Abstract
Computed tomography (CT) and magnetic resonance imaging (MRI) findings for a case of intrahepatic sarcomatoid cholangiocarcinoma is presented. A 48-year-old man with upper abdominal pain underwent contrast-enhanced CT and MRI. A 13 × 10 × 7 cm mass was seen in the left liver lobe, which had hypodense internal architecture on CT and mixed signal intensities on both T1- and T2-weighted images with an overwhelmingly hypointense signal on T1-weighted images and a hyperintense signal on T2-weighted images. The lesion had heterogeneous enhancement on both CT and MRI. A satellite nodule with the same imaging features was distinctive for the lesion.
Keywords: Cholangiocarcinoma, sarcomatous transformation, CT, MRI
Introduction
Sarcomatoid carcinomas are very rare among the solid liver tumours. Sarcomatous changes occur in both hepatocellular carcinomas (HCC) and cholangiocarcinomas (CCC). Sarcomatoid changes may affect the imaging findings, thus the unique imaging characteristics of both HCC and CCC no longer exist. In the literature, HCC with sarcomatous changes has been reported several times including the imaging findings. However, there are only a few reports of sarcomatoid cholangiocarcinomas (s-CCC) without cross-sectional imaging findings[1–3].
In this report, we present the computed tomography (CT) and magnetic resonance imaging (MRI) findings of an intrahepatic sarcomatoid cholangiocarcinoma.
Case presentation
A 48-year-old-man was admitted to our clinic with occasional left upper quadrant pain and fatigue. In his past medical history, he had a laparoscopic cholecystectomy operation 9 years previously. In his physical examination, left upper quadrant pain was noted that was aggrevated with palpation. Abnormal laboratory findings included: total bilirubin, 1.4 mg/dl (normal, <1.2 mg/dl), direct bilirubin, 0.6 mg/dl (normal, <0.4 mg/dl), alkaline phosphatases, 224 IU/l (normal, 44–155 IU/l), glutamic oxaloacetate transaminase, 45 U/dl (normal 10–30 U/dl), glutamic pyruvic transaminase, 152 U/dl (normal 7–27 U/dl), gamma-glutamyl transferase, 297 U/dl (normal, 10–49 U/dl), carbohydrate antigen 19-9, 39 U/ml (normal, <30 U/ml). Abdominal ultrasonography examination of the patient revealed a heterogeneous hypoechoic mass in the left liver lobe. Both CT and MRI of the upper abdomen were performed including magnetic resonance cholangiopancreatography (MRCP). CT was performed on a 64-slice CT scanner (Somatom Sensation; Siemens, Erlangen, Germany) without contrast, in arterial and portal venous contrast enhancement phases. MRI was performed on a 1.5-T scanner (Intera, Philips Medical Systems, Best, the Netherlands). The standard upper abdomen MRI protocol consisted of the following imaging sequences and parameters: T1-weighted spoiled gradient echo in dual phase (repetition time (TR), 140–170 ms; echo time (TE), 4.4–2.2 ms; flip angle (FA), 70°); T1-weighted fat-saturated two-dimensional spoiled gradient echo (TR, 160–180 ms; TE, 4.3 ms; FA, 70°); and T2-weighted fat-saturated echo train fast spin echo (TR, 8 ms; TE, 80 ms). All images were obtained in the axial plane with 6-mm section thickness and 160 to 190 × 256 matrix with a sensitivity encoding factor of 2. MRCP images were obtained using two-dimensional breath-hold T2-weighted fat-saturated single-shot fast spin echo images (TR, 8 ms; TE, 1200 ms) with a slab thickness of 30–50 mm in 12 paracoronal planes comprising an angle of 360°. For the acquisition of each separate plane, patients held their breath for 5 s. Gadolinium diethyltriaminepentaacetic acid (Magnevist; Schering, Berlin, Germany) was injected at a dose of 0.1 mmol/kg of body weight as a bolus injection at 2 ml/s using a power injector (Medrad, Indianola, PA). Images were acquired at 18 s (arterial-dominant phase), 45 s (portal venous phase), and 90 s (late venous phase) after contrast administration. For serial contrast-enhanced images, a T1-weighted fat-saturated two-dimensional spoiled gradient echo sequence was used.
On the upper abdomen CT examination; a 13 × 10 × 7 cm heterogeneous hypodense irregular-shaped mass, which completely occupied segments 2 and 3 of the liver and partially located in segment 4 of the liver, extending to the liver hilus and causing lobulation of liver, was seen (Fig. 1a,b). On MRI, on T1-weighted images, the lesion was hypointense relative to the liver; on T2-weighted images, it was heterogeneous and slightly hyperintense and more hyperintense areas were seen in the central region of the lesion. Also, on T2-weighted images, there was a slightly hypointense area at the anterior-superior part of the lesion, which caused irregular retraction of the liver capsule that was evaluated as scar tissue (Fig. 2a,b). On dynamic contrast-enhanced CT and MRI images, the lesion showed peripheral enhancement on the arterial phase and contrast enhancement in a progressive manner from the periphery to the centre on the venous phases. Irregular-shaped areas in the centre of the lesion that showed no contrast enhancement in late phases were evaluated as necrotic changes. In addition, at the liver hilus adjacent to this mass, satellite nodules measuring approximately 4 × 2.5 cm and 3 × 2 cm were seen. Satellite nodules showed less contrast enhancement compared with the main mass (Fig. 3a–d). At the hilar, hepatoduodenal ligament and lesser curvature levels, multiple lymph nodes were seen with the largest measuring 22 × 12 mm. On MRCP images, intrahepatic bile duct dilatation due to compression of bile ducts secondary to the main mass and satellite lesions were seen (Fig. 4a,b).
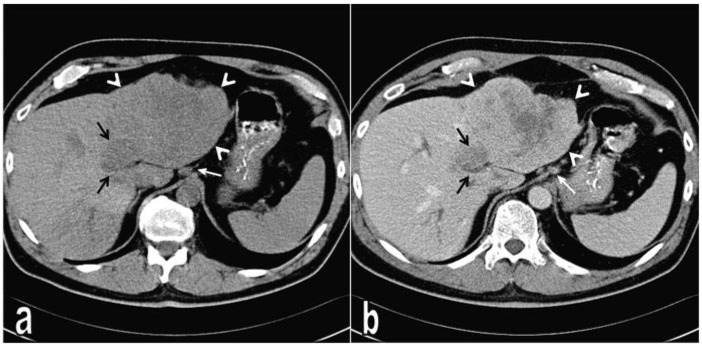
Figure 1.
Intrahepatic cholangiocarcinoma showing sarcomatous transformation in the left liver lobe. (a) On non-contrast CT, the tumour presents as a heterogeneous hypodense mass (arrow heads). (b) Portal venous phase CT reveals contrast enhancement of the tumour mass except the necrotic areas in the centre. Medial to the main tumour mass there is a more hypodense satellite tumour (black arrows), which demonstrates slight contrast enhancement on contrast-enhanced images. Note the lymph nodes at the preaortic and perigastric regions (white arrow).
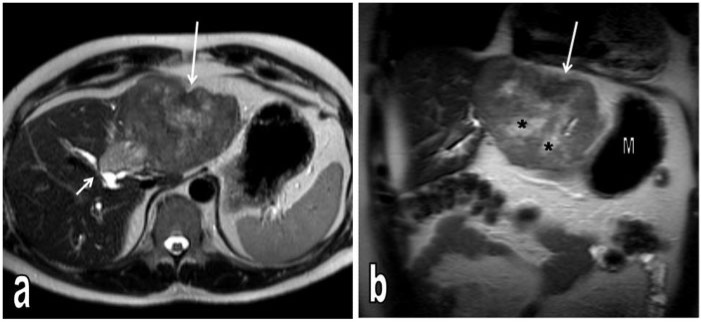
Figure 2.
T2-weighted axial (a) and coronal (b) MR images. The tumour in the left liver lobe causes contour bulging and is heterogeneously hyperintense. In the central region of the mass, there are irregular more hyperintense areas consistent with necrotic changes (*) and in the anterior-superior part of the mass, a slightly hypointense area is seen to cause retraction of the liver capsule consistent with scar (long arrows). The axial image demonstrates a more hyperintense satellite nodule adjacent to the medial part of the tumour, close to the central bile duct and causing compression (short arrow). S, stomach.
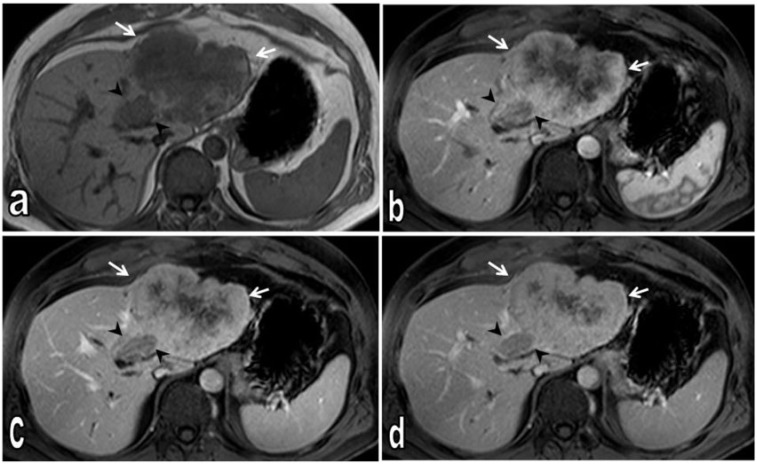
Figure 3.
Axial T1-weighted non-contrast (a), dynamic contrast-enhanced T1-weighted fat-saturated arterial (b), portal venous (c) and late venous phase (d) MR images. Non-contrast T1-weighted image demonstrates heterogeneous hypointense tumour; satellite nodule medial to the main tumour is also hypointense (arrow heads). Dynamic contrast-enhanced T1-weighted images show tumour tissue (arrows); at later phases, progressive contrast enhancement is seen from periphery to central. On late venous phases, the central region of the tumour shows no contrast enhancement consistent with necrotic changes (d). The satellite nodule shows less contrast enhancement than the main tumour on all phases (arrow heads).
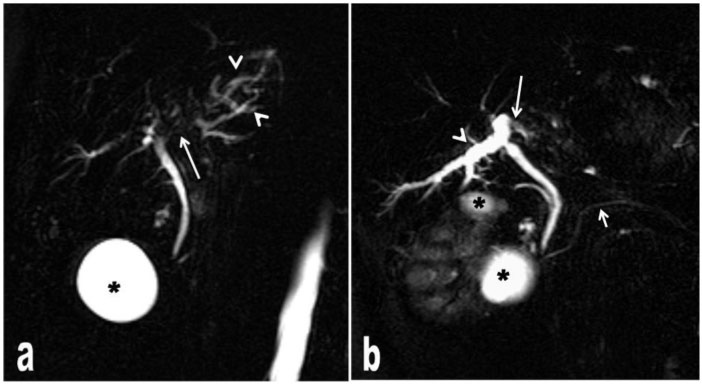
Figure 4.
MRCP images (a,b) show tumour tissue compressing the bile ducts (long arrows) extrinsically and therefore causing bile duct dilatation (arrow heads). The diameter of the common bile duct and ductus pancreaticus is normal (short arrow). *, renal cysts.
Left hemihepatectomy and lymphadenectomy was performed. There were no malignant cells at the surgical margins or in the hyperplastic lymph nodes. Histopathologic and immunohistochemical examinations were performed and cholangiocarcinoma with sarcomatous change was diagnosed. The patient received adjuvant oral chemotherapy for 6 months after the operation. On follow-up, mediastinal metastasis was suspected on thorax positron emission tomography/CT imaging at 12 months and thoracoscopic mediastinal tumour resection was performed that revealed a metastatic tumour with the same histopathology as the primary site. A new chemotherapy regimen was scheduled.
Discussion
The diagnosis of cholangiocarcinomas showing sarcomatous transformation is based on histopathologic and immunohistochemical examinations. The sarcomatous components show immune reaction with vimentin and cytokeratin. The diagnosis in our case was based on the same method. This feature is an indicator of sarcomatous transformation in a neoplasm of epithelial origin[3–8].
In the literature, cholangiocarcinomas with sarcomatous transformation have been reported as mixed echoic masses on ultrasonography in some studies[6]; whereas in other studies they have been reported as heterogeneous hypoechoic masses on ultrasonography[7,8]. In our case, the tumour appeared as a heterogeneous hypoechoic mass on ultrasonography. The common view in the literature was a hypodense tumour on CT images[5–7]. In a few previously reported cases, the tumour showed hypointensity relative to liver parenchyma on T1-weighted images and hyperintensity relative to liver parenchyma on T2-weighted images[4,7,9]. In some reports, mixed signal intensity of the mass was present on both T1- and T2-weighted images[5–7].
On dynamic contrast-enhanced CT and MRI, peripheral contrast enhancement, frequently seen in the arterial phase, the portal venous phase and the late venous phase with a progressive contrast enhancement pattern from the periphery to the centre was reported[4,7,9]. This finding is in agreement with the contrast enhancement pattern seen in classic cholangiocarcinomas. In 3 cases reported in the literature in which angiography was performed, the tumour had a hypovascular character[7]. Therefore, the typical contrast enhancement pattern of the sarcomatous component in cholangiocarcinomas with sarcomatous transformation is not yet known.
In an analytical study covering a 15-year period[6], 4 cases out of 7 patients with cholangiocarcinoma with sarcomatous transformation, a satellite nodule was present, as observed in our case. The high incidence of satellite nodules in cholangiocarcinomas with sarcomatous transformation suggest a relationship between them. In another case study of cholangiocarcinoma with sarcomatous transformation and presenting with multiple satellite nodules, sarcomatous change was reported in most of the nodules[10]. It was assumed that the more rapid growth and spread of sarcomatous elements compared with carcinomatous elements may be the cause[1]. In our case, scar tissue was observed within the lesion, which may be considered in the differential diagnosis with other central scar-containing lesions such as fibrolameller HCC. Despite these findings, imaging findings that may be helpful for comparison between cholangiocarcinomas with sarcomatous transformation and intrahepatic tumours are not yet known due to the small number of cases reported. Further reports are needed to determine the specific imaging findings.
In conclusion, we report the imaging findings for a sarcomatoid cholangiocarcinoma on CT and MRI. A satellite nodule of the tumour and mixed internal signal intensities on MRI were distinguishing features of the lesion. Histopatology was required for the definite diagnosis.
Conflict of interest
We do not have a direct financial relationship with any commercial identity. We have no such conflicts of interest.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Sasaki M, Nakanuma Y, Nonomura A. Intrahepatic cholangiocarcinoma with sarcomatous transformation: an autopsy case. J Clin Gastroenterol. 1991;13:220–225. doi: 10.1097/00004836-199104000-00022. . PMid:1851775. [DOI] [PubMed] [Google Scholar]
- 2.Nakajima T, Tajima Y, Sugano I, Nagao K, Kondo Y, Wada K. Intrahepatic cholangiocarcinoma with sarcomatous change. Clinicopathologic and immunohistochemical evaluation of seven cases. Cancer. 1993;72:1872–1877. doi: 10.1002/1097-0142(19930915)72:6<1872::AID-CNCR2820720614>3.0.CO;2-N. . PMid:7689920. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra S, Wood J, Mansy T, Singh R, Zaitoun A, Madhusudan S. Intrahepatic sarcomatoid cholangiocarcinoma. J Oncol. 2010 doi: 10.1155/2010/701476. . doi:10.1155/2010/701476. PMid:20454704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider G, Massmann A, Fries P, Kramann B, Remberger K. MRI of sarcomatoid carcinoma of the liver. Eur J Radiol. 2005;54:63–67. [Google Scholar]
- 5.Matsuo S, Shinozaki T, Yamaguchi S, et al. Intrahepatic cholangiocarcinoma with extensive sarcomatous change: report of a case. Surg Today (Jpn J Surg) 1999;29:560–563. doi: 10.1007/BF02482354. [DOI] [PubMed] [Google Scholar]
- 6.Tsou YK, Wu RC, Hung CF, Lee CS. Intrahepatic sarcomatoid cholangiocarcinoma: clinical analysis of seven cases during a 15-year period. Chang Gung Med J. 2008;31:599–605. PMid:19241900. [PubMed] [Google Scholar]
- 7.Kaibori M, Kawaguchi Y, Yokoigawa N, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol. 2003;38:1097–1101. doi: 10.1007/s00535-003-1203-y. . PMid:14673730. [DOI] [PubMed] [Google Scholar]
- 8.Matsui T, Tsuji K, Ichiya T, et al. [Sarcomatoid carcinoma of the liver: a case report and review of the literature] Nippon Shokakibyo Gakkai Zasshi. 2010;107:1175–1183. (in Japanese). PMid:20616486. [PubMed] [Google Scholar]
- 9.Lim JH, Kim JW, Heo SH, Jeong YY, Kang HK. Intrahepatic sarcomatoid cholangiocarcinoma with portal vein thrombosis: a case report. J Korean Soc Radiol. 2009;60:333–337. [Google Scholar]
- 10.Sumiyoshi S, Kikuyama M, Matsubayashi Y, et al. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809–812. doi: 10.3748/wjg.v13.i5.809. PMid:17278210. [DOI] [PMC free article] [PubMed] [Google Scholar]