Abstract
PGE2 is a major prostanoid found in the kidney and vasculature contributing to the regulation of blood pressure. The PGE2 receptor EP1 has been shown to contribute to hypertension by mediating angiotensin II-dependent vasoconstriction, although its precise role is incompletely characterized. Disruption of the EP1 receptor in C57BL/6J mice reduced the incidence of mortality during severe hypertension induced by uninephrectomy, deoxycorticosterone acetate, and angiotensin II. Mortality was dependent on all components of the model. Death was a result of aortic aneurysm rupture or occurred after development of anasarca, each of which was reduced in EP1−/− mice. Mean arterial pressure was increased in treated EP1+/+ and EP1−/− mice, however this elevation was significantly lower in EP1−/− mice. Blood pressure reduction via administration of hydralazine phenocopied EP1−/− mice. Thus reduction in blood pressure by disruption of EP1 reduced incidence of mortality and decreased organ damage suggesting that EP1 receptor blockade may be a viable target for anti-hypertensive therapy.
Keywords: prostaglandins, hypertension, aorta, aneurysm, edema
Introduction
Hypertension is a major risk factor for cardiovascular diseases (CVD), increasing the risk of stroke, myocardial infarction, arterial aneurysms, and heart failure. Approximately 30 % of adults in the US have hypertension and the incidence of CVD remains greater in hypertensive patients than normotensive patients, highlighting the need for novel therapeutic agents 1.
Prostaglandin E2 (PGE2) is a major prostanoid found in the mouse kidney and vasculature contributing to the regulation of blood pressure, where it can exert either vasopressor or vasodepressor effects 2–4. Four PGE2 receptors (EP1 through EP4) mediate these effects with the EP1 and EP3 receptors primarily mediating the pressor response of PGE2, while the EP2 and EP4 receptors mediate the depressor response 2,5–10.
Each PGE2 receptor has distinct tissue localization and elicits characteristic signal transduction pathways 11. EP1 couples to Gq-proteins, resulting in mobilization of intracellular calcium, and stimulation of phosphoinositide turnover activating protein kinase C. The EP2 and EP4 receptors couple to Gs-proteins, which increase intracellular cAMP. The EP3 receptor couples to Gi-proteins, decreasing intracellular cAMP (For reviews, see 11,12). Receptors couple to alternative signal transduction pathways as well, including arrestin-mediated signaling pathways 13,14.
Systemic infusion of PGE2 results in a vasodepressor response 2,5,15, primarily through EP2 activation. In the absence of EP2, a PGE2 pressor response is unmasked 2, mediated by the EP3 receptor 8. Agonist induced EP3 tachyphylaxis in the background of EP2 −/− mice uncovers a depressor action of EP4 8,16. EP1 does not appear to play a significant role in the blood pressure effects of systemically administered PGE2, however, it does contribute to hypertension. Genetic disruption of the EP1 receptor in mice has been shown to decrease blood pressure, particularly when mice are fed a low sodium diet 17. Furthermore, EP1 −/− mice have blunted pressor responses to both acute and chronic angiotensin II (Ang II) administration 10. In isolated vessel preparations, pre-treatment with the EP1 selective antagonist SC51322 reduced Ang II mediated vasoconstriction 10. Treatment of spontaneously hypertensive rats with SC51322 significantly reduces blood pressure 10, indicating blockade of the EP1 receptor may be a target for the treatment of hypertension.
EP1 blockade has been shown to positively affect renal function in stroke-prone spontaneously hypertensive rats 18, as well as cerebrovascular dysfunction induced by Ang II 19, implicating the EP1 receptor in hypertension and resultant end-organ damage. This has yet to be investigated in detail and in the context of other organ damage. Therefore, EP1+/+ and EP1−/− mice were studied in a model of severe hypertension.
Methods
Complete methods can be found in the supplemental materials expanded methods section.
Results
EP1−/− mice were protected against Nphx/DOCA-NaCl/Ang II mortality
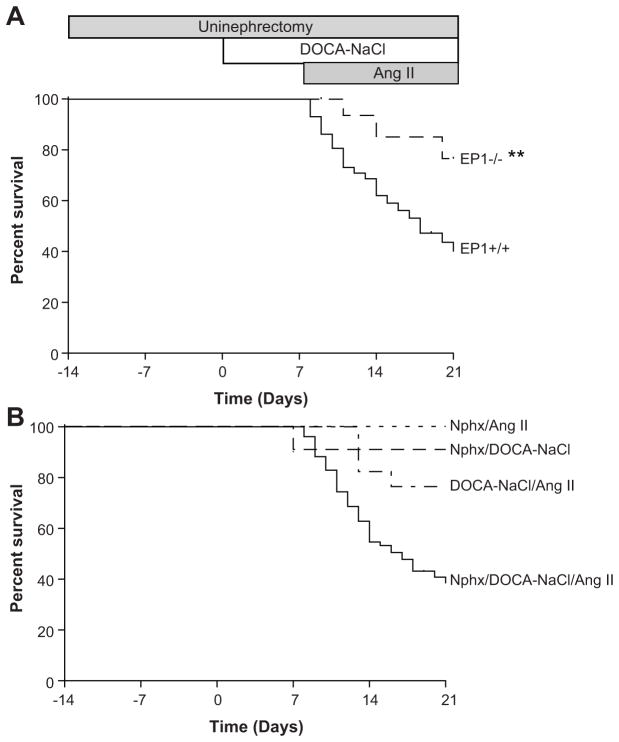
We employed a model of severe hypertension to investigate the contribution of the EP1 receptor in hypertensive end-organ damage 20. Unexpected mortality was observed following implantation of the Ang II osmotic pump (Figure 1A). Of 58 EP1+/+ mice, 60 % died within 14 days. EP1−/− mice were significantly protected against mortality; of 35 EP1−/− mice only 24 % died (P = 0.0044). Modified protocols omitting one of the three components (Nphx, DOCA-NaCl, or Ang II) demonstrated all components of the model played an essential role in causing mortality (Figure 1B, Nphx/DOCA-NaCl/Ang II vs. Nphx/DOCA-NaCl, Nphx/Ang II or DOCA-NaCl/Ang II, N = 10 per group, P = 0.011, < 0.005, or < 0.005 respectively).
Figure 1.
Survival. A. Survival of EP1+/+ and EP1−/− mice. EP1+/+ mice experience a high mortality rate after implantation of the Ang II-minipump (60 %, N=58) which is significantly reduced in EP1−/− mice (25 %, N=35). Kaplan Meier survival curves for each genotype are plotted, **P = 0.004. B. Survival of EP1+/+ modified protocol groups. Survival curves were plotted indicating reduced mortality in all modified protocol groups. Nphx/DOCA-NaCl/Ang II (data replotted from Figure 1A) vs. Nphx/DOCA-NaCl, Nphx/Ang II or DOCA-NaCl/Ang II, P = 0.011, <0.005, or < 0.005.
EP1−/− mice had reduced aortic aneurysm rupture, but comparable aortic histopathology
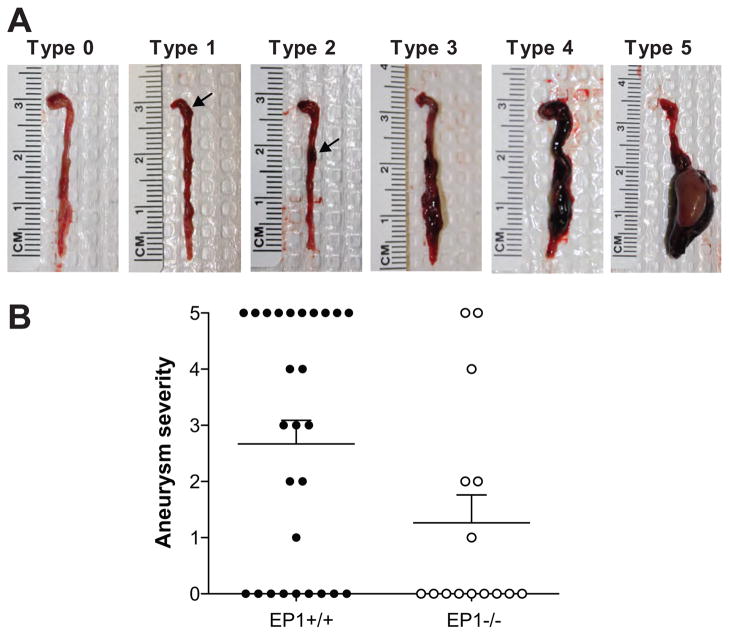
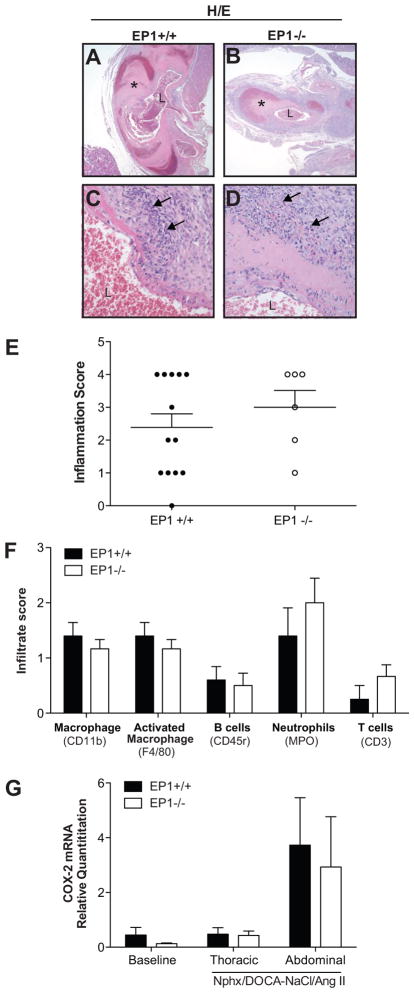
Post mortem analysis of EP1+/+ and EP1−/− mice in the full model indicated a significant portion of the mice died as a result of rupture of the aorta. Aneurysms and dissections were observed in both the thoracic and abdominal aorta. 37 % of EP1+/+ and 13 % of EP1−/− mice died due to aortic rupture. Aortic aneurysms were present in 67 % of EP1+/+ mice and 40 % of EP1−/− mice. Aneurysm severity was scored at death on a scale of 0–5 (Figure 2A). A reduction in aneurysm severity was observed in EP1−/− mice compared to EP1+/+ mice (Figure 2B, P = 0.049). Hematoxylin and eosin (H/E) staining revealed aneurysms and dissections in the wall of the thoracic and abdominal aorta were accompanied by inflammation in both EP1+/+ and EP1−/− mice (Figure 3A–E, P = 0.3975). Analysis of aortic sections from both thoracic and abdominal lesions of each genotype demonstrated macrophage and neutrophils were most abundant, with no differences observed between the genotypes in any immune cell component (Figure 3F). Cyclooxygenase-2 (COX-2) has been shown to play a significant role in aortic aneurysm formation and macrophage infiltration 23,24. COX-2 mRNA was elevated in abdominal aortae 2–5 days post Ang II administration, though differences between genotypes were not observed (Figure 3G, P > 0.774). Aortic sections stained with Masson’s Trichrome showed that regardless of genotype there was less fibrillar collagen present in vessels that ruptured, and the amount and organization of collagen surrounding the lesion was not significantly different in EP1+/+ and EP1−/− intact aneurysms (Figure S1, P = 0.1925).
Figure 2.
Aortic aneurysm formation in EP1+/+ and EP1−/− mice. A. Nphx/DOCA-NaCl/Ang II treatment resulted in formation of aneurysms and dissections in the thoracic and abdominal aorta. Aortae were scored visually upon necropsy and representative images are shown. B. Post-mortem examination revealed 18 of 27 EP1+/+ mice and 6 of 15 EP1−/− mice developed aneurysms as a result of the Nphx/DOCA-NaCl/Ang II model. Fisher’s exact test, EP1+/+ vs. EP1−/− Severity < 3 vs. ≥ 3, P = 0.049.
Figure 3.
Aortic inflammation. A–D. H/E stained aortae (40X and 400X magnification). A ruptured aneurysm in an EP1+/+ mouse (A, C) and a large aneurysm without rupture in an EP1−/− mouse (B, D). Vessel necrosis and perivascular inflammatory infiltration of macrophages and neutrophils are observed under high magnification (arrows, C and D). L = vessel lumen, * = aneurysm. E. Overall inflammation was scored based on H/E stained aortic sections. No differences in inflammation were observed between EP1+/+ and EP1−/− aortas (P = 0.3975, N = 5=6). F. Immunohistochemistry for detection of macrophage, B cells, T cells and neutrophils in aortae was performed and scored by in a blinded fashion by a comparative veterinary pathologist. No differences in infiltrate were observed (P > 0.05, N = 5–6). G. COX-2 mRNA in the thoracic and abdominal aortae 2–5 days post Ang II administration revealed a trend in increased COX-2 expression in the abdominal aorta of both EP1+/+ and EP1−/− mice post treatment. No significant differences were observed by treatment or between genotypes (P > 0.05, N = 3–8).
Anasarca was observed in EP1+/+ mice
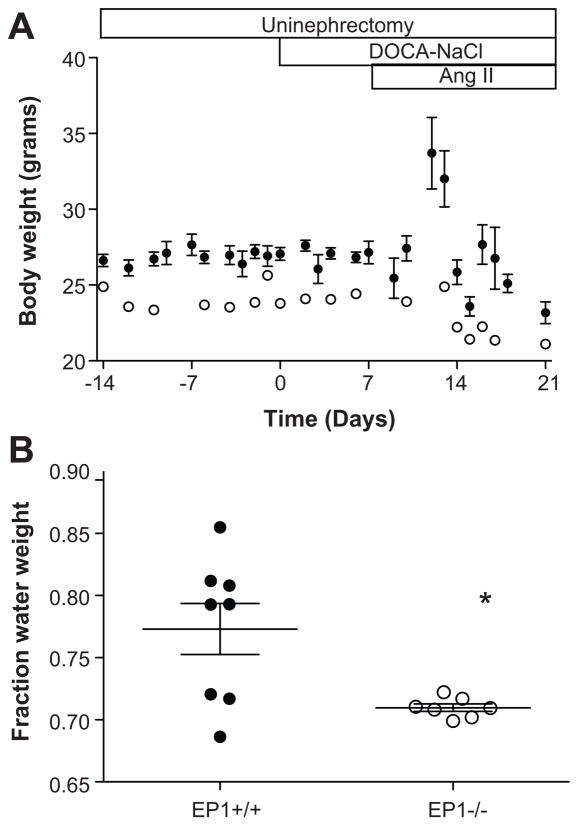
A subset of EP1+/+ mice appeared to have substantial edema and displayed an increase in body weight, peaking approximately five days post-Ang II administration (Figure 4A). Average body weight in EP1 +/+ cohort subsequently decreased due to mortality in the animals with the largest weight gain. At baseline, EP1+/+ mice weighed more than EP1−/− mice though this difference was modest (EP1+/+ 26.6 ± 0.39 grams, EP1−/− 27.9 ± 0.68 grams, P = 0.024). Body weight of EP1−/− mice was unchanged over the course of the study. EP1+/+ mice had a significantly greater fraction water weight as compared to EP1−/− mice (Figure 4B, P = 0.0138), and aneurysm incidence was lower in mice which developed anasarca compared to mice without anasarca (33 % vs 76 %). Anasarca is commonly a result of liver failure, nephrotic syndrome or heart failure 25,26. Plasma alanine transaminase (ALT) activity was analyzed as a marker of liver function in Nphx/DOCA-NaCl/Ang II treated EP1+/+ mice. ALT was not elevated above baseline values nor did they correlate with body weight (data not shown).
Figure 4.
Body weight changes during Nphx/DOCA-NaCl/Ang II treatment. A. Average body weight of EP1+/+ (N=50) and EP1−/− mice (N=22) throughout the duration of the experiment. Body weight increased in EP1+/+ mice, but not EP1−/− mice, following Ang II administration. Average body weight declined at approximately five days post-Ang II due to mortality in the subset of mice which gained the most weight (open symbols, EP1−/−; closed symbols, EP1+/+). B. Fraction water weight 5 days post-angiotensin II administration. The fraction water weight was significantly greater in EP1+/+ mice, as compared to EP1−/− mice, P = 0.0138.
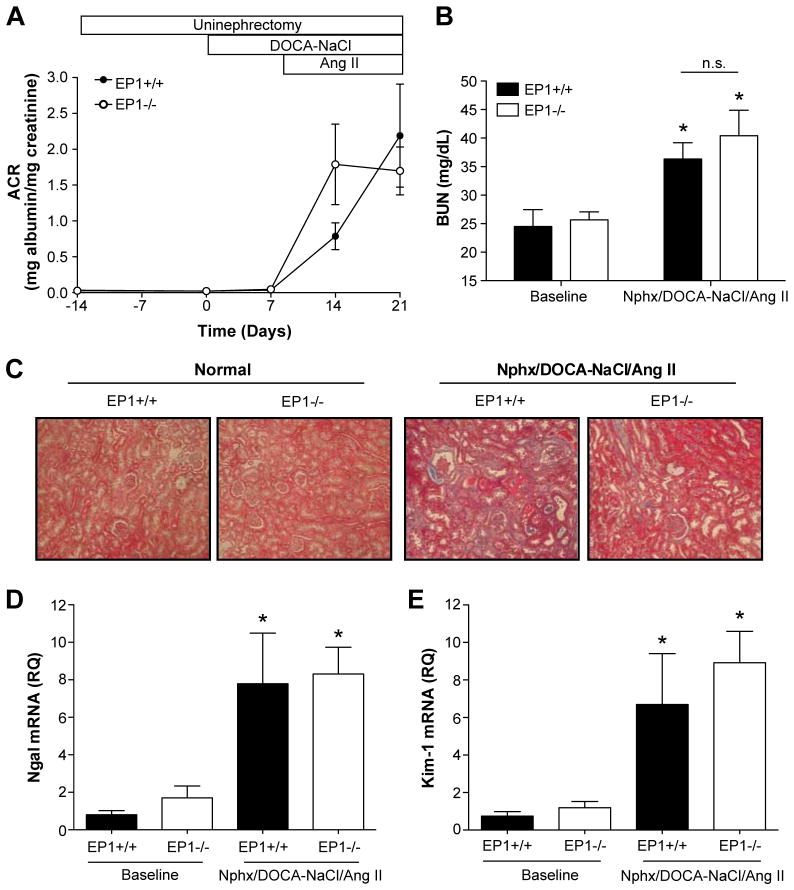
Modest renal injury was induced in EP1+/+ and EP1−/− mice
The Nphx/DOCA-NaCl/Ang II model was initially developed to induce hypertensive renal damage on the C57BL/6 background 20. To quantify renal damage in the EP1+/+ and EP1−/− mice, we monitored urinary albumin excretion, blood urea nitrogen, renal histopathology, and biomarkers of acute kidney injury, neutrophil gelatinase-associated lipocalin (Ngal) and kidney injury molecule-1 (Kim-1) mRNA expression (Figure 5). ACR and BUN were elevated but no significant differences were observed between genotypes (Figure 5A, B). Renal histopathology showed modest hypertensive renal damage compared to the contralateral kidney removed at time of uninephrectomy (Figure 5C). Dilated tubules with moderate glomerularsclerosis and tubulointerstitial fibrosis were observed. Significant increases in Ngal and Kim-1 mRNA expression in the kidney of EP1+/+ and EP1−/− mice were observed, though no differences were detected between genotypes (Figure 5D–E).
Figure 5.
Renal outcome throughout Nphx/DOCA-NaCl/Ang II model. A. Urinary albumin excretion. ACR was determined on spot urine collected throughout the duration of the experiment. High albumin levels were detected in the urine after the addition of Ang II and no significant differences were observed in ACR between the genotypes (Fisher’s Exact test P = 0.4559, N = 8–16). B. Blood urea nitrogen levels were modestly increased with Nphx/DOCA-NaCl/Ang II treatment compared to baseline (EP1+/+ P = 0.0382, EP1−/− P = 0.0132), though no differences in BUN were observed between genotypes (P = 0.5867, N = 3–7). C. Renal histopathology. Masson’s trichrome stain revealed modest hypertensive renal damage observed in both genotypes following full model treatment (right panels) and compared to normal kidneys removed at time of uninephrectomy (left panels). Specifically, dilated tubules with moderate glomerular sclerosis and tubulointerstitial fibrosis were observed. D and E. Quantification of Ngal and Kim-1 mRNA expression in whole kidneys. Expression of clinically used renal injury biomarkers, Ngal and Kim-1, revealed significant increases in both genotypes treated with the full model (Ngal: EP1+/+ P = 0.0029, EP1−/− P = 0.0008; Kim-1: EP1+/+ P = 0.007, EP1−/− P = 0.0002), though no significant differences between EP1+/+ and EP1−/− mRNA levels were observed (Ngal P = 0.8609, Kim-1 P = 0.4931, N = 3–7).
Cardiac function is reduced in EP1+/+ and EP1−/− mice
No structural differences in the heart were observed by echocardiography between EP1+/+ and EP1−/− mice at baseline. A modest increase in ejection fraction and fractional shortening was observed in EP1−/− mice at baseline. At five days post-Ang II administration, EP1+/+ had increased left ventricular posterior wall (LVPWd) and interventricular septum diastolic diameters (IVSd). EP1−/− mice had increased IVSd, though no significant change in LVPWd was observed. EP1+/+ and EP1−/− mice displayed increased left ventricular interior diameter, though no differences were observed between genotypes. Cardiac function was significantly reduced in both genotypes upon treatment with Nphx/DOCA-NaCl/Ang II, as demonstrated by decreased fractional shortening and ejection fraction (Table 1). Additionally, heart weights of EP1+/+ and EP1−/− mice after treatment with Nphx/DOCA-NaCl/Ang II showed no significant difference between the genotypes (EP1+/+: 198.9 ± 9.6 N = 21, EP1−/−: 190.0 ± 4.3 N = 17, P = 0.461).
Table 1.
Echocardiography data at baseline and 5 days post Ang II treatment.
| Baseline | Nphx/DOCA-NaCl/Ang II | |||
|---|---|---|---|---|
|
| ||||
| Parameter | EP1+/+ (N = 9) | EP1−/− (N = 8) | EP1+/+ (N = 6) | EP1−/− (N = 8) |
| LVPWd (mm) | 0.760 +/− 0.015 | 0.772 +/− 0.027 | 0.773 +/− 0.021 * | 0.794 +/− 0.028 |
| IVSd (mm) | 0.812 +/− 0.011 | 0.790 +/− 0.019 | 0.921 +/− 0.038 * | 0.895 +/− 0.010 * |
| LVIDd (mm) | 3.058 +/− 0.043 | 2.985 +/− 0.040 | 3.551 +/− 0.24 * | 3.482 +/− 0.13 * |
| FS (%) | 49.225 +/− 0.28 | 50.847 +/− 0.13 † | 31.784 +/− 2.98 * | 33.268 +/− 2.31 * |
| EF (%) | 81.917 +/− 0.27 | 83.462 +/− 0.15 † | 60.228 +/− 4.22 * | 62.467 +/− 3.20 * |
P < 0.05, Baseline vs Nphx/DOCA-NaCl/Ang II
P < 0.05, EP1+/+ vs EP1−/−
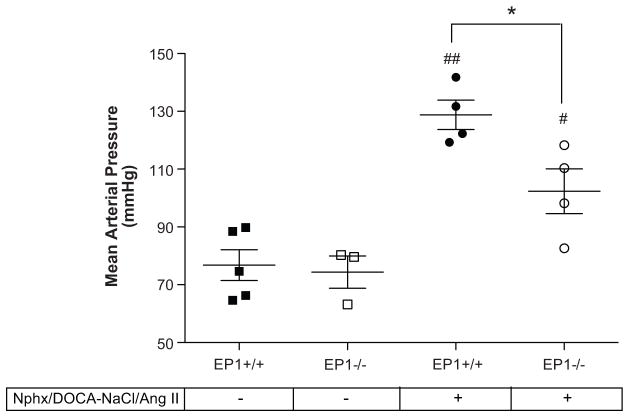
Hypertension was less severe in EP1−/− mice than EP1+/+mice
To determine the effect of disruption of the EP1 receptor on blood pressure in Nphx/DOCA-NaCl/Ang II treated animals, intracarotid blood pressure was determined in EP1+/+ and EP1−/− mice two days post-Ang II administration (Figure 6). MAP was significantly increased compared to untreated animals in both EP1+/+ (76.79 +/− 5.33 mmHg baseline vs. 128.8 +/− 5.08 mmHg, P = 0.0004) and EP1−/− mice (74.36 +/− 5.61 mmHg baseline vs. 102.4 +/− 7.77 mmHg, P = 0.0423). However, the rise in MAP was significantly lower in EP1−/− compared to EP1+/+ mice (P = 0.0295).
Figure 6.
Mean Arterial Pressure in EP1+/+ and EP1−/− mice. Intracarotid blood pressure was measured in EP1+/+ and EP1−/− mice two days post-Ang II administration under ketamine and inactin anesthesia. MAP was elevated in both groups compared to baseline (EP1+/+ ##P = 0.0004, EP1−/− #P = 0.0423) but was significantly lower in EP1−/− compared to EP1+/+ two days post-Ang II (*P = 0.0295).
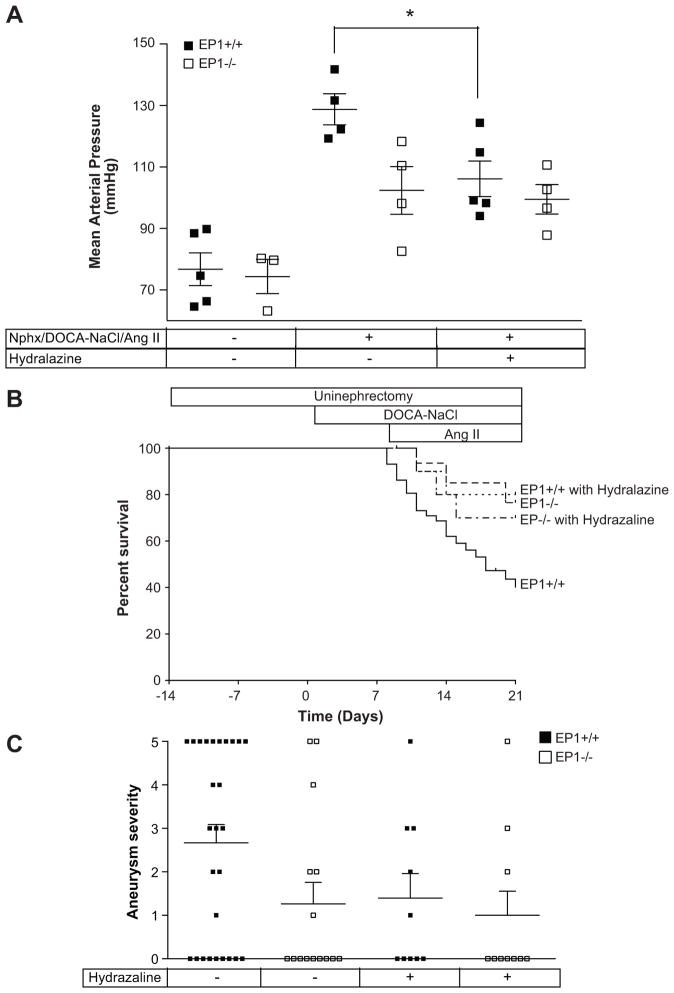
Reduction of blood pressure protected against mortality
Fifteen EP1+/+ and EP1−/− mice treated with Nphx/DOCA-NaCl/Ang II were treated with the antihypertensive agent hydralazine. Hydralazine treatment significantly reduced MAP in EP1+/+ mice (106.1 +/− 5.76 mmHg vs. 128.8 +/− 5.08 mmHg, P = 0.024), and but had no significant effect on EP1−/− mice (Figure 7A, 99.47 +/− 4.82 mmHg vs. 102.4 +/− 7.77 mmHg, P = 0.762). A significant decrease in the incidence of mortality was observed in EP1+/+ but not EP1−/− mice (Figure 7B, P = 0.007 and P = 0.642 respectively). Hydralazine treatment reduced aneurysm incidence and severity in EP1+/+ though this did not achieve statistical significance (Figure 7C, P > 0.05). Anasarca was not observed in hydralazine treated EP1+/+ (BW 5 days post-Ang II: 21.40 +/− 0.65 grams) as compared to untreated EP1+/+ mice (BW 5 days post-Ang II: 32.28 +/−1.59 grams, P < 0.0001).
Figure 7.
Hydralazine treatment reduces mortality in EP1+/+ but not EP1−/− mice. A. Mean arterial pressure was measured in EP1+/+ and EP1−/− mice treated with hydralazine at 2 days after Ang II administration and compared to existing data shown in figure 6. EP1+/+ with and without hydralazine, P = 0.024. EP1−/− with and without hydralazine, P = 0.762. B. Mortality observed in EP1+/+ mice was significantly reduced by treatment with hydralazine, ***P = 0.007. No change was observed in EP1−/− mice, P = 0.642. C. Aortic aneurysm severity. Aneurysm severity was decreased in EP1+/+ mice treated with hydralazine (P > 0.05).
Discussion
Disruption of the EP1 receptor affords substantial protection in the Nphx/DOCA-NaCl/Ang II evoked hypertension. The incidence of mortality was significantly decreased and appeared to result from reduction in MAP. Mortality was a result of ruptured aortic aneurysm or occurred after developing anasarca. The results presented here are consistent with previous studies demonstrating the role of EP1 in modulating the rise in MAP in response to Ang II10, and further reveal the protective effect disruption of the EP1 receptor has on end-organ damage.
There are several limitations to these studies which deserve mention. First, high mortality observed in EP1+/+ mice confounds the analysis of measurements taken after implantation of Ang II, such as cardiac and renal function, since the analyses are only performed on surviving mice. Second, there was a modest (<10 %), though statistically significant difference in body weight observed between EP1+/+ and EP1−/− mice. Although the dosage of Ang II was adjusted by weight, the dosage of the DOCA pellet was not. However, one would predict the genotype receiving the greater dose/weight (EP1−/−) would have the worse phenotype, and this is opposite what we observed. Lastly, EP1−/− mice were observed to have lower blood pressure than EP1+/+ mice following treatment with the Nphx/DOCA-NaCl/Ang II model. This is consistent with our previously published data10 suggesting EP1 mediates part of Ang II-induced hypertension. In this model we measured MAP at baseline or after treatment with all three model components; it is possible that EP1 also contributes to hypertension induced by Nphx or DOCA-NaCl as well.
Current models of aortic aneurysm include a combination of hyperlipidemic mice or high fat diet with modulation of the renin-angiotensin-aldosterone axis, or aberrant production of extracellular matrix components 27. Ang II-induced aortic aneurysms are characterized by accumulation of macrophages in the adventia and media, disruption of elastin fibers, expansion of the lumen, thrombus formation and disordered extracellular matrix deposition 28. These characteristics were also observed in the Nphx/DOCA-NaCl/Ang II model, although no significant differences in macrophage accumulation, matrix deposition, or COX-2 mRNA expression were detected between the two genotypes. It should be noted that the aneurysms and dissections observed in this model occur after acute severe hypertension and although the pathology appears similar to that observed in human disease, the disease genesis may not be. In humans, development of a true aneurysm is a slowly progressing disease initiating with local inflammation, disruption of the connective tissue matrix, and is often associated with atherosclerosis. In contrast, development of false aneurysm, or dissection as a result of a tear in the intima, can occur more acutely by a sudden large rise in blood pressure or direct injury and may be more representative of the damage induced by the Nphx/DOCA-NaCl/Ang II model. Our data demonstrate that protection observed when EP1 is disrupted is likely due to the prevention of a large rise in blood pressure, since treatment with hydralazine phenocopied EP1−/− mice. This does not eliminate the possibility that EP1 receptors might also provide protection directly at the target tissue.
Data exist suggesting a role for prostaglandins, in particular PGE2, in aortic aneurysm formation. COX-2 initiates the production of prostaglandins, and its expression is induced in by infusion of angiotensin II in the smooth muscle of the aorta surrounding aneurysms 23. Furthermore, either selective inhibition of COX-2 or genetic deletion of COX-2 significantly reduced aortic aneurysm formation and macrophage infiltration 23,24. Deletion of microsomal PGE synthase-1, which transforms the product of COX-2 metabolism into PGE2, has also been demonstrated to reduce aortic aneurysm formation and oxidative stress in LDLR−/− mice with an angiotensin II infusion 29, suggesting PGE2 plays an important role in development of aneurysms and the EP receptors may be viable targets for treatment of aneurysm progression.
Previous reports of the role of EP1 in renal injury are contradictory. In spontaneously hypertensive rats, treatment with an EP1 antagonist reduced proteinuria and tubulointerstitial damage 18, while in anti-GBM nephrotoxic serum nephritis genetic deletion of EP1−/− in mice resulted in enhanced mesangial expansion and tubular dilation and increased blood urea nitrogen and serum creatinine 30. In our studies, modest hypertensive renal damage was observed, although no significant differences in renal function were detected between genotypes. However, our interpretation was confounded by the differential mortalities in EP1+/+ and EP1−/− mice, potentially biasing our results. Examination of renal histopathology at time points prior to significant mortality failed to detect any severe renal damage or differences between the genotypes. This suggests the role of EP1 in renal damage is highly context dependent.
Anasarca, or extreme generalized edema, can occur in many disease settings. It is commonly a result of liver failure, nephrotic syndrome or heart failure 25,26. In our Nphx/DOCA-NaCl/Ang II model, a subset of EP1+/+ mice developed severe anasarca prior to mortality, while EP1−/− mice were protected. The EP1 receptor has previously been shown to be natriuretic10. With this paradigm, one might predict EP1−/− mice would retain more salt and water; however in our results we demonstrate that EP1+/+ mice gain excessive fluid volume that is not observed in EP1−/− mice. This contradiction leads us to conclude that alterations in kidney function by disruption of EP1 do not play a dominant role in development of the observed edema. Additionally, cardiac function was reduced to similar degrees in EP1+/+ and EP1−/− mice. Edema was prevented by treatment with hydralazine, suggesting elevation in blood pressure was responsible for development of edema. We hypothesize that hypertension induced by DOCA-NaCl and Ang II results in volume loading and enhanced vasoconstriction, which places excessive stress on the vascular wall leading to enhanced permeability, resulting in edema and susceptibility to dissections and rupture. Future experiments will be required to identify whether vascular permeability differences are observed between EP1+/+ and EP1−/− mice.
Perspectives
The EP1 receptor plays an important role in the development of hypertensive damage. In the Nphx/DOCA-NaCl/Ang II model, disruption of EP1 results in increased survival, lessened aneurysm severity and the absence of anasarca. This effect is a result of a reduced rise in blood pressure observed in EP1−/− mice, and suggests the EP1 receptor may be a viable pharmaceutical target for the treatment of hypertension and subsequent organ damage. Furthermore the Nphx/DOCA-NaCl/Ang II model may prove to be a useful tool for studying the pathology of aortic aneurysm and dissection formation in a setting of acute severe hypertension.
Supplementary Material
Novelty and Significance.
1) What is new?
Describes new model of aortic aneurysm
Anasarca is observed
Disruption of the EP1 receptor improves the outcome of each pathology and overall survival
2) What is relevant?
Studies shed light on the role of EP1 in the pathophysiology of hypertension
Identify a therapeutic target for hypertension and its sequelae
3) Summary
The Nphx/DOCA-NaCl/Ang II model induces hypertension, aortic aneurysm formation and anasarca. Disruption of the EP1 receptor reduced blood pressure in this model, leading to reduced incidence of mortality and decreased organ damage.
Acknowledgments
We thank Jason Downey for careful critique of the manuscript and Dr. Matthew Breyer for helpful discussion.
Sources of funding
This work was supported by NIH grants DK46205 (RMB), DK37097 (RMB), P50GM015431 (RMB), 2P01DK065123 (RZ), DK075594 (RZ), DK9921 (RZ), DK62794 (RCH), O’Brien P30DK79341-01 (RZ and RCH). The Mouse Metabolic Phenotyping Center is supported in part by DK059637. RZ, RCH, and RMB have Merit Awards from the Department of Veterans Affairs and RZ has an American Heart Association established investigator award.
Footnotes
Disclosures
None
References
- 1.Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13:2–8. doi: 10.18553/jmcp.2007.13.s8-a.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy CR, Zhang Y, Brandon S, Guan Y, Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD, Breyer RM. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 3.Schaaf TK, Hess HJ. Synthesis and biological activity of carboxyl-terminus modified prostaglandin analogues. J Med Chem. 1979;22:1340–1346. doi: 10.1021/jm00197a012. [DOI] [PubMed] [Google Scholar]
- 4.Hockel GM, Cowley AW., Jr Prostaglandin E2-induced hypertension in conscious dogs. Am J Physiol. 1979;237:H449–454. doi: 10.1152/ajpheart.1979.237.4.H449. [DOI] [PubMed] [Google Scholar]
- 5.Audoly LP, Tilley SL, Goulet J, Key M, Nguyen M, Stock JL, McNeish JD, Koller BH, Coffman TM. Identification of specific EP receptors responsible for the hemodynamic effects of PGE(2) Am J Physiol. 1999;277:H924–H930. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- 6.Tilley SL, Audoly LP, Hicks EH, Kim HS, Flannery PJ, Coffman TM, Koller BH. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279:F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of Murine Vasopressor and Vasodepressor Prostaglandin E(2) Receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 9.Audoly LP, Ruan X, Wagner VA, Goulet JL, Tilley SL, Koller BH, Coffman TM, Arendshorst WJ. Role of EP(2) and EP(3) PGE(2) receptors in control of murine renal hemodynamics. Am J Physiol Heart Circ Physiol. 2001;280:H327–333. doi: 10.1152/ajpheart.2001.280.1.H327. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y, Zhang Y, Wu J, Qi Z, Yang G, Dou D, Gao Y, Chen L, Zhang X, Davis LS, Wei M, Fan X, Carmosino M, Hao C, Imig JD, Breyer RM, Breyer MD. Antihypertensive effects of selective prostaglandin E(2) receptor subtype 1 targeting. J Clin Invest. 2007;117:2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 12.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 13.Kim JI, Lakshmikanthan V, Frilot N, Daaka Y. Prostaglandin E2 promotes lung cancer cell migration via EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 2010;8:569–577. doi: 10.1158/1541-7786.MCR-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun KS, Lao HC, Langenbach R. The prostaglandin E2 receptor, EP2, stimulates keratinocyte proliferation in mouse skin by G protein-dependent and {beta}-arrestin1-dependent signaling pathways. J Biol Chem. 2010;285:39672–39681. doi: 10.1074/jbc.M110.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eklund B, Carlson LA. Central and peripheral circulatory effects and metabolic effects of different prostaglandins given I.V. to man. Prostaglandins. 1980;20:333–347. doi: 10.1016/s0090-6980(80)80051-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Guan Y, Schneider A, Brandon S, Breyer RM, Breyer MD. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–1134. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- 17.Stock JL, Shinjo K, Burkhardt J, Roach M, Taniguchi K, Ishikawa T, Kim HS, Flannery PJ, Coffman TM, McNeish JD, Audoly LP. The prostaglandin E2 EP1 receptor mediates pain perception and regulates blood pressure. J Clin Invest. 2001;107:325–331. doi: 10.1172/JCI6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suganami T, Mori K, Tanaka I, Mukoyama M, Sugawara A, Makino H, Muro S, Yahata K, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats. Hypertension. 2003;42:1183–1190. doi: 10.1161/01.HYP.0000101689.64849.97. [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension. 2010;55:911–917. doi: 10.1161/HYPERTENSIONAHA.109.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff F, Krebs C, Abdulhag UN, Meyer-Schwesinger C, Maas R, Helmchen U, Hilgers KF, Wolf G, Stahl RA, Wenzel U. Rapid development of severe end-organ damage in C57BL/6 mice by combining DOCA salt and angiotensin II. Kidney Int. 2008;73:643–650. doi: 10.1038/sj.ki.5002689. [DOI] [PubMed] [Google Scholar]
- 21.Manning MW, Cassi LA, Huang J, Szilvassy SJ, Daugherty A. Abdominal aortic aneurysms: fresh insights from a novel animal model of the disease. Vasc Med. 2002;7:45–54. doi: 10.1191/1358863x02vm413ra. [DOI] [PubMed] [Google Scholar]
- 22.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 23.Gitlin JM, Trivedi DB, Langenbach R, Loftin CD. Genetic deficiency of cyclooxygenase-2 attenuates abdominal aortic aneurysm formation in mice. Cardiovasc Res. 2007;73:227–236. doi: 10.1016/j.cardiores.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 24.King VL, Trivedi DB, Gitlin JM, Loftin CD. Selective cyclooxygenase-2 inhibition with celecoxib decreases angiotensin II-induced abdominal aortic aneurysm formation in mice. Arterioscler Thromb Vasc Biol. 2006;26:1137–1143. doi: 10.1161/01.ATV.0000216119.79008.ac. [DOI] [PubMed] [Google Scholar]
- 25.Cho S, Atwood JE. Peripheral edema. Am J Med. 2002;113:580–586. doi: 10.1016/s0002-9343(02)01322-0. [DOI] [PubMed] [Google Scholar]
- 26.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012 doi: 10.1016/j.carpath.2011.11.007. In-press. [DOI] [PubMed] [Google Scholar]
- 27.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 28.Daugherty A, Cassis LA, Lu H. Complex pathologies of angiotensin II-induced abdominal aortic aneurysms. J Zhejiang Univ Sci B. 2011;12:624–628. doi: 10.1631/jzus.B1101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, Lee E, Song W, Ricciotti E, Rader DJ, Lawson JA, Pure E, FitzGerald GA. Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation. 2008;117:1302–1309. doi: 10.1161/CIRCULATIONAHA.107.731398. [DOI] [PubMed] [Google Scholar]
- 30.Rahal S, McVeigh LI, Zhang Y, Guan Y, Breyer MD, Kennedy CR. Increased severity of renal impairment in nephritic mice lacking the EP1 receptor. Can J Physiol Pharmacol. 2006;84:877–885. doi: 10.1139/y06-029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.