Abstract
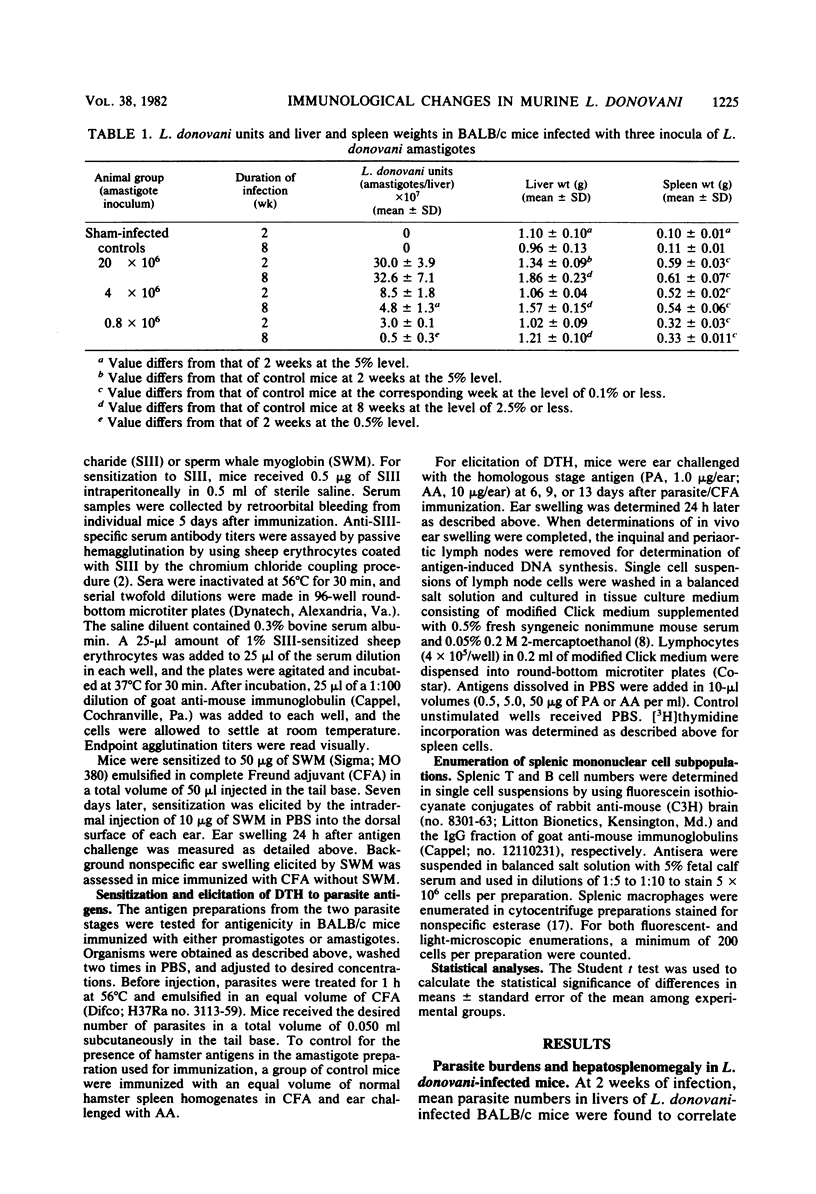
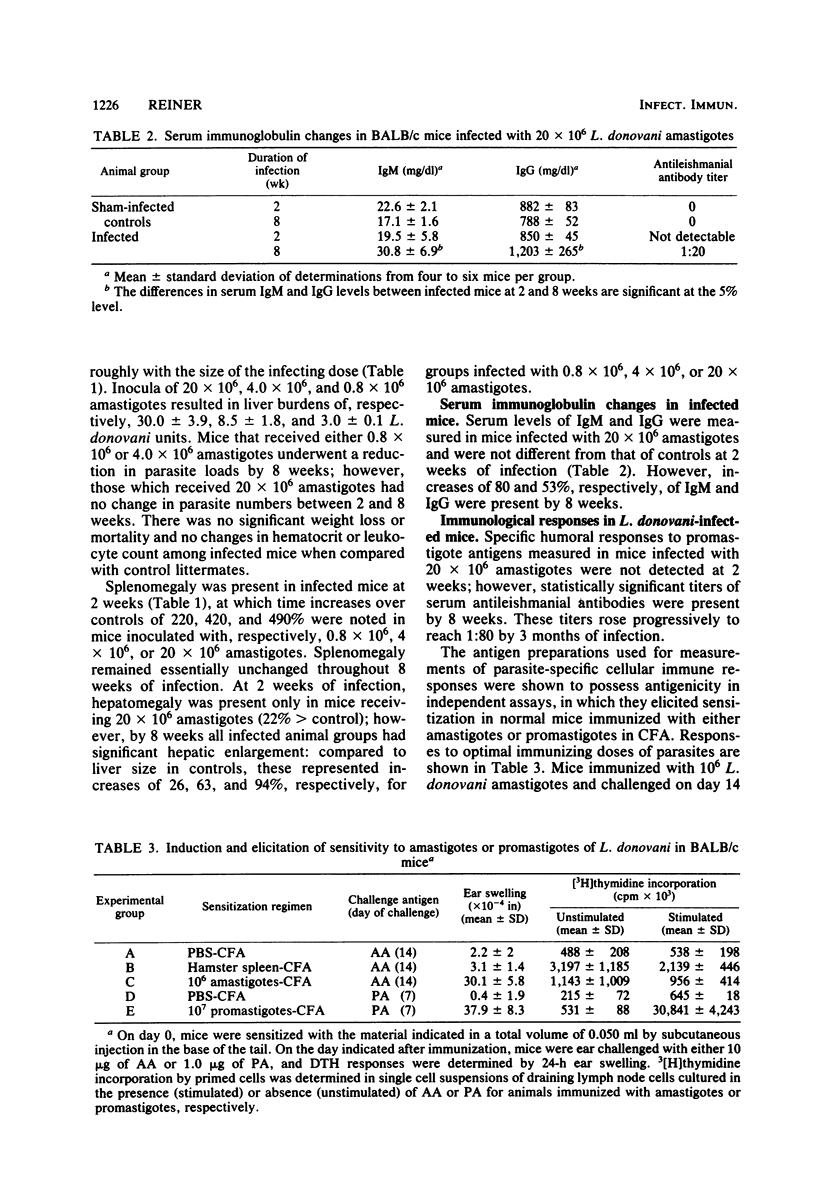
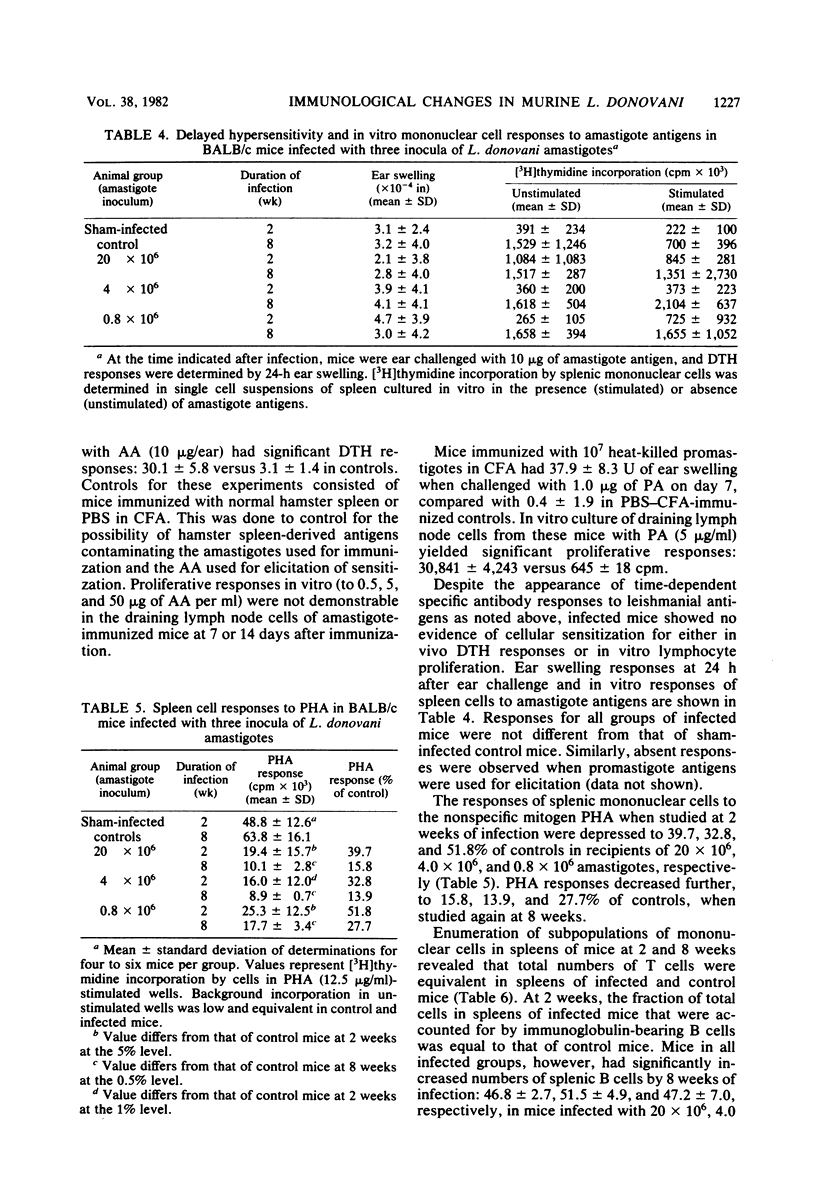
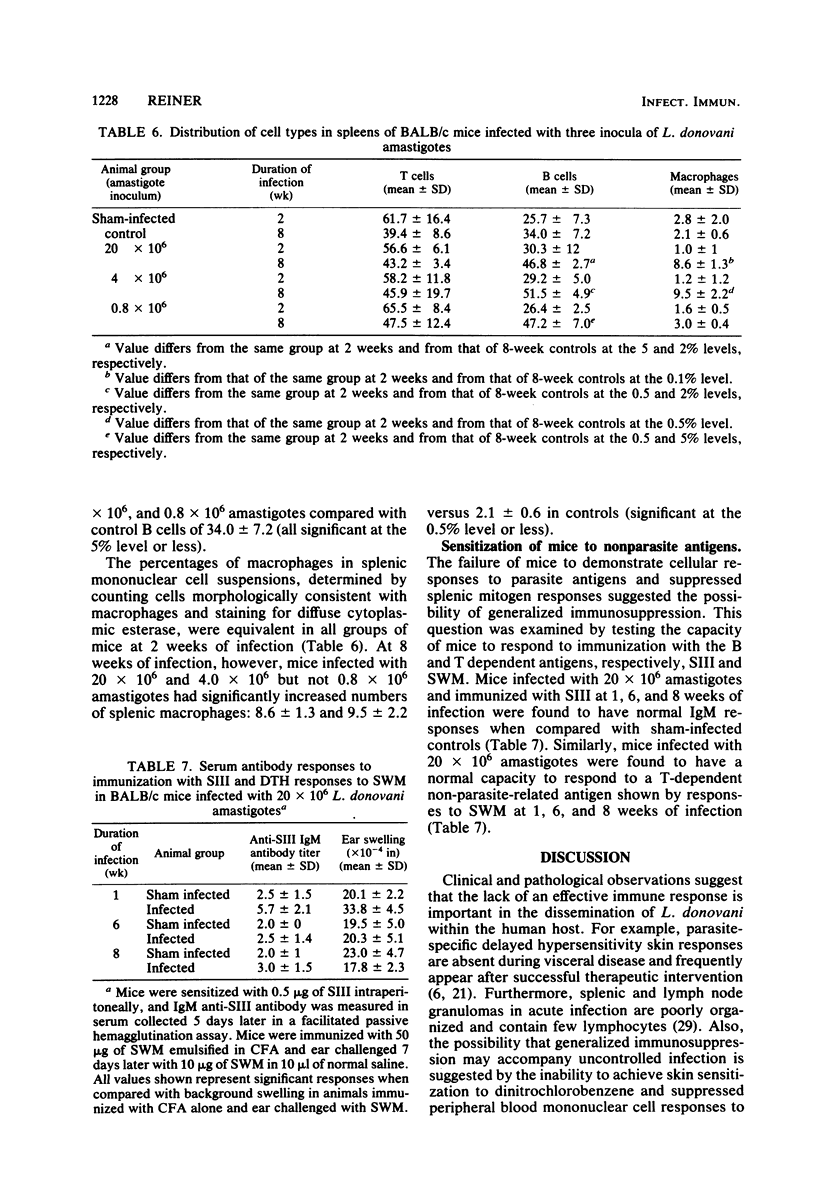
The host-parasite relationship in human visceral leishmaniasis remains poorly understood. In the present study, pathophysiological and immunological changes were examined in BALB/c mice infected with Leishmania donovani. These animals developed chronic infection with massive hepatosplenomegaly and hypergammaglobulinemia. In contrast to mice inoculated with 0.8 X 10(6) or 4 X 10(6) amastigotes, mice infected with 20 X 10(6) amastigotes failed to reduce liver parasite loads during 2 to 8 weeks of observation. At 8 weeks, liver size was increased by 26, 63, and 94%, respectively, in groups infected with 0.8 X 10(6), 4 X 10(6), or 20 X 10(6) amastigotes. Serum immunoglobulin G and M levels at 8 weeks in animals with the heaviest infection were increased by 53 and 80%, respectively, compared with controls. Specific antileishmanial antibodies were detected in the absence of antigen-specific delayed-type hypersensitivity or in vitro lymphocyte responses. Infection did not suppress the in vivo responses of mice to the non-parasite-related antigens sperm whale myoglobin or pneumococcal polysaccharide. Splenic mononuclear cell responses to phytohemagglutinin were suppressed as early as 2 weeks, and by 8 weeks, mice infected with 0.8 X 10(6), 4 X 10(6), or 20 X 10(6) amastigotes had phytohemagglutinin responses which were, respectively, 27.7, 13.9, and 15.8% of controls. Decreased phytohemagglutinin responses could not be related to reductions in splenic T cells; however, splenic B cells and macrophages were increased at 8 weeks of infection. The course of L. donovani infection and disease in BALB/c mice resembles events occurring in humans and should prove useful in defining mechanisms of immune alterations in visceral leishmaniasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikat B. K., Pathania A. G., Sehgal S., Bhattacharya P. K., Dutta U., Pasricha N., Singh S., Parmar R. S., Sahaya S., Prasad L. S. Immunological response in Indian kala-azar. Indian J Med Res. 1979 Oct;70:583–591. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J., Freeman J., Bradley D. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature. 1980 Jan 3;283(5742):72–74. doi: 10.1038/283072a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J. Letter: Genetic control of natural resistance to Leishmania donovani. Nature. 1974 Jul 26;250(464):353–354. doi: 10.1038/250353a0. [DOI] [PubMed] [Google Scholar]
- Bradley D. J., Taylor B. A., Blackwell J., Evans E. P., Freeman J. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin Exp Immunol. 1979 Jul;37(1):7–14. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Benck L., Alter B. J. Immune responses in vitro. I. Culture conditions for antibody synthesis. Cell Immunol. 1972 Feb;3(2):264–276. doi: 10.1016/0008-8749(72)90165-7. [DOI] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Phytohaemagglutinin-induced lymphocyte transformation test in Indian kala-azar. Trans R Soc Trop Med Hyg. 1979;73(6):725–726. doi: 10.1016/0035-9203(79)90032-4. [DOI] [PubMed] [Google Scholar]
- Herman R. Cytophilic and opsonic antibodies in visceral leishmaniasis in mice. Infect Immun. 1980 May;28(2):585–593. doi: 10.1128/iai.28.2.585-593.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel M., Peters W., Ranque J., Quilici M., Lanotte G. The micro-ELISA technique in the serodiagnosis of visceral leishmaniasis. Ann Trop Med Parasitol. 1978 Jun;72(3):213–218. doi: 10.1080/00034983.1978.11719308. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Chan-Liew W. L. Immunological regulation of experimental cutaneous leishmaniasis. 1. Immunogenetic aspects of susceptibility to Leishmania tropica in mice. Parasite Immunol. 1980 Winter;2(4):303–314. doi: 10.1111/j.1365-3024.1980.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J Exp Med. 1981 Mar 1;153(3):557–568. doi: 10.1084/jem.153.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S. Algorithms in the diagnosis and management of exotic diseases. XXIV. Leishmaniases. J Infect Dis. 1977 Jul;136(1):160–163. doi: 10.1093/infdis/136.1.160. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Musumeci S., Schilirò G., Li Volti S., Sciotto A. Lymphocyte changes in Mediterranean kala-azar. Trans R Soc Trop Med Hyg. 1981;75(2):304–305. doi: 10.1016/0035-9203(81)90342-4. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis. I. Nonspecific immunodepression in BALB/c mice infected with Leishmania tropica. J Immunol. 1981 Dec;127(6):2395–2400. [PubMed] [Google Scholar]
- Skov C. B., Twohy D. W. Cellular immunity to Leishmania donovani. I. The effect of T cell depletion on resistance to L. donovani in mice. J Immunol. 1974 Dec;113(6):2004–2011. [PubMed] [Google Scholar]
- Smrkovski L. L., Reed S. G., Larson C. L. Effect of cortisone and cyclophosphamide on the immunological role of BCG in Balb/c mice challenged with Leishmania donovani. Am J Trop Med Hyg. 1980 Jan;29(1):16–20. doi: 10.4269/ajtmh.1980.29.16. [DOI] [PubMed] [Google Scholar]
- Veress B., Omer A., Satir A. A., El Hassan A. M. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977 Nov;33(5):605–610. [PMC free article] [PubMed] [Google Scholar]



