Abstract
Cell walls were isolated from the mesocarp of grape (Vitis vinifera L.) berries at developmental stages from before veraison through to the final ripe berry. Fluorescence and light microscopy of intact berries revealed no measurable change in cell wall thickness as the mesocarp cells expanded in the ripening fruit. Isolated walls were analyzed for their protein contents and amino acid compositions, and for changes in the composition and solubility of constituent polysaccharides during development. Increases in protein content after veraison were accompanied by an approximate 3-fold increase in hydroxyproline content. The type I arabinogalactan content of the pectic polysaccharides decreased from approximately 20 mol % of total wall polysaccharides to about 4 mol % of wall polysaccharides during berry development. Galacturonan content increased from 26 to 41 mol % of wall polysaccharides, and the galacturonan appeared to become more soluble as ripening progressed. After an initial decrease in the degree of esterification of pectic polysaccharides, no further changes were observed nor were there large variations in cellulose (30–35 mol % of wall polysaccharides) or xyloglucan (approximately 10 mol % of wall polysaccharides) contents. Overall, the results indicate that no major changes in cell wall polysaccharide composition occurred during softening of ripening grape berries, but that significant modification of specific polysaccharide components were observed, together with large changes in protein composition.
Softening is an important part of the ripening process in most fruit, and it is widely recognized that changes in cell walls accompany fruit softening. Gross changes in wall composition may not always occur, and indeed more subtle structural modifications of constituent polysaccharides are often observed during softening (Brady, 1987; Fischer and Bennett, 1991). For example, molecular mass, solubility, and the degree of substitution (or branching) of an individual wall polysaccharide may be altered without any large change in the total amount of that polysaccharide.
Although localized alterations in pH or ion concentration can produce detectable, noncovalent changes in cell wall properties (Carpita and Gibeaut, 1993; Seymour and Gross, 1996), covalent modifications of wall polysaccharides generally result from enzymatic processes (Fischer and Bennett, 1991; Fry, 1995). In particular, an increase in water-soluble pectic polysaccharides and the loss of Gal and/or Ara from the wall occur in many fruit during softening (Huber, 1983; Gross and Sams, 1984), and this has been attributed in part to the action of PGs and PMEs. However, in nonripening mutants and in antisense experiments in tomatoes, it has become clear that these enzymes are not the only contributing factors in the observed modification of pectin solubility (Seymour et al., 1987; Smith et al., 1990). The softening process is complicated by the fact that breakdown or modifications of different components are usually accompanied by the incorporation of newly synthesized components into the wall (Gibeaut and Carpita, 1994; Seymour and Gross, 1996). The synthesis of cell wall polymers is probably continuous throughout ripening, and a change in the turnover rate of a particular component will affect the overall wall composition (Lackey et al., 1980).
Modifications of wall components might also be expected in ripening grape (Vitis vinifera L.) berries, but little is known about cell wall composition in grapes during ripening or of the mechanism of softening in this fruit. The grape berry is a nonclimacteric fruit that exhibits a double-sigmoidal growth curve characteristic of berry fruits (Coombe, 1976). The first growth phase is initially due to cell division and subsequently to cell enlargement (Harris et al., 1968). Thereafter, the grape goes through a period of little or no growth. This is followed by a second growth phase, in which the increase in berry volume is entirely due to cell expansion within the berry. The grape berry is somewhat unusual in that it softens at the same time as it expands during the second growth, or ripening, phase. The onset of the second growth phase is referred to as “veraison,” which is a viticultural term that describes the point at which a number of developmental events are initiated, including the accumulation of sugars, a decrease in organic acids, color development, berry expansion, and softening (Coombe, 1973).
The monosaccharide compositions and structures of specific pectic polysaccharide fractions from grape berries have been reported (Saulnier and Thibault, 1987; Saulnier et al., 1988), as have changes in pectin solubility as the berry ripens (Silacci and Morrison, 1990), but there have been no comprehensive analyses of intact walls during ripening. The increasing commercial importance of the wine industry internationally and the recognition that polysaccharides of cell wall origin, in particular the pectic polysaccharides, create problems during juice extraction, during filtration steps required to clarify grape extracts, and during the formation of storage hazes that decrease the shelf-life of the wine, all suggest that detailed studies of cell wall composition might reveal important changes in the walls and might point to key enzymes that mediate the process.
In the present study a recently developed procedure for the preparation of cell walls from the mesocarp of grape berries (Nunan et al., 1997) has been used to isolate walls at various stages during berry development and ripening. The appearance of the walls during ripening has been examined by light and fluorescence microscopy. The monosaccharide and polysaccharide compositions of the walls have been monitored and changes in solubility of specific polysaccharides have been observed. Significant changes in protein content and amino acid composition are also seen.
MATERIALS AND METHODS
Plant Material
Grape (Vitis vinifera L. cv Muscat Gordo Blanco, hereafter referred to simply as Gordo) berries were harvested every 2 weeks from January to March, 1994, at the South Australian Advisory Centre (Nuriootpa, South Australia). The time of anthesis was taken to be the point at which anthers were observed on 50% of the flowers (November 28, 1993). At this time, bunches that were at mid-flowering were tagged for subsequent sampling. For each sample time, a number of tagged bunches were picked and all berries were removed. A representative sample of 50 berries was used for measurements described below. The remaining grapes were rinsed thoroughly with water and stored at −80°C until required for wall isolation.
Berry Measurements
The lengths and widths of the 50 berries were recorded for the calculation of berry volume, and berry weight was also recorded. Using juice squeezed from each berry, °Brix (percent soluble sugar) was measured using a hand-held refractometer (Leica) that had been calibrated against standard Suc solutions. Deformability (the degree of softness) was measured using skin-fold calipers according to the method of Coombe and Bishop (1980).
Microscopy
Berries collected at each stage of development were prepared for microscopy, essentially as described by O'Brien and McCully (1981). Freshly picked berries were cut into small pieces and fixed in 3% (v/v) glutaraldehyde, 0.05% (w/v) caffeine, and 25 mm sodium phosphate buffer, pH 7.0, at 4°C for 16 h. The berry pieces were dehydrated for 2 h in each of two changes of methoxyethanol, ethanol, propanol, and butanol. The dehydrated berries were infiltrated with a solution of GMA containing 7% (w/v) PEG 400, 0.6% (w/v) benzoyl peroxide, and butanol (1:1, v/v) for 2 h at 4°C. The solution was removed and the berry pieces were infiltrated with the GMA mixture for a further 2 d at 4°C. The berry pieces were resuspended in fresh GMA mix and stored at 4°C prior to embedding. Each infiltrated berry piece was placed in a small plastic capsule containing GMA mixture, and the GMA was polymerized at 60°C for 2 d. Berries were sectioned to a thickness of approximately 3 μm using a Reichert-Jung Microtome 2050 Supercut (NuBloch, Germany). The sections were stained with 0.05% TBO in 10 mm sodium benzoate buffer, pH 4.4, or 0.01% (w/v) Calcofluor in water for 3 min and rinsed with water. The sections were viewed using an Axiophot Pol photomicroscope (Zeiss).
Preparation of Cell Walls
During thawing of frozen berries, skin and seeds were removed and mesocarp cell walls were isolated as described by Nunan et al. (1997). Skin and seeds were removed manually and the remaining mesocarp tissue was homogenized in 4 volumes of absolute ethanol using a household blender. The homogenate was filtered sequentially through nylon mesh (Swiss Screens, Moorabbin, Victoria, Australia) with pore sizes of 350, 280, and 71 μm. The material retained on the 71-μm mesh was stirred in phenol that had been saturated with 500 mm Tris-HCl buffer, pH 7.0, for 45 min and washed with ethanol and acetone to remove the phenol. The retained material was stirred in chloroform:methanol (1:1, v/v) twice for 1 h each, and in acetone for 1 h. The isolated cell wall preparations were dried in a vacuum oven at 40°C and stored over silica gel in a vacuum desiccator.
Fractionation of Cell Walls
Wall preparations were fractionated essentially as described by Redgwell et al. (1988). The walls (200 mg) were extracted twice in 20 mL of water at 40°C for 3 h with continuous stirring. After each solvent extraction, the wall suspension was centrifuged for 10 min at 8000g. The supernatants were combined, dialyzed exhaustively against water at 4°C, reduced in volume by rotor evaporation at 45°C, and freeze-dried. The water-insoluble pellet was suspended in 20 mL of 0.05 m CDTA and extracted twice, as described above. The combined supernatant was dialyzed against 2 changes of 0.1 m NaCl, pH 6.5, before dialyzing against water. The remaining cell walls were extracted with 0.05 m Na2CO3 at 4°C for 16 h with continuous stirring. The supernatant was neutralized with glacial acetic acid before dialysis. The pellet was extracted with 4 m KOH at room temperature for 24 h with continuous stirring. Insoluble material was washed with water and freeze-dried. Freeze-dried cell wall fractions were stored over silica gel in a vacuum desiccator.
Protein and Amino Acid Estimations
Protein in the wall preparations and amino acid compositions were determined as described by Nunan et al. (1997). Hyp in hydrolysates was measured colorimetrically by the procedure of Kivirikko and Liesmaa (1959).
Polysaccharide Linkage Analysis
Linkage analyses of cell wall preparations and extracted wall fractions were performed as described in Nunan et al. (1997). All methylation analyses were performed in duplicate, and where significant variation was observed, the analyses were repeated again in duplicate. For the reduction of uronic acid and esterified uronic acid residues, the cell wall preparations were first treated with NaBD4 (Sigma), which reduces esterified uronic acid residues (Nunan et al., 1997). Free-uronic acid residues were subsequently derivatized with 1-cyclo-3-(2-morpholinoethyl) carbodiimide metho-p-toluenesulphonate (Aldrich), and the samples were reduced either with NaBD4 for the determination of total uronic acids or with NaBH4 to determine esterified uronic acids (Sims and Bacic, 1995).
Carboxyl-reduced wall preparations and fractions were methylated using the NaOH/CH3I method of Ciucanu and Kerek (1984) as described previously (Nunan et al., 1997). The methylated polysaccharides were hydrolyzed with trifluoroacetic acid, reduced with NaBD4, and acetylated using perchloric acid as a catalyst (Harris et al., 1984). Partially methylated alditol acetates were separated on a bonded-phase BPX70 capillary column (SGE, Melbourne, Australia) in a MAT 1020B GC-MS (Finnigan, San Jose, CA). Neutral sugar and uronic acid derivatives were identified and quantitated as described previously (Sims and Bacic, 1995).
RESULTS
Berry Development
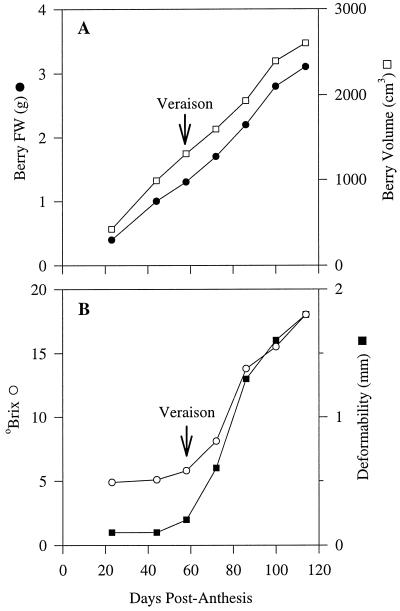
Berry weight and volume, deformability, and the accumulation of sugars during berry development are shown in Figure 1. There was a steady increase in berry weight and volume throughout the developmental period. Berry deformability and sugar content remained low until 58 dpa, when both parameters began to increase markedly (Fig. 1B). Total soluble solids remained at 5 to 6 oBrix until 58 dpa, but thereafter increased markedly and reached a value of 18 oBrix when the last sample was taken at 114 dpa (Fig. 1B). Deformability, which is a measure of berry softening, was only 0.1 to 0.2 mm until 58 dpa, but increased rapidly to 1.8 mm at 114 dpa (Fig. 1B). Based on the rapid increase in soluble solids and deformability from 58 dpa, we concluded that the inception of ripening (veraison) occurred at that time.
Figure 1.
Growth curves for Gordo grapes showing change in berry fresh weight (•) and volume (□) during development (A), and change in berry °Brix (○) and deformability (▪) (B). FW, Fresh weight.
Microscopy
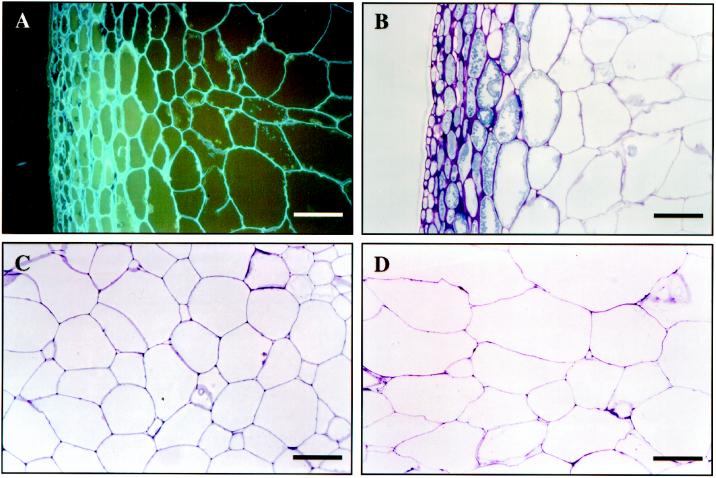
The appearance of cell walls in intact berries was monitored throughout development by fluorescence and light microscopy (Fig. 2). Examination of the walls of the skin and hypodermis and the outer mesocarp (Fig. 2, A and B) revealed few obvious differences between berries at veraison (58 dpa), with a deformability of 0.2 mm, and those at 86 dpa, with a deformability of 1.3 mm. It should be noted that the outermost layers of cells, which make up the skin and hypodermis, were removed before cell walls were prepared. It is also noteworthy that TBO, which stains polysaccharides purple and polyphenolics green, revealed a high content of phenolics in the skin cells (Fig. 2B).
Figure 2.
Microscopy of the ripening grape berry. Sections of the outermost region of the grape were prepared at 58 dpa (A) and 86 dpa (B). The sections were stained with Calcofluor and TBO, respectively, and show the epidermis to the left and the mesocarp to the right (bars = 100 μm). The sections in C and D are from approximately the same location in the central mesocarp at 58 and 114 dpa, respectively, and are stained with TBO (bars = 200 μm).
Comparison of the mesocarp tissue of a grape at 114 dpa, with a deformability of 2 mm (Fig. 2D), and the mesocarp tissue of a grape at 58 dpa, with a deformability of 0.2 mm (Fig. 2C), showed that cells were generally larger in the berry at 114 dpa compared with the 58 dpa berry. No apparent change in cell wall thickness could be detected during ripening (Fig. 2, C and D).
Cell Wall Isolation
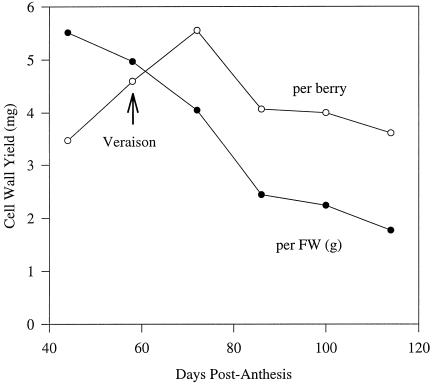
Based on the developmental patterns observed in Figure 1, six specific times of berry development were chosen for the isolation of cell walls. These were: preveraison (44 dpa), veraison (58 dpa), and four time points after veraison when berry softening was occurring (72, 86, 100, and 114 dpa). Because of the limited amount of available plant material, the low yields of cell walls from grapes generally (Nunan et al., 1997), and the time required for wall preparation, all of the grapes at each developmental stage were pooled and a single cell wall preparation was made. Nevertheless, yields for replicated wall isolations with different samples of ripe grapes varied by less than 7% (data not shown). The yields of cell walls from Gordo berries at each stage of development are shown in Figure 3. On a per gram fresh weight basis, the amount of cell wall isolated decreased steadily throughout development. However, on a per berry basis, there was an initial increase in the cell wall yield until 72 dpa, after which yields decreased to preveraison levels. A similar decrease in cell wall yield was observed when walls were isolated during the development of Ohanez, another grape cultivar (data not shown).
Figure 3.
Cell wall yield from Gordo berries during ripening, expressed on a per gram fresh weight basis (•) and on a per berry basis (○). The walls were isolated at six stages of development from preveraison (44 dpa) through veraison (58 dpa) to the fully ripe berry (114 dpa). FW, Fresh weight.
Protein and Amino Acid Content
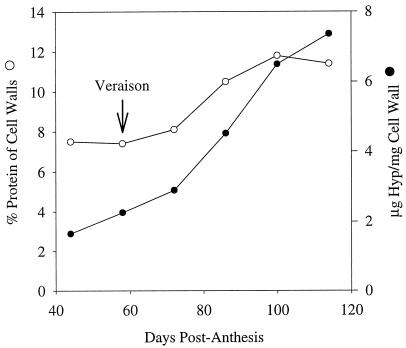
The changes in protein content of the isolated cell walls during development are shown in Figure 4. Phenol treatment has been shown to remove most adventitious proteins, and the remaining protein is assumed to be an integral component of the cell walls. The protein content of walls from young berries was less than 8% but as the berries matured, the amount of protein associated with the cell walls increased to almost 12% by weight.
Figure 4.
Protein (○) content (% by weight) and Hyp (•) content (μg/mg cell wall) of purified cell walls isolated from developing Gordo berries.
The amino acid composition of isolated cell walls is shown in Table I. The major amino acids were Glx, Arg, Hyp, and Gly. The amino acids that showed the largest changes were Arg, which decreased from 23.8 mol % to 14.5 mol % of wall protein, and Hyp, which increased from 3.6 mol % to 9.9 mol % from preveraison to 100 dpa. Over the same period the amount of Hyp increased from 1.7 to 7.4 μg per mg cell wall (Fig. 4).
Table I.
Amino acid composition of the proteins associated with cell walls isolated from developing berries from 44 to 114 dpa
| Amino Acid | 44 dpa | 58 dpa | 72 dpa | 86 dpa | 100 dpa | 114 dpa |
|---|---|---|---|---|---|---|
| mol % | ||||||
| Asx | 3.4 | 6.2 | 5.9 | 6.6 | 6.3 | 6.5 |
| Glx | 11.9 | 9.1 | 10.3 | 9.1 | 8.9 | 9.0 |
| Ser | 5.9 | 5.8 | 6.1 | 5.7 | 5.4 | 5.8 |
| His | 1.7 | 1.5 | 1.4 | 2.0 | 2.1 | 2.3 |
| Gly | 7.6 | 7.6 | 8.1 | 8.0 | 8.0 | 9.1 |
| Thr | 4.4 | 4.4 | 4.7 | 4.6 | 4.6 | 4.9 |
| Cys | 1.0 | 0.9 | 0.8 | 1.1 | 1.0 | 1.2 |
| Ala | 7.0 | 6.8 | 7.5 | 6.5 | 6.2 | 5.9 |
| Arg | 23.8 | 23.1 | 16.6 | 18.4 | 17.2 | 14.5 |
| Tyr | 2.4 | 2.3 | 2.6 | 2.7 | 2.6 | 2.9 |
| Val | 4.9 | 4.9 | 5.5 | 5.0 | 4.7 | 4.9 |
| Met | 1.4 | 1.2 | 1.2 | 1.3 | 1.0 | 1.1 |
| Phe | 3.1 | 3.1 | 3.3 | 3.0 | 2.7 | 2.7 |
| Ile | 3.9 | 3.9 | 4.1 | 3.8 | 3.5 | 3.6 |
| Leu | 6.3 | 6.5 | 6.7 | 5.9 | 5.4 | 5.5 |
| Lys | 4.0 | 4.2 | 4.5 | 5.0 | 5.1 | 5.9 |
| Pro | 3.9 | 4.1 | 4.6 | 4.7 | 5.4 | 4.9 |
| Hyp | 3.6 | 4.5 | 6.4 | 6.6 | 9.9 | 9.3 |
Wall Fractionation
The yields of various fractions of walls isolated from different stages of development are shown in Figure 5. The largest change was the 2-fold increase in the water-soluble component after veraison. A temporary decrease in the CDTA-soluble fraction at 58 dpa was observed in two separate fractionations, but otherwise the amounts stayed relatively constant throughout ripening. The Na2CO3-soluble fraction increased somewhat before veraison but decreased as the berries softened. Similarly, the KOH-soluble fraction also showed a small increase before veraison and thereafter decreased steadily. The final alkali-insoluble residue decreased throughout development, although the overall decrease in this fraction was relatively small.
Figure 5.
Changes in solubility of cell wall material isolated from developing Gordo berries, expressed on a dry weight basis. The cell walls were fractionated into water-soluble (□), 0.05 m CDTA-soluble (♦), 0.05 m Na2CO3-soluble (▪), and 4 m KOH-soluble (•) components, and a KOH-insoluble residue (○).
Linkage Analysis and Major Polysaccharides in the Cell Walls
The monosaccharide compositions and linkage types of mesocarp cell wall polysaccharides isolated at the six stages of development were analyzed by standard methylation procedures. Linkage analyses were performed in duplicate for each of the six wall preparations, and the values were averaged. The monosaccharide compositions are shown in Table II. The major change was the decrease in 4-linked galactopyranosyl (4-Galp) residues from 19 mol % before veraison to 1 mol % in fully ripe berries. The unesterified, 4-linked galacturonic acid (4-GalAp) increased during this ripening period from 10 to 20 mol %, and the esterified 4-GalAp increased from 14 to 18 mol %. The 4-linked glucopyranose (4-Glcp) showed an initial small increase, followed by a larger decrease later in development. The other derivatives constitute 5% or less of the total polysaccharide in the cell wall and did not vary significantly during ripening (Table II).
Table II.
Change in linkage composition of cell walls during the ripening of Gordo berries
| Monosaccharide | Deduced Linkagea | Linkage Composition
|
|||||
|---|---|---|---|---|---|---|---|
| 44 dpa | 58 dpa | 72 dpa | 86 dpa | 100 dpa | 114 dpa | ||
| mol % | |||||||
| Rhap | Terminal | trb | tr | tr | tr | tr | 1 |
| 2- | 1 | 1 | 1 | 1 | 1 | 2 | |
| 2,4- | 1 | tr | tr | 1 | 1 | 1 | |
| Fucf | Terminal | 1 | 1 | 1 | 1 | 1 | 1 |
| Araf | Terminal | 2 | 2 | 2 | 3 | 3 | 3 |
| 5- | 1 | 2 | 2 | 2 | 2 | 2 | |
| Xylp | Terminal | 2 | 2 | 2 | 2 | 3 | 2 |
| 2- | tr | –c | – | tr | 1 | 1 | |
| 4- | 3 | 3 | 3 | 4 | 4 | 4 | |
| 2,4- | tr | – | tr | tr | 1 | 1 | |
| Manp | 4- | 1 | tr | tr | 1 | 1 | 1 |
| 4,6- | tr | tr | – | tr | 1 | 1 | |
| Galp | Terminal | 1 | 1 | 1 | 1 | 2 | 2 |
| 2- | tr | tr | tr | tr | tr | tr | |
| 3- | 2 | 2 | 2 | 2 | 2 | 2 | |
| 4- | 19 | 12 | 9 | 3 | 1 | 2 | |
| 6- | tr | tr | – | – | tr | tr | |
| 2,4- | tr | – | – | – | tr | tr | |
| 3,4- | 1 | tr | 1 | 1 | tr | 1 | |
| 3,6- | 1 | 1 | tr | 1 | 1 | 1 | |
| Glcp | 4- | 34 | 37 | 37 | 38 | 35 | 32 |
| 2,4- | tr | – | – | tr | tr | tr | |
| 3,4- | 1 | 1 | 1 | 1 | 1 | tr | |
| 4,6- | 5 | 5 | 5 | 3 | 4 | 3 | |
| GalAp | 4- | 10 | 16 | 17 | 19 | 18 | 20 |
| GalApOMe | 4- | 14 | 15 | 17 | 16 | 17 | 18 |
| Glc/GlcAp | Terminal | 1 | 1 | 1 | 1 | 1 | 1 |
| Glc/GlcApOMe | 4- | 1 | – | tr | 1 | 1 | 1 |
Terminal Rhap is deduced from 1,2,3,4,5-penta-O-acetyl 6-deoxyhexitol, etc.
tr, Trace (< 0.5%).
–, Not detected.
The most abundant polysaccharide types found in the developing grape berry cell walls were subsequently deduced from the linkage composition (Table III), based on the totals of individual glycosyl residues that are characteristic of well-defined wall polysaccharides. The mol % values for polysaccharides referred to below and in Tables III and IV are calculated by the addition of mol % values of the appropriate methylated alditol acetates, and are expressed as mol % of total polysaccharide content of the wall; protein and other wall components are not included. These calculations embody a number of structural assumptions, but are widely used as a good indicator of trends in contents of specific polysaccharide types (Bacic et al., 1988; Shea et al., 1989; Gorshkova et al., 1996; Nunan et al., 1997). Thus, xyloglucan content was calculated from the sum of 4,6-Glcp, 4-Glcp equivalent to one-third of 4,6-Glcp, terminal and 2-Xylp, and 2-Galp and terminal l-fucopyranose. The remaining 4-Glc was assumed to be cellulose. Xylan was estimated from 4-Xylp and 2,4-Xylp, and terminal Araf or terminal GlcAp equal to the 2,4-Xylp. Arabinogalactan II was calculated from the sum of 3,6-Galp, 3-Galp, 6-Galp, and terminal Araf equivalent to the value for 3,6-Galp. Arabinogalactan I was estimated from the sum of 4-Galp, 3,4-Galp, and terminal Galp equivalent to 3,4-Galp. Arabinan was estimated from 5-Araf, mannan from the sum of 4-Manp, 4,6-Manp, and terminal Galp equivalent to 4,6-Galp. Galacturonan, which includes both rhamnogalacturonan and homogalacturonan, was the sum of 4-GalAp, 4-GalAp ester, 2-Rhap, and 2,4-Rhap (Bacic et al., 1988; Shea et al., 1989; Gorshkova et al., 1996).
Table III.
Changes in polysaccharides present in cell walls during the development of Gordo grapes
| Polysaccharide | 44 dpa | 58 dpa | 72 dpa | 86 dpa | 100 dpa | 114 dpa |
|---|---|---|---|---|---|---|
| mol %a | ||||||
| Galacturonan | 26 (58)b | 32 (49) | 35 (49) | 37 (47) | 37 (47) | 41 (48) |
| Arabinan | 1 | 2 | 2 | 2 | 2 | 2 |
| AGI | 21 | 12 | 11 | 5 | 1 | 4 |
| AGII | 3 | 4 | 2 | 4 | 4 | 4 |
| Xyloglucan | 10 | 10 | 10 | 7 | 10 | 8 |
| Heteromannan | 1 | trc | tr | 1 | 2 | 3 |
| Heteroxylan | 3 | 3 | 3 | 4 | 6 | 7 |
| α-Cellulose | 32 | 35 | 35 | 37 | 34 | 31 |
| Otherd | 3 | 2 | 2 | 3 | 4 | – |
Mol % values represent the mol % of the total wall polysaccharide, calculated from the mol % methylated alditol acetates that are characteristic of particular polysaccharides.
Numbers in parentheses indicate the percentage of esterification.
tr, Trace (<0.5%).
Linkage types that cannot be readily assigned to well-characterized polysaccharides from plant cell walls.
Table IV.
Distribution of major polysaccharides in cell wall fractions at different stages of grape berry development
| Polysaccharide Type | dpa | Total Polysaccharides | Polysaccharides in Each
Fractiona
|
||||
|---|---|---|---|---|---|---|---|
| Water | CDTA | Na2CO3 | KOH | Residue | |||
| mol %a | % | ||||||
| Galacturonan | 44 | 26 | 7 | 49 | 42 | 1 | 1 |
| 72 | 35 | 11 | 45 | 42 | 1 | 1 | |
| 114 | 41 | 26 | 41 | 32 | 1 | 0 | |
| Arabinan | 44 | 1 | trb | 13 | 53 | 13 | 20 |
| 72 | 2 | 4 | 22 | 48 | 4 | 22 | |
| 114 | 2 | 18 | 15 | 64 | 3 | tr | |
| AGI | 44 | 21 | 0 | 15 | 59 | 8 | 19 |
| 72 | 11 | 0 | 0 | 92 | 8 | 0 | |
| 114 | 4 | 0 | 0 | 100 | 0 | 0 | |
| AGII | 44 | 3 | 30 | 20 | 30 | 20 | 0 |
| 72 | 2 | 73 | tr | 23 | 4 | 0 | |
| 114 | 4 | 100 | 0 | tr | tr | 0 | |
| Xyloglucan | 44 | 10 | 0 | 0 | 0 | 80 | 20 |
| 72 | 10 | 3 | 0 | 0 | 72 | 25 | |
| 114 | 8 | 0 | 0 | 0 | 70 | 30 | |
| Heteromannan | 44 | 1 | 0 | 38 | 38 | 23 | 0 |
| 72 | tr | 5 | 26 | 29 | 8 | 32 | |
| 114 | 3 | 0 | 19 | 42 | 4 | 35 | |
| Heteroxylan | 44 | 3 | 27 | 10 | 3 | 34 | 26 |
| 72 | 3 | 27 | 16 | 10 | 44 | 3 | |
| 114 | 7 | 22 | 11 | 24 | 36 | 7 | |
| α-Cellulose | 44 | 32 | 2 | 3 | 5 | 4 | 86 |
| 72 | 35 | 3 | 0 | 0 | 1 | 97 | |
| 114 | 31 | 9 | 3 | 3 | 1 | 85 | |
Mol % values represent the mol % of the total wall polysaccharide, calculated from the mol % methylated alditol acetates that are characteristic of particular polysaccharides.
tr, Trace (<0.5%).
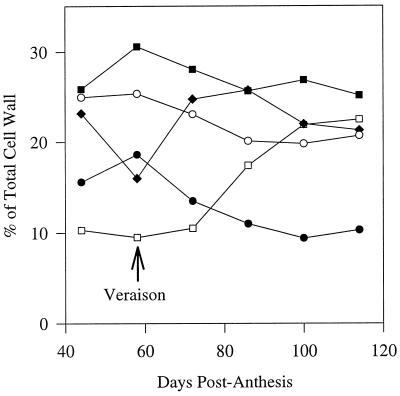
On a mol % basis, AGI decreased from 21% of total polysaccharides in preveraison walls to 1% in ripe, 100 dpa berries. Galacturonan increased from 26% preveraison to 41% in ripe berries; the DE decreased from 58% to 48% in the preveraison period and thereafter remained approximately constant. The cellulose content increased slightly early in development and decreased later. Xyloglucan was present but did not change significantly during ripening, whereas the smaller amounts of arabinan and mannan that were present remained approximately constant. Heteroxylan levels were low generally, but increased during ripening. Small amounts of material could not be readily assigned to well-characterized polysaccharides from plant cell walls and are therefore shown in Table III as “other.”
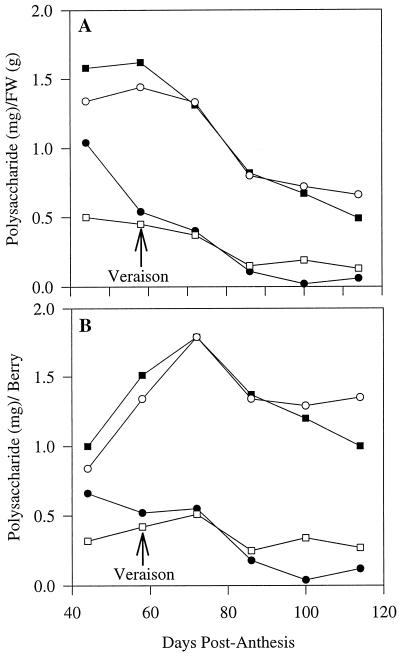
Because comparisons of mol % data are complicated by the possibility that a change in one component actually reflects a change in another, attempts were made to express the contents of specific polysaccharides on an absolute basis. From the relative proportion (mol % of total polysaccharides) of each polysaccharide in Table III and the cell wall yields of the same samples (Fig. 3), we were able to calculate the amounts of each polysaccharide on a per gram fresh weight basis and on a per berry basis. These data are presented in Figure 6, but should be viewed with some caution because changes in wall yields may be influenced not only by the absolute amount of wall material present, but also by factors such as changes in wall fragility.
Figure 6.
Changes in levels of specific cell wall polysaccharides (dry weights) during grape berry development, expressed on a tissue fresh weight basis (A) and a per berry basis (B). The four most abundant polysaccharides found in the cell walls are shown; these account for 85% to 90% of total wall polysaccharides. ▪, Cellulose; ○, galacturonan; •, AGI; and □, xyloglucan. FW, Fresh weight.
All polysaccharides decreased on a fresh weight basis (Fig. 6A), but on a per berry basis (Fig. 6B) galacturonan and cellulose contents increased up to and just after veraison before showing a slight decrease. AGI content decreased during ripening, but xyloglucan content per berry was relatively constant throughout development.
Fractionated Polysaccharides
Changing solubilities of polysaccharides, as indicated by their movement between fractions, are shown in Table IV. The increase in the water-soluble fraction was due to the shift of galacturonan from the CDTA- and Na2CO3-soluble fractions to the water-soluble fraction. AGII became completely water soluble as ripening progressed. The increase in water-soluble arabinan came from the KOH-soluble and residue fractions. The remaining AGI in the cell walls at 114 dpa was associated with the Na2CO3-soluble fraction, whereas it was found in the CDTA-, Na2CO3-, and KOH-soluble and KOH-insoluble residue fractions when the berries were young. Heteroxylans became slightly more soluble as the berry ripened; there was a decrease in the amount associated with the KOH-insoluble residue fraction and a corresponding increase in the Na2CO3-soluble fraction. The solubilities of xyloglucan and cellulose changed very little during ripening.
DISCUSSION
The onset of ripening in grape berries varies between bunches and even between individual berries in the same bunch (Coombe and Bishop, 1980; Coombe, 1992). To minimize the effects of this variation on physical and chemical analyses, bunches that flowered at the same time were tagged and used to provide samples at different stages of berry development. At each time point, a subsample of 50 berries was taken to measure berry characteristics; the remainder was used to isolate cell walls. The double-sigmoidal pattern normally seen during the development of individual berries was not obvious here (Fig. 1A) and was probably masked not only by the averaging of values for 50 berries, but also because berries were sampled only once every 2 weeks (Coombe and Bishop, 1980; Coombe, 1992). Nevertheless, the onset of ripening, or veraison, was clearly indicated by the relatively sudden increase in soluble solids and by the concomitant increase in berry deformability, which occurred at 58 dpa (Fig. 1B).
Following veraison, the size of central mesocarp cells appeared to increase (Fig. 2). The cell walls of many fruit swell as the fruit ripens (Redgwell et al., 1997). Swelling in kiwifruit cell walls during ripening is pronounced, and in some tissues a 3- to 4-fold increase in cell wall thickness has been observed (Redgwell et al., 1997). No swelling of the grape cell walls was visible by light microscopy during development (Fig. 2), in agreement with a previous study by Hardie et al. (1996).
The yield of isolated cell walls on a per-berry basis appeared to increase before veraison and into the start of the second growth phase, but decreased throughout the remainder of berry development (Fig. 3). It should be emphasized, however, that the procedure for wall isolation is not quantitative (Nunan et al., 1997) and that the decrease in yield could be explained in several ways. If there were a net decrease in cell wall synthesis, an increase in hydrolysis of wall polysaccharides, or an increase in solute accumulation relative to wall deposition, then yields would be expected to fall. Alternatively, if the walls become more fragile during ripening, they may be fragmented into smaller pieces during homogenization of the berry and be lost through the 71-μm nylon mesh.
The most significant change in the composition of grape cell walls during ripening was the dramatic decrease in the amount of (1→4)-linked Galp residues (Tables II and III). AGI is a major component of the neutral side chains of pectic polysaccharides and the loss of these side chains has been observed in other ripening fruit (Huber, 1983; Gross and Sams, 1984). The loss of AGI, which constituted 21 mol % of total polysaccharides in grape cell walls before veraison (Table III), began well before softening was detected (Table III compared with Fig. 1B), suggesting that the loss of Type I AG could be a crucial step that is associated with the initiation of softening. However, Redgwell and Harker (1995) suggested that cell wall-associated Gal loss and softening are separate processes, because inhibiting Gal loss in kiwifruit discs did not inhibit fruit softening and inhibiting the softening process did not prevent Gal loss. The enzyme β-galactosidase has been shown to degrade galactans in fruit such as apples and pears (Ross et al., 1993; Kitagawa et al., 1995), although its activity was thought not to be high enough to account for all of the degradation seen in the fruit nor was the extent of Gal loss considered high enough when isolated walls were treated with purified β-galactosidase. Additional enzymic or nonenzymic processes might therefore be involved in AGI degradation (Ross et al., 1993; Kitagawa et al., 1995).
The other significant changes in the cell walls of developing grapes were increases in galacturonan and heteroxylan content. Proportions (mol % of total wall polysaccharides) of the other major polysaccharides, cellulose and xyloglucan, remained at similar levels throughout the ripening process (Table III). The cell wall preparations were subsequently extracted with a series of increasingly harsh solvents, in the expectation that polysaccharides recovered at each stage would provide an indication as to the nature of intermolecular interactions and as to how tightly individual polysaccharides were associated with one another and with other wall components. The increase in the water-soluble fraction was attributed to an increased solubility of galacturonan that had required CDTA and Na2CO3 to extract it early in development, together with increases in AGII and arabinan that had been found in all fractions before the grapes began to soften (Table IV). An increase in water-soluble polysaccharides has also been reported in other fruit (Gross and Wallner, 1979; Ahmed and Labavitch, 1980). The movement of particular polysaccharides from one fraction to another presumably results from modifications to the fine structure of polysaccharides, which affect the way they aggregate with each other or which simply increase the inherent solubilities of the polysaccharides.
In other fruit, polyuronides that become more soluble during ripening have lower apparent Mr (Yoshioka et al., 1992; Huber and O'Donoghue, 1993). This may result from the removal of neutral side chains, as discussed above, or it may result from other modifications. Enzymes that have been implicated in these modifications, and therefore in softening, include PME, which removes the methyl groups from esterified galacturonans, and PG, which is an endo-hydrolase that cleaves unesterified galacturonans (Seymour and Gross, 1996). The removal of methyl groups by PME affects interactions of the polysaccharide with calcium and therefore changes the way it aggregates (Jarvis, 1984). The removal of some methyl groups may be required before PG can act. Hydrolysis of long polygalacturonans by PG would decrease the Mr of pectic polysaccharides and presumably increase their solubility.
In contrast to the relatively large decreases in the DE observed during ripening in tomatoes (Koch and Nevins, 1989) and pears (Martin-Cabrejas et al., 1994), it was found that there was a smaller decrease from an initial DE of 58% in the grape polygalacturonans before veraison to 48% at veraison, and thereafter there was no further decrease (Table III). The level of esterified 4-linked galacturonic acid residues increased slightly during ripening (Table II). This result suggests that the increased solubility of pectic polysaccharides as the grape softens is not due to changes in their overall DE.
As mentioned above, cellulose and xyloglucan levels remained approximately the same during ripening of grapes (Table III) and there was no apparent increase in their solubility (Table IV). This may be compared with the decrease observed in the Mr of avocado xyloglucan (O'Donoghue and Huber, 1992) and a decrease found in the Mr of alkali-soluble polysaccharides in tomatoes (Huber, 1983). It should be noted that xyloglucans might also be modified by xyloglucan endotransglycosylases (Fry, 1993), which hydrolyze and transglycosylate xyloglucans. Modification of grape cell wall xyloglucan in this way by xyloglucan endotransglycosylase might not be detected in the analyses of overall polysaccharide composition performed here.
In addition to the changes seen in polysaccharide composition and solubility in walls of ripening grape berries, levels of wall-associated proteins were found to increase from approximately 7% (w/w) at veraison to almost 12% later in ripening (Fig. 4). Although it might be argued that the increase could be attributed simply to increasing contamination of wall preparations with protein of cytoplasmic origin (Nunan et al., 1997), the concomitant increase in Hyp content from 2 to 7 μg/mg cell wall (Fig. 4) and from 2.6 to 3.6 μg/berry (data not shown) suggests that wall proteins such as the Hyp-rich glycoproteins (extensins) are being deposited in the walls as the berries develop (Fig. 4). Hyp-rich glycoproteins are believed to form a fibrillar network in plant cell walls that is independent of the cellulosic network, and this second, Hyp-rich glycoprotein network is likely to considerably strengthen the cell wall (Varner and Lin, 1989; Carpita and Gibeaut, 1993). It is possible that expansion of mesocarp cells in the ripening berry (Fig. 2, C and D) necessitates the reinforcement of the walls with Hyp-rich glycoproteins so that cellular integrity can be maintained during the softening process. The changes in amino acid composition during ripening, such as the decrease in Arg content, suggest that other modifications are also occurring (Table I).
In summary, the changes in cell wall polysaccharides during grape berry ripening suggest that a limited number of enzymes play an important role in the softening process. In particular, these would include endo- or exo-hydrolases capable of depolymerizing the (1→4)-β-galactan constituents of pectic polysaccharides. Low but significant levels of PG or PME, which could be responsible for the partial solubilization of galacturonans (Table IV), might also be present, but only low levels of cellulase, xyloglucanase, and xylanase would be expected. An understanding of the role of these enzymes in the modification of grape berry cell wall polysaccharides could provide opportunities to improve grape and wine quality through genetic engineering.
ACKNOWLEDGMENTS
We thank Dr. Robert Redgwell, Mr. Jelle Lahnstein, and Dr. Meredith Wallwork for their assistance with various aspects of the work.
Abbreviations:
- AGI and AGII
type I and type II arabinogalactan, respectivelyAraf, l-arabinofuranose
- CDTA
trans-1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid
- DE
degree of esterification
- dpa
days postanthesis
- GalAp
d-galacturonic acid
- Galp
d-galactopyranoseGlcAp, d-glucuronic acid
- Glcp
d-glucopyranose
- GMA
glycol methacrylateManp, d-mannopyranose
- PG
endo-polygalacturonase
- PME
pectin methylesterase
- Rhap
l-rhamnopyranose
- TBO
Toluidine Blue O
- Xylp
d-xylopyranose
Footnotes
This work was supported by the Cooperative Research Centre for Viticulture.
LITERATURE CITED
- Ahmed AE, Labavitch JM. Cell wall metabolism in ripening fruit I. Cell wall changes in ripening “Bartlett” pears. Plant Physiol. 1980;65:1009–1013. doi: 10.1104/pp.65.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacic A, Harris PJ, Stone BA (1988) Structure and function of plant cell walls. In J Preiss, ed, The Biochemistry of Plants: a Comprehensive Treatise. Vol 14, Carbohydrates. Academic Press, New York, pp 297–371
- Brady CJ. Fruit ripening. Annu Rev Plant Physiol. 1987;38:155–178. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr Res. 1984;131:209–217. [Google Scholar]
- Coombe BG. The regulation of set and development of the grape berry. Acta Hortic. 1973;34:261–273. [Google Scholar]
- Coombe BG. The development of fleshy fruits. Annu Rev Plant Physiol. 1976;27:207–228. [Google Scholar]
- Coombe BG, Bishop GR. Development of the grape berry. II. Changes in diameter and deformability during veraison. Aust J Agric Res. 1980;31:499–509. [Google Scholar]
- Coombe BG. Research on development and ripening of the grape berry. Am J Enol Vitic. 1992;43:101–110. [Google Scholar]
- Fischer RL, Bennett AB. Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:675–703. [Google Scholar]
- Fry SC. Loosening the ties: a new enzyme, which cuts and then re-forms glycosidic bonds in the cell wall, may hold the key to plant cell growth. Curr Biol. 1993;3:355–357. doi: 10.1016/0960-9822(93)90199-x. [DOI] [PubMed] [Google Scholar]
- Fry SC. Polysaccharide-modifying enzymes in the plant cell wall. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:497–520. [Google Scholar]
- Gibeaut DM, Carpita NC. Biosynthesis of plant cell wall polysaccharides. FASEB J. 1994;8:904–915. doi: 10.1096/fasebj.8.12.8088456. [DOI] [PubMed] [Google Scholar]
- Gorshkova TA, Wyatt SE, Salnikov VV, Gibeaut DM, Ibragimov MR, Lozovaya VV, Carpita NC. Cell-wall polysaccharides of developing flax plants. Plant Physiol. 1996;110:721–729. doi: 10.1104/pp.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross KC, Sams CE. Changes in cell wall neutral sugar composition during fruit ripening: a species survey. Phytochemistry. 1984;23:2457–2461. [Google Scholar]
- Gross KC, Wallner SJ. Degradation of cell wall polysaccharides during tomato fruit ripening. Plant Physiol. 1979;63:117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie WJ, O'Brien TP, Jaudzems VG. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust J Grape Wine Res. 1996;2:97–142. [Google Scholar]
- Harris JM, Kriedemann PE, Possingham JV. Anatomical aspects of grape berry development. Vitis. 1968;7:106–119. [Google Scholar]
- Harris PJ, Henry RJ, Blakeney AB, Stone BA. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984;127:59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Huber DJ. Polyuronide degradation and hemicellulose modifications in ripening tomato fruit. J Amer Soc Hort Sci. 1983;108:405–409. [Google Scholar]
- Huber DJ, O'Donoghue EM. Polyuronides in avocado (Persea americana) and tomato (Lycopersicon esculentum) fruits exhibit markedly different patterns of molecular weight downshifts during ripening. Plant Physiol. 1993;102:473–480. doi: 10.1104/pp.102.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant Cell Environ. 1984;7:153–164. [Google Scholar]
- Kitagawa Y, Kanayama Y, Yamaki S. Isolation of β-galactosidase fractions from Japanese pear. Activity against native cell wall polysaccharides. Physiol Plant. 1995;93:545–550. [Google Scholar]
- Kivirikko KI, Liesmaa M. A colorimetric method for determination of hydroxyproline in tissue hydrolysates. Scand J Clin Lab Invest. 1959;11:128–133. [Google Scholar]
- Koch JL, Nevins DJ. Tomato fruit cell wall. I. Use of purified tomato polygalacturonase and pectinmethylesterase to identify developmental changes in pectins. Plant Physiol. 1989;91:816–822. doi: 10.1104/pp.91.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey GD, Gross KC, Wallner SJ. Loss of tomato cell wall galactan may involve reduced rate of synthesis. Plant Physiol. 1980;66:532–533. doi: 10.1104/pp.66.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Cabrejas M, Waldron KW, Selvendran RR. Cell wall changes in Spanish pear during ripening. J Plant Physiol. 1994;144:541–548. [Google Scholar]
- Nunan KJ, Sims IM, Bacic A, Robinson SP, Fincher GB. Isolation and characterization of cell walls from the mesocarp of mature grape berries (Vitis vinifera) Planta. 1997;203:93–100. [Google Scholar]
- O'Brien TP, McCully ME (1981) In: The study of plant structure principles and selected methods. Termarcarphi Pty., Ltd., Melbourne, Australia
- O'Donoghue EM, Huber DJ. Modification of matrix polysaccharides during avocado (Persea americana) fruit ripening: an assessment of the role of Cx-cellulase. Physiol Plant. 1992;86:33–42. [Google Scholar]
- Redgwell RJ, Harker R. Softening of kiwifruit discs; effect of inhibition of galactose loss from cell walls. Phytochemistry. 1995;39:1319–1323. [Google Scholar]
- Redgwell RJ, MacRae E, Hallet I, Fischer M, Perry J, Harker R. In vivo and in vitro swelling of cell walls during fruit ripening. Planta. 1997;203:162–173. [Google Scholar]
- Redgwell RJ, Melton LD, Brasch DJ. Cell-wall polysaccharides of kiwifruit (Actinidia deliciosa): chemical features in different tissue zones of the fruit at harvest. Carbohydr Res. 1988;182:241–258. [Google Scholar]
- Ross GS, Redgwell RJ, MacRae EA. Kiwifruit β-galactosidase: isolation and activity against specific fruit cell-wall polysaccharides. Planta. 1993;189:499–506. [Google Scholar]
- Saulnier L, Brillouet J-M, Joseleau J-P. Structural studies of pectic substances from the pulp of grape berries. Carbohydr Res. 1988;182:63–78. [Google Scholar]
- Saulnier L, Thibault J-F. Extraction and characterization of pectic substances from pulp of grape berries. Carbohydr Poly. 1987;7:329–343. [Google Scholar]
- Seymour GB, Gross KC. Cell wall disassembly and fruit softening. Postharvest News and Information. 1996;7:45N–52N. [Google Scholar]
- Seymour GB, Harding SE, Taylor AJ, Hobson GE, Tucker GA. Polyuronide solubilization during ripening of normal and mutant tomato fruit. Phytochemistry. 1987;26:1871–1875. [Google Scholar]
- Shea EM, Gibeaut DM, Carpita NC. Structural analysis of the cell walls regenerated by carrot protoplasts. Planta. 1989;179:293–308. doi: 10.1007/BF00391074. [DOI] [PubMed] [Google Scholar]
- Silacci MW, Morrison JC. Changes in pectin content of Cabernet Sauvignon grape berries during maturation. Am J Enol Vitic. 1990;41:111–115. [Google Scholar]
- Sims IM, Bacic A. Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochemistry. 1995;38:1397–1405. [Google Scholar]
- Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE, Arnold C, Tucker GA, Schuch W, Harding S and others. Inheritance and effect on ripening of antisense polygalacturonase genes in transgenic tomatoes. Plant Mol Biol. 1990;14:369–379. doi: 10.1007/BF00028773. [DOI] [PubMed] [Google Scholar]
- Varner JE, Lin L-S. Plant cell wall architecture. Cell. 1989;56:231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Aoba K, Kashimura Y. Molecular weight and degree of methoxylation in cell wall polyuronide during softening in pear and apple fruit. J Am Soc Hort Sci. 1992;117:600–606. [Google Scholar]