Abstract
Our understanding of biological mechanisms and treatment options for traumatic brain injury (TBI) is limited. Here, we employed quantitative real-time PCR (QRT-PCR) and immunohistochemical analyses to determine the dynamic expression of cell proliferation and apoptosis in an effort to provide insights into the therapeutic window for developing regenerative strategies for TBI. For this purpose, young adult Sprague–Dawley rats were subjected to experimental TBI using a controlled cortical impactor and then euthanized 1–48 h after TBI for QRT-PCR and immunohistochemistry. QRT-PCR revealed that brains from TBI-exposed rats initially displayed nestin mRNA expression that modestly increased as early as 1 h post-TBI, then significantly peaked at 8 h, but thereafter reverted to pre-TBI levels. On the other hand, caspase-3 mRNA expression was slightly elevated at 8 h post-TBI, which did not become significantly upregulated until 48 h. Immunofluorescent microscopy revealed a significant surge in nestin-immunoreactive cells in the cortex, corpus callosum, and subventricular zone at 24 h post-TBI, whereas a significant increase in the number of active caspase-3-immunoreactive cells was only found in the cortex and not until 48 h. These results suggest that the injured brain attempts to repair itself via cell proliferation immediately after TBI but this endogenous regenerative mechanism is not sufficient to abrogate the secondary apoptotic cell death. Treatment strategies designed to amplify cell proliferation and to prevent apoptosis are likely to exert maximal benefits when initiated at the acute phase of TBI.
Keywords: Cell proliferation, Neurogenesis, Apoptosis, Head trauma, Nestin, Caspase-3
INTRODUCTION
Traumatic brain injury (TBI) represents a significant unmet medical condition in the US. According to the National Institutes of Health, about 5 million new head injuries occur in the US each year, with 2 million patients who suffer from brain injuries that result in lifelong difficulties in daily activities (44). Annually, the economic cost for TBI is $56 billion (28). Over the last two decades, an increase in TBI cases is associated with combat injuries, including blast, impact, or acceleration/deceleration injuries to the head, sustained in Iraq and Afghanistan may result in TBI characterized by damage to the frontal and temporal lobes (5,16,20,40). Despite the recognition that TBI is a serious public health problem, there is currently no proven effective therapy for this disorder. The numbers of affected individuals and the costs necessary to facilitate their care and rehabilitation coupled with the lack of therapies indicate that TBI requires urgent research attention for better understanding of the disease pathophysiology and its treatment.
Two commonly employed in vivo TBI models are the fluid percussion and the controlled cortical impact (CCI) injury techniques; both models produce motor and cognitive impairments resembling many of the behavioral abnormalities seen in TBI patients (23,43). The brain injury produced by the CCI model is reminiscent of the clinical TBI pathology, which presents initially as a necrotic cell death in the underlying cortical tissue and white matter axonal injury, but also followed by an apoptotic cell death in surrounding tissue due to multiple subsequent events such as edema, ischemia, excitotoxicity, and altered gene expression (11,32,36,46).
Neurogenesis is a potent endogenous repair mechanism that the brain mounts against injury, which has been shown to accompany TBI (41,47). However, this self-repair process of the injured brain is transient and partial, thus neither abrogating the disease progression nor translating into stable functional recovery. To this end, we posit that gaining insights into the temporal pattern of TBI-induced apoptosis and neurogenesis should allow a better understanding of disease pathophysiology and its treatment. Thus, in the present study, we employed quantitative real-time PCR and immunohistochemical assays in order to unravel the early molecular and cellular responses of the brain to severe TBI produced by the controlled cortical impact (CCI) model. Here, we tested our hypothesis that TBI acutely entails neurogenesis, which fails to prevent the secondary apoptotic cell death. If this hypothesis holds true, the subsequent goal is to amplify neurogenesis and/or block apoptosis as a therapeutic strategy for treating TBI.
MATERIALS AND METHODS
Surgical Procedures
All animal procedures followed approved IACUC guidelines for use of animals in research. Ten-week-old Sprague–Dawley rats (n = 3–5 from triplicate independent experiments) were subjected to TBI using a controlled cortical impactor (Pittsburgh Precision Instruments). Animals initially received buprenorphine (0.05 mg/kg, SC) at the time of anesthesia induction (ketamine, 100 mg/kg, IP, mixed with 5 mg/kg xylazine, IP). Once deep anesthesia was achieved (by checking for pain reflexes), individual animals were fixed in a stereotaxic frame (David Kopf Instruments). After exposing the skull, a craniectomy (4 mm) was performed over the right frontoparietal cortex (0.5 mm anterior and +2.8 mm lateral to the midline). The pneumatically operated TBI device (diameter = 3 mm) impacted the brain at a velocity of 6.0 m/s, reaching a depth of 2.0 mm below the dura matter layer and remained in the brain for 150 ms. The impactor rod was angled 15° to the vertical to maintain a perpendicular position in reference to the tangential plane of the brain curvature at the impact surface. A linear variable displacement transducer (Macrosensors), which was connected to the impactor, measured velocity and duration to verify consistency. Bone wax was used to cover the craniectomized region, and the skin incision was sutured thereafter. Sham injury surgeries (i.e., uninjured controls; n = 8) consisted of animals exposed to anesthesia, scalp incision, craniectomy, and suturing. A computer-operated thermal blanket pad and a rectal thermometer allowed maintenance of body temperature within normal limits. All animals were closely monitored until recovery from anesthesia and over the next 48 h. Animals were randomly selected and euthanized between 1 and 48 h after TBI.
Quantitative Real-Time PCR Analysis (QRT-PCR) of Caspase-3 and Nestin Gene Expression
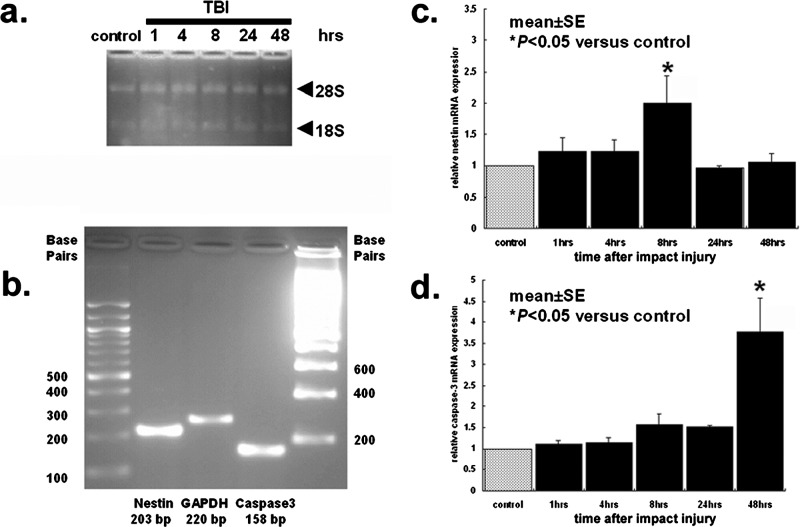
Brains from euthanized rats were instantaneously frozen in liquid nitrogen and stored at –80°C until processing for quantitative real-time PCR analysis (QRT-PCR). QRT-PCR was performed using the entire brain. Total RNA was extracted from the frozen brain using mirVana™ miRNA isolation kit (Ambion) according to the manufacturer's instructions and the A260/280 ratio of RNA extraction corresponded to 2.2, which is considered high quality. RNA integrity was confirmed under UV light by visualization of 28S- and 18S-rRNA bands on a denaturing gel containing ethidium bromide (Fig. 1A).
Figure 1.
QRT-PCR analyses of nestin and caspase-3 expression in TBI brains. QRT-PCR was conducted using the entire brain. (A) confirms RNA integrity under UV light by visualization of 28S- and 18S-rRNA bands on a denaturing gel containing ethidium bromide. (B) shows amplified PCR productions visualized with ethidium bromide under UV light. (C, D) reveals QRT-PCR analyses of nestin and caspase-3 gene expression (n = 3–5 from triplicate independent experiments) performed at 1, 4, 8, 24, and 48 h after TBI (nestin, p < 0.05 for control vs. 8 h; caspase-3, p < 0.05 for control vs. 48 h). Bars represent mean values ± SEM. *p < 0.05.
For cDNA synthesis, total RNA (2 µg) was reverse-transcribed in a 20-µl volume of reaction mixture, using a RETROscript (Ambion) according to the manufacturer's instructions. Transcript reactions without the reverse transcriptase enzyme were performed for negative controls in subsequent PCR reactions. The primer sequences of nestin (NM047626) and caspase-3 (NM047473) were as follows: nestin (NM047626) forward 5′-CTGAGGCCTCTCTTCTTCCA-3′, reverse 5′-ACTCCTGTACCGGGTCTCCT-3′ and caspase-3 (NM047473) forward 5′-GGACCTGTGGACCTGAAAAA-3′, reverse 3′-GCATGCCATATCATCGTCAG-3′. For quantitative nestin and caspase-3 gene expression, PCR amplification was performed in each reaction mixture containing 300 ng cDNA sample, 200 nM of each primer, 0.1 Unit Taq DNA polymerase (Ambion), 200 µM dNTPs, and 1.5 mM MgCl2 (total volume, 25 µl) using mirVana™ qRT-PCR miRNA detection kit (Ambion). The reaction mixture was heated at 94°C for 3 min, followed by 40 cycles, each consisting of incubation for 30 s at 94°C, 40 s at 72°C, and 30 s at 55°C. Amplification and Sybr Green I (Applied Biosystems) detection were performed on iCycler iQ™ Real-Time PCR Detection System (Bio-Rad). Primers specific for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as internal control for the amounts of cDNA generated from each sample. The primer sequences of GAPDH (NW047696) were as follows: forward 5′-ATGGGAAGCTGGTCATCAAC-3′ and reverse 5′-GTGGTTCACACCCATCACAA-3′. For each gene, PCRs were run in triplicate, and the amplified transcripts were quantified using the comparative Ct method. Briefly, threshold cycle (Ct) values were calculated by the equation Δ ΔCt = ΔCt nestin or caspase-3 – ΔCt GAPDH, where ΔCt is the difference in Ct values between nestin or caspase-3 and the GAPDH. The relative quantity of mRNA expression in the right hemisphere (i.e., TBI target region) was compared with the control hemisphere (i.e., sham operated, intact brain). Xn can be calculated by the formula Xn = 2meanΔΔCt. Differences between Ct values were less than 2%. The amplified PCR productions were electrophoresed on a 2% agarose gel containing 0.2 µg/µl ethidium bromide, and the sequences were confirmed using ABI 3730 XL 96-capillary sequencer (data not shown). The productions were visualized under UV light, saved digitally with AlphaImager 2000 (Alpha Innoteck Corporation), and represented as single and theoretical base pair bands (Fig. 1B). For all experiments, controls without template were incubated. Each primer pair was, when possible, designed to span an exon–exon boundary.
Immunohistochemical Analysis of Caspase-3 and Nestin-Positive Cells
Rats were randomly euthanized between 1 and 48 h post-TBI using carbon dioxide overdose. The rats were perfused transcardially with 200 ml of cold phosphate-buffered saline (PBS) and 200 ml of 4% paraformaldehyde in PBS. Brains were removed and postfixed in the same fixative overnight at 4°C and then embedded in paraffin. The brains were coronally sectioned at the thickness of 8 µm. All sections were mounted on glass slides for immunohistochemical examinations and were first deparaffinized through three washes in xylene and then rehydrated through graduated alcohol solution, followed by washing three times for 5 min in PBS. Sections were then incubated overnight at 4°C with mouse anti-nestin antibody (1:200, Millipore) or rabbit anti-active caspase-3 antibody (1:500, Abcam), primary antibody, and washed three times in PBS. The sections were then incubated with the corresponding secondary antibodies (goat anti-mouse IgG-Alexa 594 or goat anti-rabbit IgG-Alexa 488, Invitrogen) for 90 min. Finally, sections were washed three times for 5 min each, washed in distilled water, and cover-slipped with Fluoromount (Sigma). Control studies included exclusion of the primary antibody substituted with 10% normal goat serum in PBS. No immunoreactivity was observed in these controls. For morphological analyses, immunoreactive cells in the cortex, corpus callosum, and subventricular zone (SVZ) within the ipsilateral to the TBI hemisphere were examined using Axio Imager.Z1 (Carl Zeiss). Specifically, six coronal sections at every 300 µm that approximately captured the entire damaged cortex (AP −2.0 to +2.0 mm from bregma) were examined from each rat, the number of positive cells was counted in each six high-power fields, and the averages were used for statistical analyses.
Statistical Analyses
Repeated measures of ANOVA followed by post hoc test using Fisher's protected least significant difference (PLSD) was used to reveal statistical significance in both QRT-PCR and histological data. A statistically significant difference was preset at p < 0.05.
RESULTS
TBI Induces the Gene Expression of Nestin and Caspase-3
ANOVA revealed significant timing effects for both nestin mRNA and caspase-3 mRNA expressions after TBI (p's < 0.01) (Fig. 1C and D). The levels of nestin mRNA expression, a marker of neurogenesis, were slightly increased at 1 and 4 h post-TBI but did not reach statistical significance compared with controls (p's > 0.05). At 8 h post-TBI, there was a onefold increase in nestin mRNA expression that was significantly higher than controls (p < 0.05). However, such elevated neurogenic response was transient in that at 24 and 48 h post-TBI, the levels of nestin mRNA expression reverted to pre-TBI levels and did not differ from the controls (p's > 0.05). On the other hand, the levels of caspase-3 mRNA expression, a marker of apoptosis, were not altered at 1 and 4 h post-TBI (p's > 0.05); although modest elevations were detected at 8 and 24 h post-TBI, they remained not statistically significant compared with the controls (p's > 0.05). However, a dramatic twofold increase (p < 0.05 vs. controls) in caspase-3 mRNA expression was detected at 48 h post-TBI.
Time Dependence of TBI-Induced Neural Progenitor Cell Proliferation
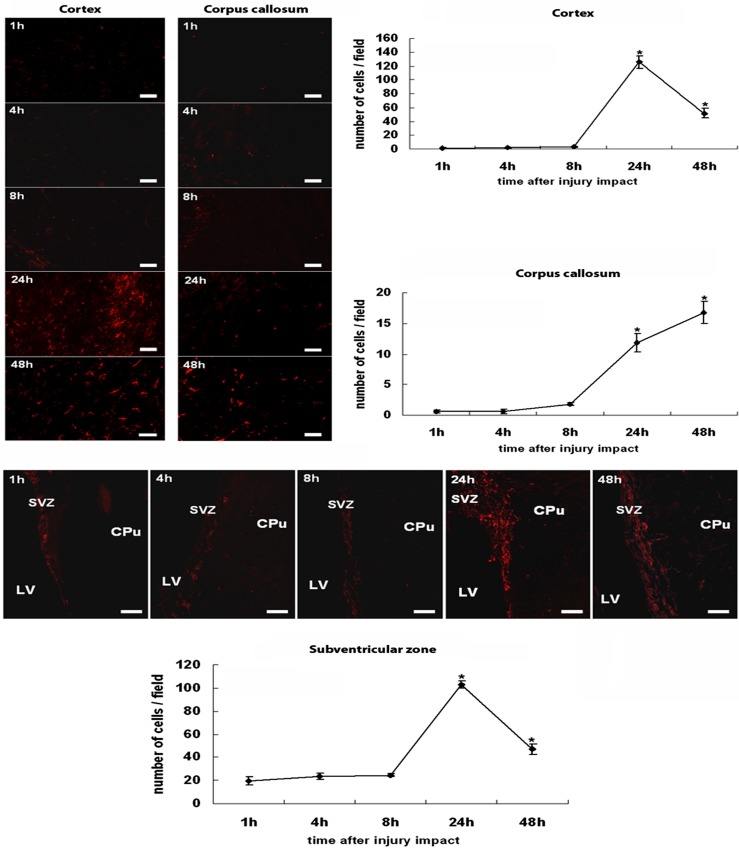
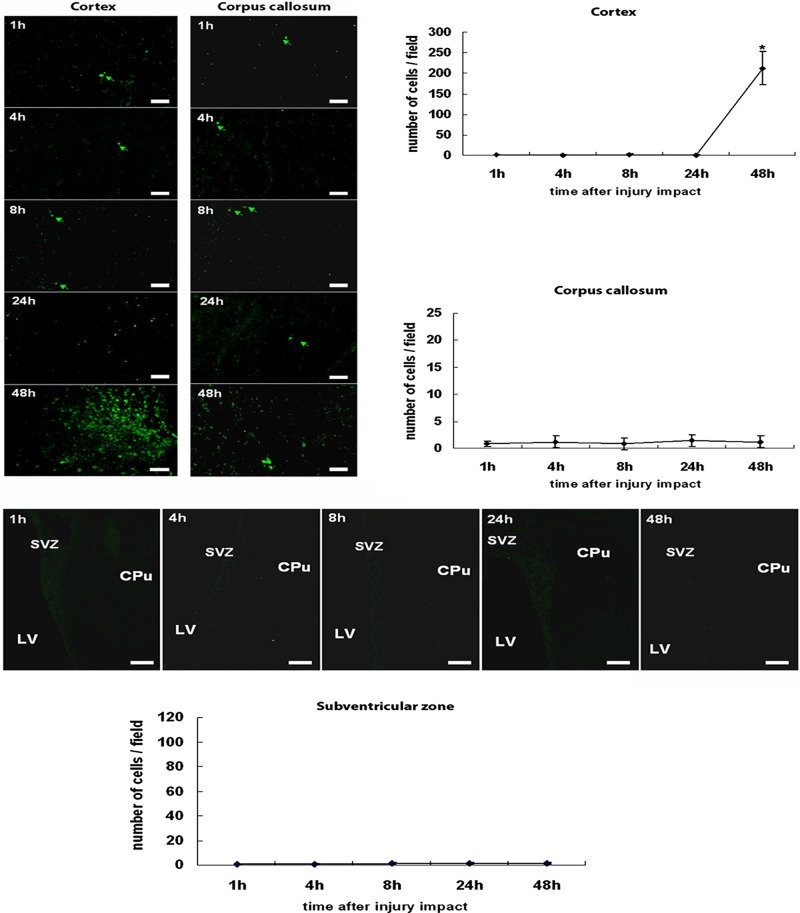
In general agreement with differential mRNA expressions of nestin and caspase-3 after TBI, immunofluorescent microscopic examination revealed an earlier post-TBI increase in the number of neurogenic nestin-immunoreactive cells compared with apoptotic active caspase-3-immunoreactive cells within the TBI hemisphere (Figs. 2 and 3). ANOVA again revealed significant timing effects on nestin and active caspase-3 immunoreactivity following TBI induction (p's < 0.01). Few nestin-immunoreactive cells were detected between 1 and 8 h post-TBI in all three brain regions, namely the cortex, the corpus callosum, and the SVZ ipsilateral to TBI, examined here (p's > 0.05). At 24 h post-TBI, both the cortex and the SVZ displayed a significant surge in nestin immunoreactive cells, which was less pronounced at 48 h post-TBI compared with the controls (p's < 0.05). A significant increase in nestin-immunoreactive cells in the corpus callosum was also recognized at 24 h post-TBI (p < 0.05 vs. controls). On the other hand, a significant increase in active caspase-3 expression was only found in the cortex and limited to the 48 h post-TBI period (p < 0.05 vs. controls). Across all time points post-TBI, both the corpus callosum and the SVZ did not exhibit any detectable increments in active caspase-3 expression (p's > 0.05 vs. controls).
Figure 2.
Nestin expression in the cortex, the corpus callosum, and the subventricular zone after TBI. Nestin-positive cells were hardly seen in both cortex and corpus callosum at 1, 4, and 8 h post-TBI. At 24 h post-TBI, the cortex was highly populated with nestin-positive cells, and while still detected, nestin immunoreactivity was less pronounced at 48 h post-TBI. In the corpus callosum, the number of nestin-positive cells increased gradually at 24 h and peaked at 48 h post-TBI. A pattern of nestin immunoreactivity in the subventricular zone resembles that seen in the cortex. Very few nestin-positive cells were detected at 1, 4, and 8 h post-TBI, and a robust nestin immunoreactivity was recognized at 24 h post-TBI, which became less pronounced at 48 h post-TBI. Quantitative cell counts are presented in the graphs, and error bars represent mean values ± SEM. *p < 0.05 versus controls. LV, lateral ventricle; CPu, caudate putamen; SVZ, subventricular zone. Scale bars: 30 µm. Nondetectable immunofluorescence accompanied the controls.
Figure 3.
Active caspase-3 expression in cortex, corpus callosum, and subventricular zone after TBI. Throughout the entire 48 h post-TBI, the corpus callosum displayed sporadic active caspase-3-positive cells. The cortex also remained relatively negative for active caspase-3 immunoreactivity up to 24 h post-TBI, but a massive surge in active caspase-3-positive cells was detected at 48 h post-TBI. In parallel with near complete absence of caspase-3 immunoreactivity in the corpus callosum, the subventricular zone also exhibited very few active caspase-3-positive cells at all time points post-TBI. Quantitative cell counts are presented in the graphs, and bars represent mean values ± SEM. *p < 0.05 versus controls. LV, lateral ventricle; CPu, caudate putamen; SVZ, subventricular zone. Scale bars: 30 µm. Nondetectable immunofluorescence accompanied the controls.
DISCUSSION
The present study demonstrates a discrete temporal pattern of cell proliferation and apoptosis produced by TBI as revealed by both mRNA and immunohistochemical analyses of nestin and caspase-3 expression, respectively. Here, we show that cell proliferation precedes apoptosis, suggesting that the host brain in response to injury immediately activates a self-repair mechanism. However, the rapid switch from cell proliferation (peaks between 8 and 24 h post-TBI) to apoptotic cell death (48 h post-TBI) indicates that endogenous processes alone are not capable of retarding cell death progression. In addition, the observed distinct onset of nestin and caspase-3 gene and protein expression offers insights into the optimal timing post-TBI for initiating treatment strategies designed to enhance cell proliferation, hopefully resulting in neurogenesis, and to suppress apoptosis.
Accumulating scientific evidence has demonstrated that following injury the adult brain undergoes remodeling reminiscent of ontogeny, characterized by tissues proximal to the site of injury as well as distinct neurogenic sites (i.e., SVZ and dentate gyrus), exhibiting enhanced cell proliferation, as seen here, and subsequent neurogenesis that are normally inherent in embryonic or neonatal stages of development (4,41,47,48). Unfortunately, the host brain's attempt to repair itself via this ontogenic remodeling is incomplete, thus neither abrogating the cell death progression nor translating into a significant clinical improvement epitomizing the limited plasticity of the adult brain. Accordingly, restorative therapies, such as exogenous stem cell transplantation and endogenous stem cell mobilization (12,15,19,26,35), have been introduced to enhance the host brain remodeling repair mechanism.
The purpose of this study is to investigate the time course of cell proliferation and apoptosis after TBI. Although it has been shown that apoptosis peaks 1–2 days after injury (2,31), for TBI-induced cell proliferation, however, it is less clear. The present observation that maximal cell proliferation occurs 8 h after injury is the most novel aspect of this study. This observed nestin expression likely reflects an early attempt of the brain to initiate neurogenesis in response to injury. Nestin is used widely as a cell type marker for stem/progenitor cells, and recently its activation has been implicated as critical to neurogenesis (22,47). However, equating expression of brain nestin with neurogenesis needs clarification. In fact, nestin expression is not limited to neural stem/progenitors but is also found in endothelial cells, mesenchymal derivatives, such as muscle, and reactive astrocytes (34,49). Nestin, an intermediate filament protein, is expressed in reactive astrocytes in the injury areas including cerebral cortex and white matter tract (6,14,21,30,37). The reactive astrocyte expression is relevant in this study because TBI causes significant reactive astrocytosis (10,13). In addition, we concur that our quantitative data come from the entire brain of injured animals when there is only a partial portion of the brain that is relevant to injury-induced neurogenesis. However, the present immunohistochemical assessment adds corroboration with this QRT-PCR analysis, allowing delineation of astrocytosis from cell proliferation, at least in discrete brain regions. Nonetheless, we agree that further investigations are warranted to fully reveal the neurogenic cell phenotype, including QRT-PCR assays of specific brain areas, as well as immunohistochemical double labeling of nestin with neuronal or astrocytic markers. Delayed expression of nestin (i.e., 1–4 weeks) following TBI has been reported by Kaya and colleagues (18). These findings suggest that further investigations examining nestin expression following TBI during a longer time course are warranted.
Cell proliferation (i.e., neurogenesis) and apoptosis following TBI are two separate events located in different regions in the brain. Numerous studies have shown that TBI-induced neurogenesis occurs predominantly in the neurogenic regions, i.e., the SVZ and the dentate gyrus of the hippocampus, areas that are relatively away from the injury impact (3,9,33,41,42). Whereas injury-induced neurogenesis in the hippocampus may play a role in regeneration, the role of neurogenesis in the SVZ following TBI remains poorly understood. It is well known that neurons generated from the SVZ migrate along rostral migrate stream to the olfactory bulb (1,17). Unlike ischemic injury model, in TBI models, no study has demonstrated that the SVZ-generated cells migrate to the injury areas. Due to mitochondrial dysfunction in the injured areas, TBI-induced apoptosis as demonstrated by TUNEL staining and caspase-3 mRNA and protein expression is mostly located in areas at the site directly under the injury impact (2,7,45). The caspase-3 expression in this study also showed a similar pattern. Accordingly, while it is recognized that injury-induced neurogenesis and apoptosis are two different entities, many newly generated cells in the hippocampus undergo apoptosis during disease progression, which appears to be the same temporal profile displayed by the injured cortex. Because the cortex is considered a nonneurogenic site, the source of enhanced nestin-expressing cells in this area might have resulted from the migration of newly formed cells from SVZ via the corpus callosum; both regions shown here are also highly populated by nestin-positive cells.
The therapeutic window for targeting neurogenesis and apoptosis in TBI appears critical if stable functional recovery is desired. The logical timing post-TBI is to coincide treatment intervention with the onset of endogenous repair mechanism. Based on the present mRNA expression of nestin, the 8-h post-TBI corresponds to the maximal upregulation of this cell proliferative gene, while the translation of this genetic signal into a phenotypic protein expression occurs at 24 h post-TBI. Taken together, these results suggest that the period between 8 and 24 h post-TBI represents an appropriate time frame to amplify host neurogenic effects. That amplifying nestin expression is key to endogenous neurogenesis concurs with recent studies demonstrating that activation of nestin is necessary for brain remodeling after injury, including TBI (29,47). On the other hand, both mRNA and immunohistochemical data revealed that apoptosis commences not until 48 h post-TBI, suggesting that antiapoptotic treatments may have a wider therapeutic window than neurogenesis-enhancing regimens. Indeed, antiapoptotic drugs have been shown to inhibit caspase-mediated apoptosis after TBI (8,24). A combination of cell proliferative (i.e., neurogenic) and antiapoptotic therapies may further improve the prognosis of TBI.
The brain region-specific expression of nestin and caspase-3 following TBI reveals that the endogenous remodeling process involves cell proliferation in both cortex and SVZ and likely cell migration along the corpus callosum, whereas apoptosis appears limited to the cortex. The absence of apoptotic signals in the SVZ and corpus callosum suggests that the migratory pathway between the SVZ and the injured cortex via the corpus callosum is intact at least up to 48 h post-TBI. However, many of the newly formed cells remained lodged within the corpus callosum at 48 h when a massive apoptotic cell death had already commenced in the cortex. Thus, proper migration of the endogenously proliferated cells to the site of injury may not be optimal at this 48-h post-TBI period, implying that initiation of therapeutic intervention at a much earlier time point may be more beneficial.
CONCLUSION
Our study provides evidence of cell proliferation prior to apoptosis after TBI. Amplification of the brain self-regeneration process, either via cell replacement therapy (27,38) or pharmacologic mobilization of endogenous stem/progenitor cells (e.g., G-CSF or erythropoietin) (25,39), in combination with antiapoptotic medications (8,24), within the acute postinjury period may prove effective in treating TBI.
ACKNOWLEDGMENTS
Financial support for this study was partially through the Draper Laboratory and the University of South Florida Department of Neurosurgery and Brain Repair funds. CVB is funded by the James and Esther King Biomedical Research Foundation 1KG01-33966, NIH 5U01NS055914-04, and NIH 1R01NS071956-01A1. The authors declare no conflict of interest.
REFERENCES
- 1. Alonso M.; Ortega-Perez I.; Grubb M. S.; Bourgeois J. P.; Charneau P.; Lledo P. M. Turning astrocytes from the rostral migratory stream into neurons: A role for the olfactory sensory organ. J. Neurosci. 28(43):11089–11102; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beer R.; Franz G.; Schopf M.; Reindl M.; Zelger B.; Schmutzhard E.; Poewe W.; Kampfl A. Expression of Fas and Fas ligand after experimental traumatic brain injury in the rat. J. Cereb. Blood Flow Metab. 20(4):669–677; 2000. [DOI] [PubMed] [Google Scholar]
- 3. Chirumamilla S.; Sun D.; Bullock M. R.; Colello R. J. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma 19(6):693–703; 2002. [DOI] [PubMed] [Google Scholar]
- 4. Chopp M.; Li Y.; Zhang J. Plasticity and remodeling of brain. J. Neurol. Sci. 265(1–2):97–101; 2008. [DOI] [PubMed] [Google Scholar]
- 5. Chuck Y. Mild traumatic brain injury screening and evaluation implemented for OEF/OIF veterans, but challenges remain. Washington, DC: United States Government Accountability Office; 2008. [Google Scholar]
- 6. Clarke S. R.; Shetty A. K.; Bradley J. L.; Turner D. A. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport 5(15):1885–1888; 1994. [DOI] [PubMed] [Google Scholar]
- 7. Conti A. C.; Raghupathi R.; Trojanowski J. Q.; McIntosh T. K. Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 18(15):5663–5672; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cutler S. M.; Cekic M.; Miller D. M.; Wali B.; VanLandingham J. W.; Stein D. G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma 24(9):1475–1486; 2007. [DOI] [PubMed] [Google Scholar]
- 9. Dash P. K.; Mach S. A.; Moore A. N. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63(4):313–319; 2001. [DOI] [PubMed] [Google Scholar]
- 10. Di Giovanni S.; Movsesyan V.; Ahmed F.; Cernak I.; Schinelli S.; Stoica B.; Faden A. I. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA 102(23):8333–8338; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dikranian K.; Cohen R.; Mac Donald C.; Pan Y.; Brakefield D.; Bayly P.; Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 211(2):551–560; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emerich D. F.; Silva E.; Ali O.; Mooney D.; Bell W.; Yu S. J.; Kaneko Y.; Borlongan C. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 19(9):1063–1071; 2010. [DOI] [PubMed] [Google Scholar]
- 13. Hilton G. D.; Stoica B. A.; Byrnes K. R.; Faden A. I. Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood. Flow. Metab. 28(11):1845–1859; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmin S.; Almqvist P.; Lendahl U.; Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur. J. Neurosci. 9(1):65–75; 1997. [DOI] [PubMed] [Google Scholar]
- 15. Hung H. S.; Shyu W. C.; Tsai C. H.; Hsu S. H.; Lin S. Z. Transplantation of endothelial progenitor cells as therapeutics for cardiovascular diseases. Cell Transplant. 18(9):1003–1012; 2009. [DOI] [PubMed] [Google Scholar]
- 16. Inglese M.; Makani S.; Johnson G.; Cohen B. A.; Silver J. A.; Gonen O.; Grossman R. I. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J. Neurosurg. 103(2):298–303; 2005. [DOI] [PubMed] [Google Scholar]
- 17. Inta D.; Alfonso J.; von Engelhardt J.; Kreuzberg M. M.; Meyer A. H.; van Hooft J. A.; Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc. Natl. Acad. Sci. USA 105(52):20994–20999; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaya S. S.; Mahmood A.; Li Y.; Yavuz E.; Chopp M. Expression of nestin after traumatic brain injury in rat brain. Brain Res. 840(1–2):153–157; 1999. [DOI] [PubMed] [Google Scholar]
- 19. Kern D. S.; Maclean K. N.; Jiang H.; Synder E. Y.; Sladek Jr. J. R.; Bjugstad K. B. Neural stem cells reduce hippocampal tau and reelin accumulation in aged Ts65Dn Down syndrome mice. Cell Transplant. 20(3):371–379; 2011. [DOI] [PubMed] [Google Scholar]
- 20. Kraus M. F.; Susmaras T.; Caughlin B. P.; Walker C. J.; Sweeney J. A.; Little D. M. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130(10):2508–2519; 2007. [DOI] [PubMed] [Google Scholar]
- 21. Krum J. M.; Rosenstein J. M. Transient coexpression of nestin, GFAP, and vascular endothelial growth factor in mature reactive astroglia following neural grafting or brain wounds. Exp. Neurol. 160(2):348–360; 1999. [DOI] [PubMed] [Google Scholar]
- 22. Lagace D. C.; Whitman M. C.; Noonan M. A.; Ables J. L.; DeCarolis N. A.; Arguello A. A.; Donovan M. H.; Fischer S. J.; Farnbauch L. A.; Beech R. D. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27(46):12623–12629; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. LaPlaca M. C.; Simon C. M.; Prado G. R.; Cullen D. K. CNS injury biomechanics and experimental models. Prog. Brain Res. 161:13–26; 2007. [DOI] [PubMed] [Google Scholar]
- 24. Lau A.; Arundine M.; Sun H. S.; Jones M.; Tymianski M. Inhibition of caspase-mediated apoptosis by peroxynitrite in traumatic brain injury. J. Neurosci. 26(45):11540–11553; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lieutaud T.; Andrews P. J.; Rhodes J. K.; Williamson R. Characterization of the pharmacokinetics of human recombinant erythropoietin in blood and brain when administered immediately after lateral fluid percussion brain injury and its pharmacodynamic effects on IL-1beta and MIP-2 in rats. J. Neurotrauma 25(10):1179–1185; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Lin S. Z. Advances in translational stem cell research and therapies. Cell Transplant. 20(1):1–3; 2011. [DOI] [PubMed] [Google Scholar]
- 27. Lu D.; Sanberg P. R.; Mahmood A.; Li Y.; Wang L.; Sanchez-Ramos J.; Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 11(3):275–281; 2002. [PubMed] [Google Scholar]
- 28. Mammis A.; McIntosh T. K.; Maniker A. H. Erythropoietin as a neuroprotective agent in traumatic brain injury. J. Surg. Neurol. 71(5):527–531; 2009. [DOI] [PubMed] [Google Scholar]
- 29. Miles D. K.; Kernie S. G. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus 18(8):793–806; 2008. [DOI] [PubMed] [Google Scholar]
- 30. Moreels M.; Vandenabeele F.; Dumont D.; Robben J.; Lambrichts I. Alpha-smooth muscle actin (alpha-SMA) and nestin expression in reactive astrocytes in multiple sclerosis lesions: Potential regulatory role of transforming growth factor-beta 1 (TGF-beta1). Neuropathol. Appl. Neurobiol. 34(5):532–546; 2008. [DOI] [PubMed] [Google Scholar]
- 31. Newcomb J. K.; Zhao X.; Pike B. R.; Hayes R. L. Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in the rat. Exp. Neurol. 158(1):76–88; 1999. [DOI] [PubMed] [Google Scholar]
- 32. Reiss P. Z. C.; Saatman K. E. Transplanted neural cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 51:1043–1054; 2002. [DOI] [PubMed] [Google Scholar]
- 33. Rice A. C.; Khaldi A.; Harvey H. B.; Salman N. J.; White F.; Fillmore H.; Bullock M. R. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp. Neurol. 183(2):406–417; 2003. [DOI] [PubMed] [Google Scholar]
- 34. Ridet J. L.; Malhotra S. K.; Privat A.; Gage F. H. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 20(12):570–577; 1997. [DOI] [PubMed] [Google Scholar]
- 35. Sanberg P. R.; Eve D. J.; Willing A. E.; Garbuzova-Davis S.; Tan J.; Sanberg C. D.; Allickson J. G.; Cruz L. E.; Borlongan C. V. The treatment of neurodegenerative disorders using umbilical cord blood and menstrual blood-derived stem cells. Cell Transplant. 20(1):85–94; 2011. [DOI] [PubMed] [Google Scholar]
- 36. Sandhir R.; Onyszchuk G.; Berman N. E. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 213(2):372–380; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmidt-Kastner R.; Humpel C. Nestin expression persists in astrocytes of organotypic slice cultures from rat cortex. Int. J. Dev. Neurosci. 20(1):29–38; 2002. [DOI] [PubMed] [Google Scholar]
- 38. Shear D. A.; Tate M. C.; Archer D. R.; Hoffman S. W.; Hulce V. D.; Laplaca M. C.; Stein D. G. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res. 1026(1):11–22; 2004. [DOI] [PubMed] [Google Scholar]
- 39. Sheibani N.; Grabowski E. F.; Schoenfeld D. A.; Whalen M. J. Effect of granulocyte colony-stimulating factor on functional and histopathologic outcome after traumatic brain injury in mice. Crit. Care Med. 32(11):2274–2278; 2004. [DOI] [PubMed] [Google Scholar]
- 40. Suh M.; Kolster R.; Sarkar R.; McCandliss B.; Ghajar J. Deficits in predictive smooth pursuit after mild traumatic brain injury. Neurosci. Lett. 401(1–2):108–113; 2006. [DOI] [PubMed] [Google Scholar]
- 41. Sun D.; Bullock M. R.; McGinn M. J.; Zhou Z.; Altememi N.; Hagood S.; Hamm R.; Colello R. J. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 216(1):56–65; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun D.; McGinn M. J.; Zhou Z.; Harvey H. B.; Bullock M. R.; Colello R. J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204(1):264–272; 2007. [DOI] [PubMed] [Google Scholar]
- 43. Tehranian R.; Rose M. E.; Vagni V.; Pickrell A. M.; Griffith R. P.; Liu H.; Clark R. S.; Dixon C. E.; Kochanek P. M.; Graham S. H. Disruption of Bax protein prevents neuronal cell death but produces cognitive impairment in mice following traumatic brain injury. J. Neurotrauma 25(7):755–767; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thurman D. J.; Alverson C.; Dunn K. A.; Guerrero J.; Sniezek J. E. Traumatic brain injury in the United States: A public health perspective. J. Head Trauma Rehabil. 14(6):602–615; 1999. [DOI] [PubMed] [Google Scholar]
- 45. Yakovlev A. G.; Knoblach S. M.; Fan L.; Fox G. B.; Goodnight R.; Faden A. I. Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17(19):7415–7424; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. You Z.; Savitz S. I.; Yang J.; Degterev A.; Yuan J.; Cuny G. D.; Moskowitz M. A.; Whalen M. J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 28(9):1564–1573; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu T. S.; Zhang G.; Liebl D. J.; Kernie S. G. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 28(48):12901–12912; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang R. L.; Zhang Z. G.; Chopp M. Ischemic stroke and neurogenesis in the subventricular zone. J. Neuropharm. 55(3):345–352; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zimmerman L.; Parr B.; Lendahl U.; Cunningham M.; McKay R.; Gavin B.; Mann J.; Vassileva G.; McMahon A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12(1):11–24; 1994. [DOI] [PubMed] [Google Scholar]