCCTAA | Sense | 12993-13011 | Exon 7 | C1/C2 | |
| 2DL3-SEQ-E7R | CCCACATGGCCCTGAGC | Antisense | 11966-11982 | Exon 7 | C2 | |
| 2DL3-SEQ-E89F | TGCTTATGAAATGAGGGCCC | Sense | 12336-12355 | Exons 8, 9 | C2 | |
| 2DL3-SEQ-E89R | AGGGCTCAGCATTTGGAAG | Antisense | 12683-12701 | Exons 8, 9 | C2 | |
| 2DL4 | 2DL4-SEQ-E1R | CATCCTCACCACTCACTTGC | Antisense | 126-145 | Exon 1 | A2 |
| 2DL4-SEQ-E2F | GGCTCAGGAGGAAAGGGTAG | Sense | 177-196 | Exon 2 | A/A2 | |
| 2DL4-SEQ-E2R | CAGGCCTTCCCATGGTCAG | Antisense | 374-392 | Exon 2 | A/A2 | |
| 2DL4-SEQ-E3F | GGGGAGAATCTTCTGAGCAC | Sense | 1063-1082 | Exon 3 | A/A2 | |
| 2DL4-SEQ-E3R | CACCAGAAGCTCTGGGACTC | Antisense | 1469-1488 | Exon 3 | A/A2 | |
| 2DL4-SEQ-E5F | AGAGCAGGGCAGTGAGTTCT | Sense | 2217-2236 | Exon 5 | A/A2 | |
| 2DL4-SEQ-E5R | TCCACATCTGTCCATGCTTC | Antisense | 2677-2696 | Exon 5 | A | |
| 2DL4-SEQ-E6F | CCAGGGCCCAACATTAGATA | Sense | 5074-5093 | Exon 6 | A | |
| 2DL4-SEQ-E6R | ATCACAGAGCTGGCAGGTG | Antisense | 5316-5334 | Exon 6 | A/B | |
| 2DL4-SEQ-E7F | CCTGGCAACCAAGAAATGAG | Sense | 9400-9419 | Exon 7 | B | |
| 2DL4-SEQ-E7R | AGACTTTCCTGCCAGTGAGG | Antisense | 9663-9682 | Exon 7 | B | |
| 2DL4-SEQ-E89F | CCCCCTGTGTGTTGGTATCT | Sense | 9965-9984 | Exons 8,9 | B | |
| 2DL4-SEQ-E89R | TAAGCAAGAGACAGGCACCA | Antisense | 10519-10538 | Exons 8, 9 | B | |
| 2DL5 | 2DL5-SEQ-E1F | ATCTTGTGTTCGGGAGGTTG | Sense | 5UTR, (-274) – (-256) | 5’ noncoding region | A |
| 2DL5-SEQ-E1R | AACTCCACCTCCAGGCCTAT | Antisense | I1,101-120 | Exon 1 | A | |
| 2DL5-SEQ-E2F | ACCAAGACTCACAGCCCAGT | Sense | I1,706-725 | Exon 2 | A | |
| 2DL5-SEQ-E2R | TCCCTCCTGTTTCAGGAAAAT | Antisense | I2,873-893 | Exon 2 | A | |
| 2DL5-SEQ-E3F | GGGGAGAATCTTCTGAGCACT | Sense | I2,1510-1529 | Exon 3 | A | |
| 2DL5-SEQ-E3R | TGCTCTGGGATTCAGGAAGT | Antisense | I3,1908-1927 | Exon 3 | A | |
| 2DL5-SEQ-E5F | GGGAGCTGTGACAAGGAAGA | Sense | I3,2697-2716 | Exon 5 | A/B | |
| 2DL5-SEQ-E5R | AGCAGGAAGCTCCTCAGCTA | Antisense | I5,3088-3107 | Exon 5 | B | |
| 2DL5-SEQ-E6F | GCCATGAACCAACCTCAAAG | Sense | I5,5131-5150 | Exon 6 | B | |
| 2DL5-SEQ-E6R | CTGAGCCAATGCTTGAATCC | Antisense | I6,5321-5340 | Exon 6 | B | |
| 2DL5-SEQ-E7F | GCTGGCAACCAAGAAATGAG | Sense | I6,7950-7969 | Exon 7 | B | |
| 2DL5-SEQ-E7R | ACCAGTGTGCTCCCATCCT | Antisense | I7,8187-8205 | Exon 7 | B | |
| 2DL5-SEQ-E89F | CCCTTCCAGCTGTTTTGATG | Sense | I7,8562-8581 | Exons 8, 9 | B | |
| 2DL5-SEQ-E89R | TGATGCCTTCAGATTCCAGC | Antisense | I9,9010-9029 | Exons 8, 9 | B | |
| 2DS1 | 2DS1-SEQ-E1R | GGCCCATCACTCCATCTCT | Antisense | 470-488 | Exon 1 | A |
| 2DS1-SEQ-E2F | CAAGACTCACAGCCCAGTG | Sense | 1220-1238 | Exon 2 | A | |
| 2DS1-SEQ-E2R | GGAGGCAAGGTCAGAAATGT | Antisense | 1464-1483 | Exon 2 | A | |
| 2DS1-SEQ-E4F | GAYGCCTTCTRAACTCACAAC | Sense | 3753-3773 | Exon 4 | A | |
| 2DS1-SEQ-E4R | AATTCCTGGATCATTCACTC | Antisense | 4128-4147 | Exon 4 | A/B | |
| 2DS1-SEQ-E5F | AAGGGAGCTGTGACAAGGAA | Sense | 5581-5600 | Exon 5 | A/B | |
| 2DS1-SEQ-E5R | TCTGCATCTGTCCATGCTTC | Antisense | 6008-6027 | Exon 5 | B | |
| 2DS1-SEQ-E6F | GCCTTTCTTTATGCCAGTGTC | Sense | 8785-8805 | Exon 6 | B | |
| 2DS1-SEQ-E6R | CTGAGTCAACGCCTGAATCC | Antisense | 9166-9185 | Exon 6 | B | |
| 2DS1-SEQ-E7F | CCAATCAAGAAATGCGAGACA | Sense | 13295-13315 | Exon 7 | B | |
| 2DS1-SEQ-E7R | CAGGGGAAGGGAATCTGGT | Antisense | 13609-13620 | Exon 7 | B | |
| 2DS1-SEQ-E89F | TCCCCCTGTTTGTTGGTATC | sense | 13882-13901 | Exons 8, 9 | B | |
| 2DS1-SEQ-E89R | AAGGGCGAGTGATTTTTCTCT | Antisense | 14155-14175 | Exons 8, 9 | B | |
| 2DS2 | 2DS2-SEQ-E1R | GGCCCATCACTCCATCTCT | Antisense | 129-147 | Exon 1 | A |
| 2DS2-SEQ-E2F | CAAGACTCACAGCCCAGTG | Sense | 745-763 | Exon 2 | A | |
| 2DS2-SEQ-E2R | GGAGGCAAGGTCAGAAATGT | Antisense | 989-1008 | Exon 2 | A | |
| 2DS2-SEQ-E4F | AAGGGGAAGCCTCACTCATT | Sense | 3216-3235 | Exon 4 | A | |
| 2DS2-SEQ-E4R | GCCCAATGACAATGAGAATG | Antisense | 3614-3633 | Exon 4 | A/B | |
| 2DS2-SEQ-E5F | TGAAGAGAGATGGGGTGGAG | Sense | 4977-4996 | Exon 5 | A/B | |
| 2DS2-SEQ-E5R | CTCTCTGCATCTGTCCATGC | Antisense | 5491-5510 | Exon 5 | B | |
| 2DS2-SEQ-E6F | CAGAGTGTTGGCCATGAACC | Sense | 8486-8505 | Exon 6 | B | |
| 2DS2-SEQ-E6R | CTGAGTCAACGCCTGAATCC | Antisense | 8686-8705 | Exon 6 | B | |
| 2DS2-SEQ-E7F | CCAATCAAGAAATGCGAGACA | Sense | 12818-12838 | Exon 7 | B | |
| 2DS2-SEQ-E7R | CAGGGGAAGGGAATCTGGT | Antisense | 13143-13161 | Exon 7 | B | |
| 2DS2-SEQ-E89F | CCTCCGAGCTCTTTTGTTGA | Sense | 13427-13446 | Exons 8, 9 | B | |
| 2DS2-SEQ-E89R | TTATGCGTATGACACCTCCTGAT | Antisense | 13633-13655 | Exons 8, 9 | B | |
| 2DS3 | 2DS3-SEQ-E1R | AGGCCTATATCTCCACCTCTG | Antisense | 88-108 | Exon 1 | A |
| 2DS3-SEQ-E2F | GCCTGGCTACCAAGACTCAC | Sense | 1247-1266 | Exon 2 | A | |
| 2DS3-SEQ-E2R | AGAGACTCCCCGACAGGACT | Antisense | 1443-1462 | Exon 2 | A | |
| 2DS3-SEQ-E4F | GGAAGCCTCACTCAATCCAG | Sense | 3739-3758 | Exon 4 | A | |
| 2DS3-SEQ-E4R | CCTCCAAGTCCTGGATCATT | Antisense | 4165-4184 | Exon 4 | A | |
| 2DS3-SEQ-E5F | AAGGGAGCTGTGACAAGGAA | Sense | 5581-5600 | Exon 5 | A | |
| 2DS3-SEQ-E5R | TCTGCATCTGTCCATGCTTC | Antisense | 6008-6027 | Exon 5 | A/B | |
| 2DS3-SEQ-E6F | CCCAGGGCCCAATATTAGAT | Sense | 8969-8988 | Exon 6 | B | |
| 2DS3-SEQ-E6R | GGTGGAAGACAGGGGTACAA | Antisense | 9229-9248 | Exon 6 | B | |
| 2DS3-SEQ-E7F | TCAATCAAGAAATGCGAGACA | Sense | 13321-13341 | Exon 7 | B | |
| 2DS3-SEQ-E7R | CACACCCACGTGCTAACATC | Antisense | 13556-13575 | Exon 7 | B | |
| 2DS3-SEQ-E89F | TCCCCCTGTTTGTTGGTATC | Sense | 13882-13901 | Exons 8, 9 | B | |
| 2DS3-SEQ-E89R | TTATGCGTATGACACCTC | Antisense | 14141-14158 | Exons 8, 9 | B | |
| 2DS4 | 2DS4-SEQ-E1R | CAGGCCCATATCTCCACCT | Antisense | 91-109 | Exon 1 | A |
| 2DS4-SEQ-E2F | GGGCTGGCTATCAAGACTCA | Sense | 2222-2241 | Exon 2 | A | |
| 2DS4-SEQ-E2R | TCCCGTTTCAGGAAAATCC | Antisense | 2396-2414 | Exon 2 | A | |
| 2DS4-SEQ-E4F | AGGCTCACTCATTCCAGGTG | Sense | 4736-4755 | Exon 4 | A | |
| 2DS4-SEQ-E4R | TTACAACCACCTGGGTCTCC | Antisense | 5174-5193 | Exon 4 | A/B | |
| 2DS4-SEQ-E5F | GGGAGCTGTGACAAGGAAGA | Sense | 6610-6630 | Exon 5 | B/C | |
| 2DS4-SEQ-E5R | CATGCTGCGTCTTCTCTCTG | Antisense | 7025-7044 | Exon 5 | B | |
| 2DS4-SEQ-E6F | GGCCATGAACCAAACTCAAA | Sense | 10016-10035 | Exon 6 | B | |
| 2DS4-SEQ-E6R | CAGGCGTACAATGTCAGAGC | Antisense | 10236-10256 | Exon 6 | B | |
| 2DS4-SEQ-E7F | GTGGTTACCTGCCAATCAAGA | Sense | 14327-14347 | Exon 7 | B | |
| 2DS4-SEQ-E7R | ATCCTGCTGGTGAGGAACAC | Antisense | 14592-14611 | Exon 7 | B | |
| 2DS4-SEQ-E89F | AAATGAGGACCCAGAAGTGC | Sense | 14927-14946 | Exons 8, 9 | B | |
| 2DS4-SEQ-E89R | TTATGCGTATGACACCTCCTGAT | Antisense | 15153-15175 | Exons 8, 9 | B | |
| 2DS5 | 2DS5-SEQ-E2R | AGACTCCCTGACAGGACTTC | Antisense | 1613-1632 | Exon 2 | A |
| 2DS5-SEQ-E4F | AGCCTCACTCAATCCAGGTG | Sense | 3915-3934 | Exon 4 | A | |
| 2DS5-SEQ-E4R | ACCTGTGATCACGATGTCCA | Antisense | 4273-4292 | Exon 4 | A/B | |
| 2DS5-SEQ-E5F | CAGAGCAGGGGAGTGAGTTC | Sense | 5731-5750 | Exon 5 | A/B | |
| 2DS5-SEQ-E5R | AGCAGGAAGCTCCTCAGCTA | Antisense | 6159-6178 | Exon 5 | B | |
| 2DS5-SEQ-E6F | CCCAGGGCCCAATATTAGAT | Sense | 9145-9164 | Exon 6 | B | |
| 2DS5-SEQ-E6R | GGTGGAAGACAGGGGTACAA | Antisense | 9405-9424 | Exon 6 | B | |
| 2DS5-SEQ-E7F | GCTAGGTCTCCCACCATTTG | Sense | 133440-13459 | Exon 7 | B | |
| 2DS5-SEQ-E7R | ATCCTGCCTGTGAGGAACAC | Antisense | 13752-13771 | Exon 7 | B | |
| 2DS5-SEQ-E89F | TCCCCCTGTTTGTTGGTATC | Sense | 14059-14079 | Exons 8, 9 | B | |
| 2DS5-SEQ-E89R | TTATGCGTATGACACCTC | Antisense | 14318-14335 | Exons 8, 9 | B | |
| 3DL1 | 3DL1-SEQ-E1R | CTCCACTTCAGGCCCATAAC | Antisense | 138-157 | Exon 1 | A |
| 3DL1-SEQ-E2F | CAAGACKCACAGCCCAGTG | Sense | 953-971 | Exon 2 | A | |
| 3DL1-SEQ-E2R | TGGAGCACCCTAGTCTCACC | Antisense | 1262-1281 | Exon 2 | A | |
| 3DL1-SEQ-E3F | GAGAATCTTCTGGGCACTGG | Sense | 1739-1758 | Exon 3 | A | |
| 3DL1-SEQ-E3R | ATTCAGGAGGTGGGACAGTG | Antisense | 2126-2145 | Exon 3 | A/M | |
| 3DL1-SEQ-E4F | ACCCTCACTCATTCCAGGTG | Sense | 3136-3155 | Exon 4 | A/M | |
| 3DL1-SEQ-E4R | AAGTCCTRGATCATTCACTC | Antisense | 3555-3574 | Exon 4 | A/B/M | |
| 3DL1-SEQ-E5F1 | GGTCATAGAGCAGGGGAGTG | Sense | 4970-4989 | Exon 5 | B | |
| 3DL1/2-SEQ-E5F2 | GGTCATAGAGCAGGGGAGTG[ch1] | Sense | 5080-5097 | Exon 5 | B | |
| 3DL1-SEQ-E5R | TGCATCTGTCCATGCTTTTC | Antisense | 5434-5453 | Exon 5 | B | |
| 3DL1-SEQ-E6F | GCCTTTCTTTATGCCAATGT | Sense | 8254-8273 | Exon 6 | B | |
| 3DL1-SEQ-E6R | CCCTTTCACTGTTGGAGTGT | Antisense | 8708-8727 | Exon 6 | B | |
| 3DL1-SEQ-E7F | AGGGGTCAAACATCTCAACT | Sense | 12638-12657 | Exon 7 | B | |
| 3DL1-SEQ-E7R | AGCTGTGTGCTCCCATCCT | Antisense | 13016-13034 | Exon 7 | B | |
| 3DL1-SEQ-E89F | AAATGAGGACCCAGAAGTGC | Sense | 13372-13391 | Exons 8, 9 | B | |
| 3DL1-SEQ-E89R | GCCTCTGAGAAGGGCGA | Antisense | 13676-13692 | Exons 8, 9 | B | |
| 3DL1/2-SEQ-E89F | GGAGACAGAATCAATGGGAT | Sense | 15619-15638 | Exon 8, 9 | B | |
| 3DL1/2-SEQ-E89R | GGCTGTTGTCTCCCTAGAAA | Antisense | 16178-16197 | Exons 8, 9 | B | |
| 3DL2 | 3DL2-SEQ-E1R | CGAGATCTCCATCCCCACT | Antisense | 66-84 | Exon 1 | A2 |
| 3DL2-SEQ-E2F | AGTTTACCTTCAGCCCAGCA | Sense | 631-650 | Exon 2 | A/A2 | |
| 3DL2-SEQ-E2R | GAGACTCCCCGACAGGACTT | Antisense | 848-867 | Exon 2 | A/A2 | |
| 3DL2-SEQ-E3F | AGCGGAAATGGGAGAATCTT | Sense | 1436-1455 | Exon 3 | A/A2 | |
| 3DL2-SEQ-E3R | CAGAAGCTCTGGGATTCAGG | Antisense | 1847-1866 | Exon 3 | A/A2 | |
| 3DL2-SEQ-E4F | ACCCTCACTCATTCCAGGTG | Sense | 3196-3215 | Exon 4 | A/A2 | |
| 3DL2-SEQ-E4R | TCTGTGTCCCAATGACAATGA | Antisense | 3595-3615 | Exon 4 | A/A2 | |
| 3DL2-SEQ-E5F | CTCAGGTATGAGGGGAGCTG | Sense | 5078-5097 | Exon 5 | A/A2 | |
| 3DL2-SEQ-E5R | TCTGCATCTGTCCATGCTTC | Antisense | 5515-5534 | Exon 5 | A | |
| 3DL2-SEQ-E6F | AGGGTCCAACATTAGATAACA | Sense | 8492-8512 | Exon 6 | A/B | |
| 3DL2-SEQ-E6R | CCAGGTTTCCAAAAGCAGAG | Antisense | 8677-8696 | Exon 6 | B | |
| 3DL2-SEQ-E7F | GTCAATCAAGAAATGAGACAA | Sense | 15253-15273 | Exon 7 | B | |
| 3DL2-SEQ-E7R | GCAATGGTCTGTGAGCTGAA | Antisense | 15598-15617 | Exon 7 | B | |
| 3DL2-SEQ-E89F | TGAAATGAGGACCCAGAAGG | Sense | 15837-15856 | Exons 8, 9 | B | |
| 3DL2-SEQ-E89R | AACCCCCTCAAGACCTGACT | Antisense | 16231-16250 | Exons 8, 9 | B | |
| 3DL3 | 3DL3-SEQ-E1R | CTCGATTCCCTTCCAGGACT | Antisense | 38-57 | Exon 1 | A2 |
| 3DL3-SEQ-E2F | GAGATGTTGGCTTGGAGTGC | Sense | 442-461 | Exon 2 | A2 | |
| 3DL3-SEQ-E2R | ATCAGTCAACCCCCTGTGTC | Antisense | 820-839 | Exon 2 | A/A2 | |
| 3DL3-SEQ-E3F | AGAAACGTGGAAATGGGAGA | Sense | 1426-1445 | Exon 3 | A/A2 | |
| 3DL3-SEQ-E3R | GAGGTGGGACAGTGAGAAGC | Antisense | 1823-1842 | Exon 3 | A/A2 | |
| 3DL3-SEQ-E4F | TAGACACCATGGAGGGGAAG | Sense | 2982-3001 | Exon 4 | A/A2 | |
| 3DL3-SEQ-E4R | AAGTCCTRGATCATTCACTC | Antisense | 3418-3437 | Exon 4 | A | |
| 3DL3-SEQ-E5F | AGCTCAGGTGTGAGGAGAGC | Sense | 4890-4909 | Exon 5 | A | |
| 3DL3-SEQ-E5R | TGAGCCTAAGTTCACCGGC | Antisense | 5083-5101 | Exon 5 | A | |
| 3DL3-SEQ-E5F2 | ATCTATCCAGGGAGGCAGAG | Sense | 5063-5082 | Exon 5 | B | |
| 3DL3-SEQ-E5R2 | TGGCTCTAGGATCACAAGACA | Antisense | 5277-5297 | Exon 5 | A/B | |
| 3DL3-SEQ-E7F | CTCCTTGGGACAGCATTGAT | Sense | 10395-10414 | Exon 7 | B | |
| 3DL3-SEQ-E7R | AGAAAGTCCTGCCTCTGTGG | Antisense | 10938-10957 | Exon 7 | B | |
| 3DL3-SEQ-E89F | AAATGAGGACCCAGAAGTGC | Sense | 11231-11250 | Exons 8, 9 | B | |
| 3DL3-SEQ-E89R | CAGCATTTGGAAGTTCCGTGTT | Antisense | 11562-11583 | Exons 8, 9 | B | |
| 3DS1 | 3DS1-SEQ-E1R | AGGCCCATAACTCCACCTCT | Antisense | 109-128 | Exon 1 | A |
| 3DS1-SEQ-E2F | AGTTTACCTTCAGCCCAGCA | Sense | 920-939 | Exon 2 | A | |
| 3DS1-SEQ-E2R | ACAGGACTTCCCTCCCATTT | Antisense | 1126-1145 | Exon 2 | A | |
| 3DS1-SEQ-E3F1 | TCTATGCAGGATGGGTCCTT | Sense | 1664-1683 | Exon 3 | A | |
| 3DS1-SEQ-E3F2 | CAACATGAGCCCTGTGACCA | Sense | 1982-2001 | Exon 3 | B | |
| 3DS1-SEQ-E3R1 | CAGAAGCTCTGGGATTCAGG | Antisense | 2137-2157 | Exon 3 | B | |
| 3DS1-SEQ-E3R2 | GGTGTGAACCCCGACATG | Antisense | 2023-2040 | Exon 3 | A | |
| 3DS1-SEQ-E4F | ACCCTCACTCATTCCAGGTG | Sense | 3509-3528 | Exon 4 | B | |
| 3DS1-SEQ-E4R | TCCAAGTCCTGGATCATTCAC | Antisense | 3929-3949 | Exon 4 | B | |
| 3DS1-SEQ-E5F | GGTCATAGAGCAGGGGAGTG | Sense | 5370-5389 | Exon 5 | B | |
| 3DS1-SEQ-E5R | ATGAAGGAGGGTTTGGAGGT | Antisense | 5911-5930 | Exon 5 | B | |
| 3DS1-SEQ-E6F | ACTCCCAGGGTCCAACATTA | Sense | 8811-8830 | Exon 6 | B | |
| 3DS1-SEQ-E6R | TTCACAGAGCTGGGAGGTTT | Antisense | 9055-9074 | Exon 6 | B | |
| 3DS1-SEQ-E7F | CATCTGGGTGCTTGTCCTAAA | Sense | 13138-13158 | Exon 7 | B | |
| 3DS1-SEQ-E7R | ATCCTGCTTCCCCACATGG | Antisense | 13402-13420 | Exon 7 | B | |
| 3DS1-SEQ-E89F | TCCCCCTGTTTGTTGGTATC | Sense | 13744-13763 | Exons 8,9 | B | |
| 3DS1-SEQ-E89R | CTCTGAGAAGGGCGAGTG | Antisense | 14051-14068 | Exons 8,9 | B |
Numbering is based on the genomic sequences in the LRC database (http://www.ncbi.nlm.nih.gov/gv/lrc/). Nucleotide 1 is the first base of exon 1.
Exon numbering is based on 9 total exons for each locus. Some of the KIR loci are missing an exon or have a pseudo exon that is not analyzed. KIR2DL1-3 and KIR2DS1-5 have a pseudo-exon 3 while KIR2DL4 and KIR2DL5 lack exon 4 (24).
2.9 Sequence analysis including preparation of locus-specific KIR libraries
Analysis software: Assign SBT 3.2.7 (Conexio Genomics, Applecross, Western Australia), HLA Librarian (Conexio Genomics), Sequencher 4.6 (Ann Arbor, MI, USA) with manuals (see Note 9)
KIR nucleotide sequence databases: IPD-KIR curated coding region sequence database at http://www.ebi.ac.uk/ipd/kir/index.html; Leukocyte Receptor Complex (LRC) database alignment viewer for genomic sequences at http://www.ncbi.nlm.nih.gov/gv/lrc/
3. Methods
3.1. DNA preparation
Label the appropriate number of 1.5 ml microcentrifuge tubes and QIAamp spin columns with sample identifier. See Note 10 on laboratory.
Add 200 µl whole blood sample to the tube (see Note 11). If the sample volume is less than 200 µl, add PBS to bring sample to volume.
Pipet 20 µl protease into the blood sample in the tube.
Add 200 µl Buffer AL to the sample (see Note 12). Immediately mix by vortexing for 15 seconds.
Incubate at 56°C for 10 minutes.
Briefly centrifuge the microcentrifuge tube to remove condensation drops from the inside of the lid (See Note 13).
Add 200 µl 96–100% ethanol to the sample and mix again by vortexing for 15 seconds. Again briefly centrifuge the microcentrifuge tube.
Carefully apply the sample to the QIAamp spin column in a collection tube without wetting the rim of the spin column. Centrifuge at 6000 × g (8000 rpm) for 1 minute. Place the QIAamp spin column into a clean 2 ml collection tube and discard the tube containing the filtrate.
After placing the spin column into a clean collection tube, carefully add 500 µl Buffer AW1 without wetting the rim of the spin column. Centrifuge at 6000 × g (8000 rpm) for 1 min.
Place the spin column into a clean 2 ml collection tube and discard tube with the filtrate. Carefully add 500 µl Buffer AW2 without wetting the rim. Centrifuge at 20,000 × g (14,000 rpm) for 3 minutes.
Place the QIAamp spin column in a clean 1.5 ml microcentrifuge tube and discard the tube with the filtrate. Add 200 µl water and incubate at room temperature for 1–5 minutes.
Centrifuge at 6000 × g (8000 rpm) for 1 min. The isolated DNA is in the liquid fraction.
Discard the spin column. Make sure the sample tube is labeled correctly. Store at 4°C for short term, or –20°C to −80°C for long term storage (see Note 14). See Note 15 for a discussion of potential problems.
3.2. Polymerase chain reaction amplification of individual KIR loci—General
-
1.
See Table 1 for a listing of those KIR loci that should be amplified following this protocol (see Note 16).
-
2.
Thaw 10X High Fidelity PCR buffer, 50mM MgSO4, dNTP mix, primer solutions, and DMSO or 5 M betaine solution (see Note 17). Mix the solutions thoroughly before use.
-
3.
Prepare the reaction mix in a 1.5 ml tube as described in Table 4.
-
4.
Vortex the reaction mix and dispense 45 ul volumes into each well of a semi-skirted PCR tray.
-
5.
Add 5 µl of genomic DNA (50–200 ng), purified as described in Section 3.1, to each well containing reaction mix (see Note 18).
-
6.
Set up positive and negative amplification control wells. The positive control for each primer pair is 5 µl DNA (50–200 ng) from a cell carrying that KIR locus. The negative control for each primer pair is 5 µl DNA (50–200 ng) from a cell lacking that KIR gene. For primers amplifying framework genes (KIR2DL4, KIR3DL2, and KIR3DL3), use 5 ul water as a negative control instead of DNA.
-
10.
Place tape seal over entire tray and quick spin the plate in the centrifuge to ensure all the liquid is at the bottom of the wells. Place in the thermal cycler.
-
11.
Polymerase chain reaction (PCR) conditions are described in Table 5. See Note 19.
-
12.
Prepare a 1.5% agarose gel in 1X TBE. Ethidium bromide (2 µl) should be added to the gel solution.
-
13.
After the amplification cycles are complete, confirm amplification by electrophoresis. Mix 5 µl of each amplification reaction with 2 µl of 5X sucrose cresol solution and load the entire sample into one well of the polymerized agarose gel. Electrophorese the DNA ladder as a molecular weight marker. Electrophorese at 100 volts for 20 min until the cresol red dye has reached the bottom of the gel.
-
14.
Visualize the bands by placing the gel on a UV translluminator. Photograph the gel. Using the molecular weight markers, determine the approximate molecular weight of the amplicons by comparison. The expected sizes of the amplicons for each locus are listed in Table 2. The presence of additional bands indicates a potential problem (see Note 20).
-
15.
Add the AMPure solution directly to each PCR reaction in the PCR plate. The volume of AMPure to add is 1.8 X the reaction volume. See Note 21.
-
16.
Mix thoroughly by pipeting and place the PCR plate onto a magnetic plate to separate the AMPure beads from the solution. Incubate at room temperature for approximately 5–10 minutes.
-
17.
With the PCR plate on the magnet, aspirate the cleared solution with a pipet and discard.
-
18.
Keeping the PCR plate on the magnet, dispense 200 µl of 70% ethanol to each well. Allow to sit at least 30 seconds at room temperature. Aspirate the wash solution with a pipet, discard and repeat. Be sure to remove as much ethanol as possible to shorten the drying time. Dry at room temperature for 10 min.
-
19.
To elute the purified DNA, add 30–50 ul (see Note 22) of reagent grade water to each well and mix well by pipeting up and down. Place the plate back on the magnet.
-
20.
Remove the eluate containing the amplified DNA to a clean 96 well plate to begin the DNA sequencing reactions (Section 3.8).
Table 4.
Composition of reaction master mix for Platinum Taq DNA Polymerase High Fidelity
| Component | Volume in Each Reactiona |
|---|---|
| 10X High Fidelity PCR buffer | 5 µl |
| MgSO4 (50 mM) | Variable (see Table 2) |
| dNTP (10 mM each) | 1 µl |
| Sense primer (10 µM) (see Table 2) | 2 µl |
| Antisense primer (10 µM) (see Table 2) | 2 µl |
| DMSO or 5 M betaine solution | Variable (see Table 2) |
| Platinum Taq DNA Polymerase High Fidelity (5 U/µl) | 0.5 µl |
| Template DNA | Added in later step in protocol |
| Water | Bring final volume including DNA to 50 µl |
The volume for a single reaction is 50 µl so multiple the number of amplification reactions desired by 50 to determine how much reaction master mix to make. Always make more than you need to account for losses during pipetting.
Table 5.
Polymerase chain reaction amplification conditions
| General PCR Conditions (Section 3.2) |
Nested PCR (Section 3.3) |
Long Template PCR (Section 3.7) |
|
|---|---|---|---|
| Denaturation | 95°C for 5 min | 92°C for 4 min | 92°C for 2 min |
| Initial cycles | 10 cycles: | 10 cycles:
|
10 cycles:
|
| Secondary cycles | 30 cycles: | 30 cycles
|
30 cycles:
|
| Final extension | 68°C for 10 min | 72°C for 10 min | 68°C for 10 min |
| Final hold | 4°C | 4°C | 4°C |
3.3 Nested PCR for KIR2DL2 amplicon B, KIR2DL3 amplicon A, and KIR2DS4 amplicon B
See Table 1 for a listing of those KIR loci that should be amplified following this protocol.
Thaw Taq DNA Polymerase, 10X PCR buffer with MgCl2, dNTP mix, 5 M betaine solution, and appropriate primer solutions (Table 2). Mix the solutions thoroughly before use. See Note 23.
Prepare the nested PCR reaction master mix as shown in Table 6.
Aliquot 45 ul of master mix into each well of a semiskirted PCR tray.
Add 5 ul of each purified PCR product (i.e., KIR2DL2 amplicon B, KIR2DL3 amplicon A, and KIR2DS4 amplicon B) to each well containing reaction mix.
Place in the thermal cycler and perform PCR using the protocol in Table 5.
Purify the nested PCR product of KIR2DL2 and KIR2DL3 for DNA sequencing with AMPure as described in Section 3.2.15. Purify the nested PCR product of KIR2DS4 with AMPure as described in Section 3.2.15. If required, clone the KIR2DS4 alleles as described in Section 3.6.
Table 6.
Composition of reaction master mix for nested polymerase chain reaction amplification
| Components | Volume in Each Reactiona |
|---|---|
| 10X PCR Buffer with MgCl2 | 5 µl |
| dNTP (10mM each) | 1 µl |
| Sense primer (10 µM) (Table 2) | 2 µl |
| Antisense primer (10 µM) (Table 2) | 2 µl |
| 5M betaine solution | 10 µl |
| Taq DNA Polymerase | 0.25 µl |
| Template DNA | Added at later step |
| Water | Bring final volume including DNA to 50 µl |
The volume for a single reaction is 50 µl so multiple the number of amplification reactions desired by 50 to determine how much reaction master mix to make. Always make more than you need to account for losses during pipetting.
3.4 Isolation of KIR2DL2 and KIR2DL3 using HaploPrep
Haplotype-specific extraction is performed using genomic DNA from some cell lines shown to carry KIR2DL2 and KIR2DL3 as described in Table 1.
Thaw HaploPrep KIR2DL2 and KIR2DL3 locus probes and hybridization buffer on ice (See Notes 24 and 25).
Prepare HaploPrep reaction mix as described in Table 7.
Pipet up and down to mix the reaction mix thoroughly and dispense the volume listed in Table 7 into 1.5 ml tubes.
Add 5 ul genomic DNA (30–150 ng) to each tube containing reaction mix. See Note 26.
Cap the tubes, mix well by vortexing and centrifuge briefly. Place the tubes in a heating block with a heated lid at 95°C and incubate for 15 min to denature the DNA.
Insert the EZ1 HaploPrep card into the BioRobot EZ1 following instructions from the instrument manual.
Switch on the EZ1 instrument and prepare the instrument as described in the instrument manual.
Allow the internal heating block of the EZ1 instrument to heat up to 64°C . After the 15 min incubation in step 6 is complete, remove the tubes from external heating block. Remove the caps, and place opened sample tube containing denatured samples immediately into the EZ1 instrument heating block. See Note 27.
Close the instrument door and continue to follow the instruction manual.
Once the HaploPrep-isolated DNA has been prepared, perform PCR amplification as described Section 3.2 and proceed with DNA sequencing in Section 3.8.
Table 7.
HaploPrep reaction master mix
| Components | Volume in Each Reaction |
|---|---|
| Hybridization buffer H | 15 µl |
| HaploPrep Extraction Probe: 2DL2-999T or 2DL3-1316T |
2 µl |
| Water | 8 µl |
| Genomic DNA | 5 µl (added at a later step) |
The volume for a single reaction is 25 µl without the DNA added so multiple the number of reactions desired by 25 to determine how much reaction master mix to make. Always make more than you need to account for losses during pipetting.
3.5. Isolation of KIR2DL3 locus—Restriction enzyme digestion
This protocol is performed for some cells carrying KIR2DL3 as described in Table 1.
Prepare the restriction enzyme reaction mix according to Table 8.
Mix the reaction thoroughly and dispense indicated volume from Table 8 into a 1.5 ml tube.
Add 2 ug genomic DNA to each tube containing reaction mix. Incubate at 50°C for 1 hour.
Isolate DNA by adding 200 ul phenol:chloroform:isoamyl alcohol to each tube and vortexing (see Note 7).
Centrifuge briefly (1–2 minutes) and transfer the aqueous (top) phase to a clean tube.
Add 100 ul reagent grade water to the aqueous phase and vortex. Briefly centrifuge and transfer the aqueous phase (approximately 300 ul) to a clean tube.
Add 30 ul 3M sodium acetate to the aqueous phase and place the solution at −20°C for at least 30 min.
Centrifuge at 14,000 rpm for 20 to 30 min at room temperature. Remove the liquid with a pipettor.
Wash pellet by adding 200 ul cold 70% ethanol (see Note 28).
Centrifuge for 10 min, remove the liquid with a pipettor, and air dry the pellet for approximately 20 min at room temperature.
Re-dissolve the pellet in 20 ul reagent grade water.
Perform PCR amplification as performed as described Section 3.2 and proceed with DNA sequencing in Section 3.8.
Table 8.
Restriction enzyme reaction master mix
| Components | Volume in Each Reactiona |
|---|---|
| 10X NE Buffer 3 | 20 µl |
| Bc1I | 3 µl |
| Genomic DNA | 20 µl (approximately 2 µg) |
| Water | Bring volume to 200 µl |
The volume for a single reaction is 200 µl so multiple the number of digestion reactions desired by 200 to determine how much reaction master mix to make. Always make more than you need to account for losses during pipetting.
3.6 KIR2DS4 allele isolation by cloning
-
1.
Cloning is required only for PCR amplicons containing both a full length allele and an allele with a deletion (see Note 29). Prepare a nested KIR2DS4 amplicon by PCR as described in Section 3.3.
-
2.
Verify amplified products on a 1.5% agarose gel with 1Kb DNA ladder as described in Section 3.2.13.
-
3.
Purify the PCR products using AMPure as described in Section 3.2.15.
-
4.
Using the TOPO TA cloning kit, clone the PCR product into the pCR 2.1-TOPO vector following the manufacturer’s instructions. See Note 30.
-
5.
Add 2µl of the TOPO cloning reaction to a vial of One Shot Chemical E.coli and mix gently. Incubate on ice for 5–30 minutes.
-
6.
Heat-shock the cells for 30 seconds at 42°C.
-
7.
Add 250 µl of SOC at room temperature to the tube.
-
7.
Incubate in a 37°C shaker (250 rpm) for 1 hr before plating on LB agar.
-
8.
Apply 40 µl Xgal (40 mg/ml) and 40 µl 100 mM IPTG to the surface of an LB agar plate containing ampicillin and let dry.
-
9.
To optimize distinct colonies, plate 50 ul and 100 µl of each transformation onto two separate agar plates. Incubate at 37°C overnight.
-
10.
Pick several isolated white colonies from the agar plate using a sterile toothpick. Transfer each colony of bacteria into a 0.5 ml tube containing 50 µl sterile water. See Note 31.
-
11.
Place the tubes in a heating block at 94°C for 5 min to lyse the bacteria and to inactivate nucleases. Centrifuge at 2000 rpm for 5 minutes.
-
12.
Use 5 µl of the supernatant in a 50 µl PCR reaction with the same 2DS4 nested primers and protocol as described Section 3.3.
-
13.
Verify amplification on a 1% agarose gel as described in Section 3.2.12
-
14.
Purify the PCR fragments using AMPure as described in Section 3.2.15 and proceed with DNA sequencing in Section 3.8.
3.7. Long template PCR for KIR3DL1 B and KIR3DS1 B amplicons
Amplification of long segments of DNA from KIR3DL1 and KIR3DL2 will require this protocol (Table 1). Thaw 10X Expand Long Template buffer 3, dNTP mix, and primer solutions for KIR3DL1 B and KIR3S1 B amplicons (Table 2). Vortex the solutions thoroughly before use (See Note 32).
Assemble the reaction mix for the Expand Long Template PCR System as described in Table 9.
Vortex the reaction mix thoroughtly and dispense 45 ul volumes into each well of semi-skirted PCR tray.
Add 5 µl template DNA (100–200 ng) to each well containing reaction mix (See Note 33).
Set up positive and negative control wells as described in Section 3.2.6.
Place in the thermal cycler and perform PCR using the protocol in Table 5.
Check for amplification of a band of appropriate size by electrophoresis on a 1.0% agarose gel stained with ethidium bromide as described in Section 3.2.12.
Purify and elute the PCR product with AMPure as described in Section 3.2.15 and proceed with DNA sequencing in Section 3.8.
Table 9.
Composition of reaction master mix for Expand Long Template PCR Reaction
| Components | Volume in Each Reactiona |
|---|---|
| 10X Expand Long Template Buffer 3 | 5 µl |
| dNTP (10mM) | 2.5 µl |
| Forward primer (10 µM) (Table 2) | 1.5 µl |
| Reverse primer (10 µM) (Table 2) | 1.5 µl |
| Expand Long Template Enzyme mix | 0.75 µl |
| Template DNA | Added at later step |
| Water | Bring final volume including DNA to 50 µl |
The volume for a single reaction is 50 µl so multiple the number of amplification reactions desired by 50 to determine how much reaction master mix to make. Always make more than you need to account for losses during pipetting.
3.8 DNA sequencing
Sequence the amplicons using KIR loci sequencing primers (Table 3). For each locus, both sense and antisense primers are used to cover the complete sequence of the exons (Figure 1) (See Note 34).
To each well, add 2 ul of diluted Big Dye Terminator, 1 ul of the appropriate primer (Table 3) and 3 ul of the purified PCR product. For exon 1 sequences for all KIR loci, add 0.3 ul DMSO to the reaction (see Note 35).
Place tape seal over entire tray and quick spin the plate in the centrifuge to ensure all liquid is at the bottom of the wells. Place in the thermal cycler.
Perform the DNA sequencing reaction using the protocol in Table 10.
Use the Agencourt CleanSEQ kit to remove excess dye terminators from the sequence reaction by adding 10 µl of CleanSEQ magnetic beads solution to each well of the sequencing plate.
For a 10 µl sequencing reaction, add approximately 75 µl 73% ethanol to each well and mix thoroughly.
Place the sequencing plate onto the magnet to separate the beads from the solution. Incubate approximately 3 minutes at room temperature.
With the sequencing plate on the magnet, aspirate the cleared solution with a pipet and discard.
Keeping the plate on the magnet, dispense 100 µl 73% ethanol to each well and allow it to sit for at least 30 seconds at room temperature. Aspirate the solution and discard.
Add 30 ul of water to each well. The reactions are now ready to electrophorese on the DNA analyzer.
Follow the instructions for operation of the DNA analyzer. The samples are electrophoresed using ABI RunModule “Rapidseq 36_POP7” with the default values. Longer electrophoresis times may be required for some sequences.
Sample files are analyzed as described in Section 3.9.
Table 10.
DNA sequencing reaction conditions
| Conditions for All Exons Except Exon 1 |
Conditions for Exon 1 |
|---|---|
30 cycles:
|
30 cycles:
|
| Hold at 4°C | |
3.9 Sequence analysis including preparation of locus-specific KIR libraries
Locus-specific KIR libraries must be created prior to analysis of KIR sequencing data. Go to the IPD-KIR database downloads and open up the FTP directory. Obtain the nucleotide coding region sequences of all known alleles at each KIR locus as nuc.fasta files (e.g., KIR2DL1_nuc.fasta; one file for each locus). Create two separate libraries for KIR2DS4, one library with the full length allele sequences and a second library with the sequences of the alleles exhibiting the 22 base pair deletion.
Manually add the intron 8 genomic sequence from one representative allele from each locus to the nucleotide sequence of every allele at the locus. Use the database of the Leukocyte Receptor Complex to obtain the intron sequence from the genomic DNA. See Note 36.
Manually add the 247 base pair genomic sequences found 5’ of exon 1 to the KIR2DL5 locus allele sequences (see Note 37).
Use HLA Librarian to create a sequence library and reference file for each locus following the Library Builder user’s guide.
Import each nuc.fasta file containing intron 8 sequences into HLA Librarian assigning a name for the library and reference files (e.g., 2DL1). Enter information into the reference file as indicated including the position of nucleotides at the 5’ and 3’ ends of each exon.
Output the files to the Assign directory following instructions in the Assign user’s guide.
The library should be validated by interpreting the sequences of multiple known KIR alleles obtained by sequencing both homozygous and heterozygous reference cell DNA.
Once the library has been created, use Assign SBT 3.2.7 software to interpret sequencing results and assign alleles (see Notes 38 and 39).
The library should be updated with newer versions of the IPD-KIR database as required (see Note 40).
Footnotes
Blood (8.5 ml) is collected by venipuncture into a yellow top ACD-A tube. ACD is the preferred anticoagulant. Other anticoagulants (e.g., heparin) may inhibit DNA amplification during the polymerase chain reaction. Blood can be aliquoted into 2 ml tubes and stored at −20°C until use. An alternative sample source is a[0] buccal swab but it is likely that the yield of DNA will be low and insufficient for sequencing of all KIR loci. Blood should be treated as a biohazard and handled with caution.
The panel of reference cells should include cells that lack specific KIR genes as well as cells that carry specific KIR genes. It is helpful to know the KIR alleles carried by the cells so that they can serve as controls for the assignment of KIR alleles.
Aliquot diluted primers. Repeated freezing and thawing of diluted oligonucleotide primers should be avoided.
The DNA ladder should range in size between 400 base pairs (bp) and 13,000 bp. It is helpful to have markers every 500 bp to 1000 bp. A high DNA mass ladder (Invitrogen) is also helpful when judging the approximate quantity of amplicon present.
Handle carefully; ethidium bromide is a carcinogen.
It is critical to have a heated lid for the Haploprep protocol.
Handle phenol:chloroform:isoamyl alcohol carefully and work in a fume hood. Alternatives to phenol:chloroform:isoamyl alcohol extraction might be use of the Agencourt AMPure kit (Beckman Coulter, Beverly, MA, USA) or Amicon Ultra centrifugal filters (Millipore, Billerica, MA, USA) but the authors have not tested these products in this protocol.
Aliquot diluted primers. Repeated freezing and thawing of diluted oligonucleotide primers should be avoided.
Assign is used to obtain KIR allele assignments from the DNA sequences obtained. HLA Librarian is used to create the locus specific KIR libraries. Sequencher with its library of full length genomic sequences and coding region sequences is used to confirm the annealing site of PCR and sequencing primers, to design new primers, and to aid in assigning alleles in unusual sequences.
Amplicons generated in previous PCR reactions are a[0] source of sample contamination. By separating the source of the amplicons (i.e., post-PCR activities as defined by thermal cycling and subsequent steps) from the pre-PCR activities (as defined by all steps up to and including assembly of the PCR reaction just prior to placing in the thermal cycler), the potential for contamination is greatly reduced. Ideally, the pre-PCR and post-PCR procedures should be performed in two different rooms, but, if not available, different areas of the laboratory should be set aside. If all activities are to be performed in a single room, pre-PCR activities should occur inside a laminar flow hood, preferably equipped with a UV light. The walls of the hood should be wiped with a freshly made 10% bleach solution (1 part regular bleach: 9 parts tap water) before processing samples or preparing PCR samples. Dedicated equipment (e.g., pipettors, test tube racks) and lab coats should be set aside for pre-PCR procedures.
Typically, 200 ul of whole blood from a healthy individual will yield 3–12 ug of DNA. Sequencing of each KIR locus requires approximated 500 ng DNA. To sequence all the KIR loci, 5–10 µg of genomic DNA is required.
Never add Buffer AL directly to the protease. To obtain complete lysis, the sample and the Buffer AL must be mixed immediately and thoroughly.
The speed of the quick spin should be above 1000 rpm. Set the speed to 8000 rpm; press the button for 5 seconds and release to achieve this speed.
DNA should be stored in a neutral to slightly basic buffered solution to prevent degradation. Tris EDTA (TE) buffer can be used for storage. TE contains EDTA which has a high affinity towards divalent ions like Ca+2 and Mg+2. These ions are cofactors for many enzymes including nucleases that digest DNA molecules. Since repeated access to a tube of genomic DNA may introduce nucleases, TE buffer will protect DNA from degradation during long term storage. However, since EDTA can bind divalent ions, it can inhibit Taq polymerase in the PCR reaction. If DNA is stored in deionized water which is often at an acidic pH, DNA degradation can occur by acid hydrolysis.
Refer to the QIAampR DNA Mini Kit handbook for troubleshooting problems.
It is helpful to initially assay for the presence or absence of KIR genes using a sequence-specific priming assay as described in Chapter ????. This will facilitate the selection of protocols to use to isolate KIR genes for sequencing as described in Table 1. Methods described in this chapter have been published (10),(11),(12),(13) (Hou, in preparation).
Some KIR haplotypes include fusion genes. For example, KIR3DL1/KIR3DL2 hybrid alleles have been found in populations of recent African origin (14),(13). These alleles carry the first five exons of KIR3DL1 and exons 6–9 of KIR3DL2. The KIR3DL1 primer pairs in this protocol will amplify this chimeric gene. When sequencing amplicon B of KIR2DL4, be alert for a single nucleotide deletion that removes the last nucleotide (811) of exon 7 in some alleles (e.g., KIR2DL4*008). When sequencing KIR2DL5, it is possible that a cell may carry three or four alleles i.e., two alleles of KIR2DL5A and two alleles of KIR2DL5B are potentially possible. An additional two primer pair pairs listed in Table 1 will assist in clarifying the allele calls in this situation. These pairs are each specific for a subset of KIR2DL5 alleles. Sequencing primers used with KIR2DL5 amplicon A will anneal to these two amplicons.
The polymerase and buffer used in the PCR reaction vary for different loci and are described in Table 2. DMSO or 5 M betaine solution can improve and enhance the specificity of the polymerase chain reaction. The volumes in each reaction of MgSO4, DMSO, and 5 M betaine solution are provided in Table 2.
It is critical to have high quality DNA for the PCR reaction. To quantify the DNA and to determine its purity, read its optical density (OD) using a spectrophotometer. The NanoDrop spectrophotometer (e.g., NanoDrop ND-1000, NanoDrop Technologies, Inc. Wilmington, DE USA) uses very small quantities of the solution so it or a similar instrument is recommended. The DNA concentration at OD 260 nm should be >10 ng /µl (OD260 × dilution factor × 50 = ng/µl). The purity as measured by the ratio of the absorbance at 260 nm/absorbance at 280 nm (measuring protein contamination) should be in the 1.65-1.9 range.
The thermal cycler should be calibrated at regular intervals to insure that the temperatures required for PCR are achieved in all of the wells of the thermal cycler. This should be done at least every 6 months or more frequently depending on the usage. The Driftcon Temperature Verification System (CYCLERtest, Landgraaf, Netherlands) is one instrument that might be used if this calibration is performed in-house.
The molecular weight markers should be present as single sharp bands. The cresol red dye runs at approximately 125 base pairs. Each PCR reaction should yield a single bright band of the expected size (Table 2). [The deletion present in some KIR2DS4 alleles does not make a visible difference in the mobility of the band compared to alleles without the deletion.] The presence of additional bands suggests that the amplification conditions were less stringent than required and the primer annealing temperature should be raised until a single band is produced. The absence of a band may indicate that the gene is absent (see Note 16) or that the amplification conditions are too stringent. To reduce stingency, lower the annealing temperature until a single strong band is produced. Amplification of a locus or of one of two alleles at a locus may fail if the allele carries a nucleotide sequence variation in a primer annealing site.
The AMPure kit will remove unincorporated primers, dNTPs and salts following the PCR reaction.
Comparison of the intensity of staining of a reference mass ladder (See Note 4) to the staining intensity of an amplicon following gel electrophoresis can be used to estimate the amount of amplified DNA in the reaction. In turn, this information can be used to determine the amount of water used to elute purified DNA from the AMPure beads. If the concentration of DNA is low, elute with 30 µl instead of 50 µl of water.
Perform the protocol in the post-PCR laboratory since nested PCR uses amplified DNA as a template. Use aliquots of PCR reagents and do not return them to the pre-PCR room.
Probe 2DL2-999T targets nucleotide position 708 in exon 6 shared by all known KIR2DL2 alleles except KIR2DL2*004. Probe 2DL3-1316T targets nucleotide position 1024T in exon 9 shared by all known KIR2DL3 alleles. If KIR2DL2*004 is present, the allele can be assigned based on amplicon A but cloning or allele-specific nested PCR of the B and C amplicons must be used to obtain the complete allele sequence. The strategies used will depend on the other KIR genes found in the sample and co-amplifying with KIR2DL2*004.
It is critical that the buffer be thawed on ice. HaploPrep reagents must be always kept on ice when working with them on the bench.
It is critical that the DNA is not sheared so avoid excessive pipetting or vortexing.
It is critical that the solution be maintained at a high temperature to prevent renaturation of the DNA prior to exposure to the HaploPrep reagents.
Be careful not to lose the pellet.
A known 22 base pair deletion in some alleles of KIR2DS4 will make sequencing difficult if such an allele is found together with an allele lacking the deletion. The reading frame will be shifted resulting in uninterpretable sequences in the region of the deletion. In these cases, it is necessary to separate the two alleles by cloning in order to obtain a clear sequence of each allele in this region.
The amplified DNA should be obtained by PCR just prior to cloning.
The efficiency at which inserts are obtained should be at least 70–80%. The white colonies contain inserted DNA (e.g., KIR2DS4); the blue colonies do not contain an insert.
It is essential to vortex buffer 3 until the salt is in solution.
Ensure that template DNA is of sufficiently high quality and is not degraded. Avoid vigorous mixing or pipetting of the solution to prevent DNA from shearing.
The KIR sequencing primers flank each exon with the exception of exon 1 and the last two exons (exon 8 and exon 9). The sequences of exon 1 for all loci except KIR2DL5 are obtained using only an antisense primer. Since the PCR amplification primers anneal just 5’ of exon 1, it is not possible to obtain a complete “read” of exon 1 sequence using either internal forward primers or the forward PCR primers as sense strand sequencing primers. The KIR2DL5 A amplicon includes 274 base pairs of the 5’ upstream region so that transcription factor binding sites impacting gene expression (15) can be evaluated. For exons 8 and 9, one sequencing primer anneals 5’ of exon 8 and the second anneals 3’ of exon 9 so that the resultant sequence includes intron 8.
All exon 1 sequence reactions require 5% DMSO. The thermal cycler profile for the sequencing reaction for exon 1 is shown in Table 10 and does not include a primer annealing step. The sequence of exon 1 is very short and the antisense primer site has repeated sequences so that higher denaturation and annealing temperatures are required.
It is recommended that locus specific libraries be created to facilitate the interpretation of KIR nucleotide sequences. The intron 8 data are not analyzed so it doesn’t matter that the intron 8 sequence in the library comes from a single allele. It is also helpful to have the same length of nucleotides in the intron 8 library sequence so don’t insert the intron 8 sequences from multiple alleles.
The 5’ sequences for each KIR2DL5 allele can be found in the IPD-KIR database, in GenBank and in publications.
The primarily heterozygous sequences are compared to a database of known KIR sequences created in this section to identify alleles. The library does not need to be created each time DNA sequencing is performed. Manual inspection of the chromatograph should be performed to confirm assigned sequences and to exclude closely related sequences. Be alert to the presence of novel alleles.
The allele assignments for multiple loci should be consistent with known telomere and centromere haplotype structures (summarized by (5)). For example, essentially all KIR haplotypes carry the framework genes, KIR3DL3, KIR2DL4, and KIR3DL2. Since KIR2DL2 and KIR2DL3 are alleles at a single locus, the cell should not carry more than a total of 2 alleles (e.g., two alleles of KIR2DL2 with KIR2DL3 absent, not two alleles at KIR2DL2 and one allele at KIR2DL3). The same is true for KIR3DL1 and KIR3DS1. The KIR2DL5 locus has been duplicated; the two genes are termed KIR2DL5A and KIR2DL5B. KIR2DL5A and KIR2DL5B should be associated with either KIR2DS3 or KIR2DS5 and specific combinations of alleles at these loci have been observed (16),(11). It should be noted that other KIR haplotypes have been described at lower frequencies, for example, a haplotype with a duplication so that an individual carries two KIR3DL1 alleles and a KIR3DS1 allele (14),(17),(18).
Poor quality sequences should not be interpreted and sequencing of those samples should be repeated.
The known KIR allele database, IPD-KIR, is updated at least annually with new, modified or deleted alleles.
References
- 1.Bashirova AA, et al. The Killer Immunoglobulin-Like Receptor Gene Cluster: Tuning the Genome for Defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 2.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khakoo SI, Carrington M. KIR and disease: a model system or system of models? Immunol Rev. 2006;214:186–201. doi: 10.1111/j.1600-065X.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 4.Tomblyn M, et al. Decreased Infections in Recipients of Unrelated Donor Hematopoietic Cell Transplantation from Donors with an Activating KIR Genotype. Biol Blood Marrow Transplant. 2010;16:1155–1161. doi: 10.1016/j.bbmt.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooley S, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010 doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moesta AK, et al. Synergistic polymorphism at two positions distal to the ligandbinding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180:3969–3979. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 7.Yawata M, et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing selfresponse. Blood. 2008;112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma D, et al. Dimorphic motifs in D0 and D1+D2 domains of killer cell Ig-like receptor 3DL1 combine to form receptors with high, moderate, and no avidity for the complex of a peptide derived from HIV and HLA-A*2402. J Immunol. 2009;183:4569–4582. doi: 10.4049/jimmunol.0901734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou L, et al. African Americans exhibit a predominant allele in the midst of extensive KIR2DL1 allelic diversity. Tissue Antigens. 2010;76:31–34. doi: 10.1111/j.1399-0039.2010.01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou L, et al. Thirty allele-level haplotypes centered around KIR2DL5 define the diversity in an African American population. Immunogenetics. 2010;62:491–498. doi: 10.1007/s00251-010-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L, et al. In contrast to other stimulatory natural killer cell immunoglobulin-like receptor loci, several KIR2DS5 alleles predominate in African Americans. Hum Immunol. 2009;70:733–737. doi: 10.1016/j.humimm.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang B, et al. The profile of KIR3DL1 and KIR3DS1 alleles in an African American population resembles that found in African populations. Tissue Antigens. 2010;76:64–66. doi: 10.1111/j.1399-0039.2010.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman PJ, et al. Meiotic recombination generates rich diversity in NK cell receptor genes, alleles, and haplotypes. Genome Res. 2009;19:757–769. doi: 10.1101/gr.085738.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilches C, Gardiner CM, Parham P. Gene structure and promoter variation of expressed and nonexpressed variants of the KIR2DL5 gene. J Immunol. 2000;165:6416–6421. doi: 10.4049/jimmunol.165.11.6416. [DOI] [PubMed] [Google Scholar]
- 16.Ordonez D, et al. Duplication, mutation and recombination of the human orphan gene KIR2DS3 contribute to the diversity of KIR haplotypes. Genes Immun. 2008;9:431–437. doi: 10.1038/gene.2008.34. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Lozano N, et al. The silent KIR3DP1 gene (CD158c) is transcribed and might encode a secreted receptor in a minority of humans, in whom the KIR3DP1, KIR2DL4 and KIR3DL1/KIR3DS1 genes are duplicated. Eur J Immunol. 2005;35:16–24. doi: 10.1002/eji.200425493. [DOI] [PubMed] [Google Scholar]
- 18.Martin MP, et al. Cutting edge: expansion of the KIR locus by unequal crossing over. J Immunol. 2003;171:2192–2195. doi: 10.4049/jimmunol.171.5.2192. [DOI] [PubMed] [Google Scholar]
- 19.Uhrberg M, et al. Human diversity in killer cell inhibitory receptor genes. Immunity. 1997;7:753–763. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 20.Vilches C, et al. Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70:415–422. doi: 10.1111/j.1399-0039.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Lozano N, Vilches C. Genotyping of human killer-cell immunoglobulin-like receptor genes by polymerase chain reaction with sequence-specific primers: An update. Tissue Antigens. 2002;59:184–193. doi: 10.1034/j.1399-0039.2002.590302.x. [DOI] [PubMed] [Google Scholar]
- 22.Murdoch S, et al. Detailed gene and allele content analysis of three homozygous KIR haplotypes. Tissue Antigens. 2006;68:72–77. doi: 10.1111/j.1399-0039.2006.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Sun JY, Oki A, Senitzer D. Alleles and intron polymorphism of KIR3DL1 shown by combination of allele group-specific primers and sequencing. Tissue Antigens. 2008;72:578–580. doi: 10.1111/j.1399-0039.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 24.Vilches C, Pando MJ, Parham P. Genes encoding human killer-cell Ig-like receptors with D1 and D2 extracellular domains all contain untranslated pseudoexons encoding a third Ig-like domain. Immunogenetics. 2000;51:639–646. doi: 10.1007/s002510000184. [DOI] [PubMed] [Google Scholar]