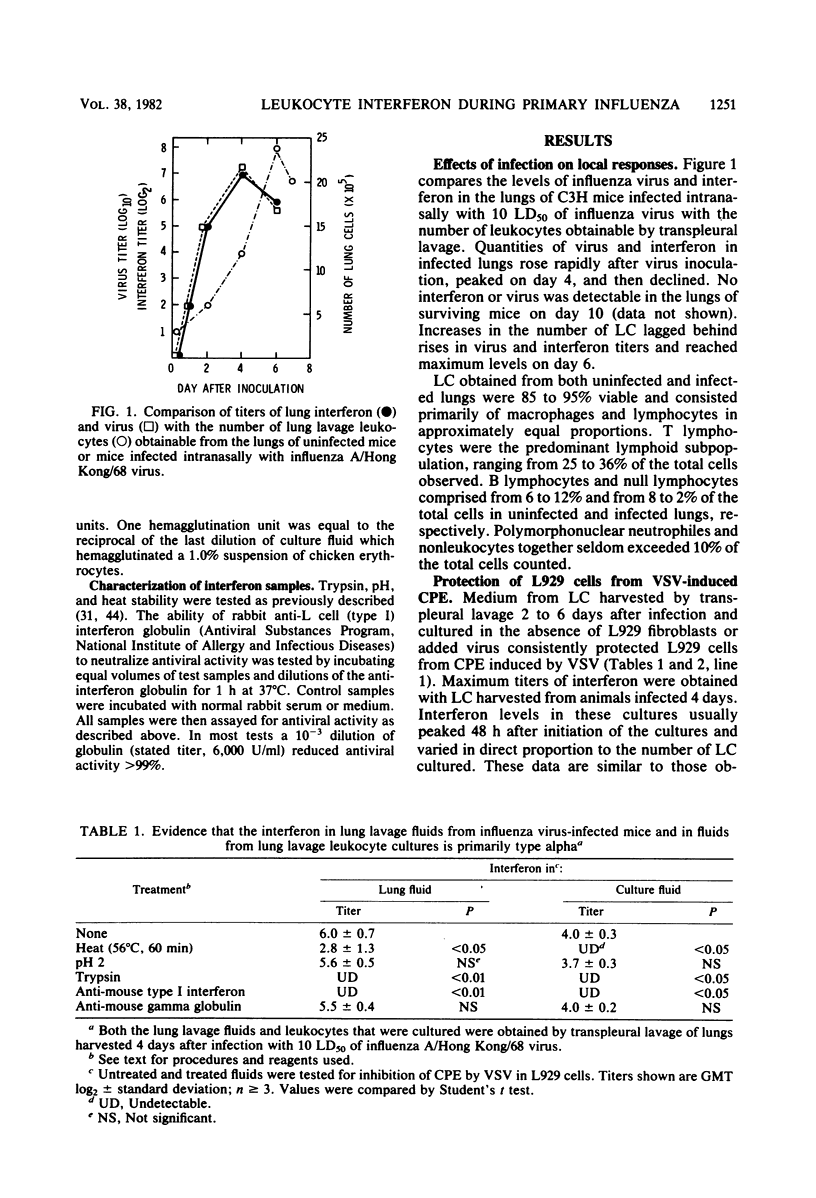
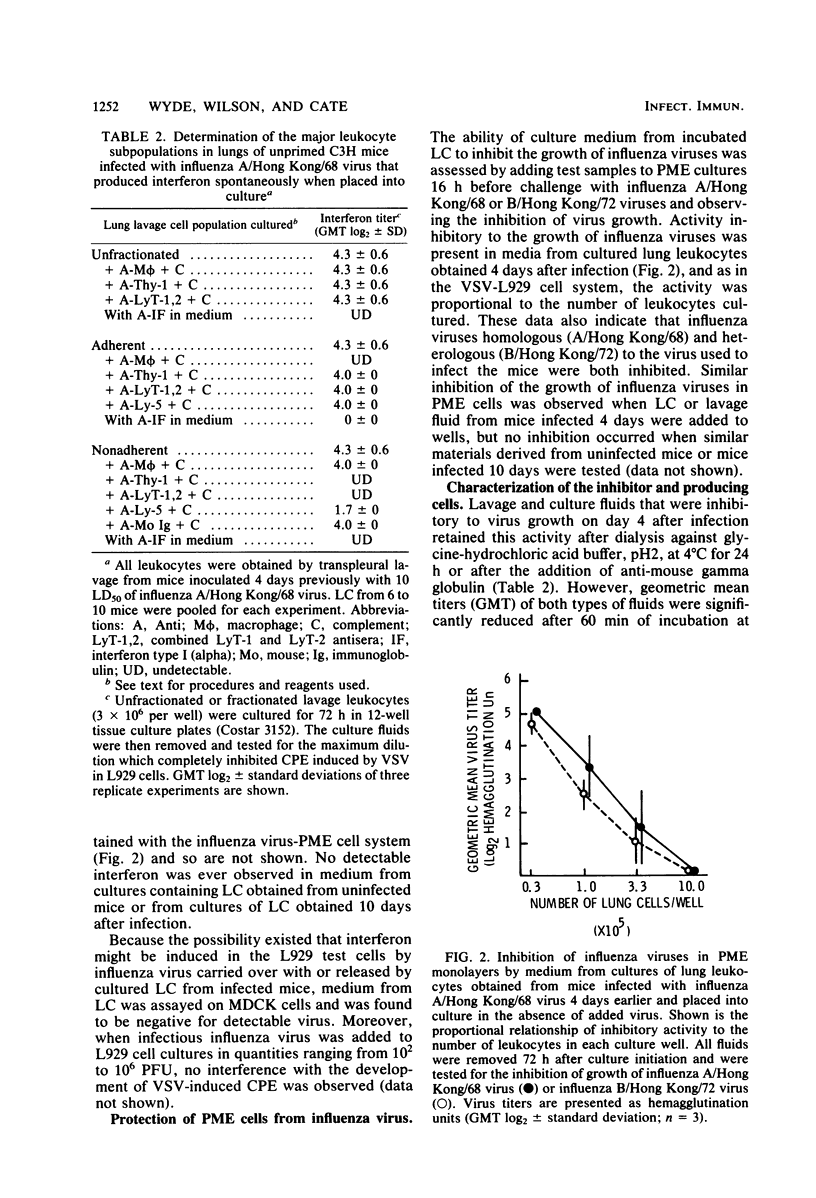
Abstract
Lung fluids and leukocytes were obtained from unprimed C3H mice by transpleural lavage at intervals after infection with influenza A/Hong Kong/68 virus and were tested for interferon activity. Lavage fluid interferon titers correlated directly with lung virus titers and with initial increases in leukocyte yields from infected lungs. In contrast to cultured lymph node cells from infected animals or leukocytes from lungs of uninfected mice, washed leukocytes obtained from the lungs of mice infected 2 to 6 days earlier produced interferon spontaneously in culture. The physiochemical, biological, and antigenic properties of both the interferon in lavage fluids and that produced by lung lavage leukocytes were similar and characteristics of alpha interferon. Fractionation studies indicated that macrophages and T lymphocytes were primarily responsible for the interferon produced in culture. The early presence and significant numbers of interferon-producing leukocytes in infected lungs suggests that these cells have an early role in defense against influenza virus infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton J. D., Myrvik Q. N. Production of interferon by alveolar macrophages. J Bacteriol. 1966 Jun;91(6):2300–2304. doi: 10.1128/jb.91.6.2300-2304.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Dianzani F. General considerations of the interferon system. Tex Rep Biol Med. 1977;35:1–10. [PubMed] [Google Scholar]
- Barth R. F., Malmgren R. A., Friedman R. M. Depression of interferon production in mice after treatment with anti-lymphocyte serum. Lancet. 1969 Oct 4;2(7623):723–724. doi: 10.1016/s0140-6736(69)90431-0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cate T. R., Douglas R. G., Jr, Couch R. B. Interferon and resistance to upper respiratory virus illness. Proc Soc Exp Biol Med. 1969 Jun;131(2):631–636. doi: 10.3181/00379727-131-33941. [DOI] [PubMed] [Google Scholar]
- Davies H. W., Appleyard G., Cunningham P., Pereira M. S. The use of a continuous cell line for the isolation of influenza viruses. Bull World Health Organ. 1978;56(6):991–993. [PMC free article] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J., Jullien P. Inter- feron synthesis in x-irradiated animals. 3. The high radiosensitivity of myxovirus-induced circulating interferon production. Proc Soc Exp Biol Med. 1969 May;131(1):36–41. doi: 10.3181/00379727-131-33799. [DOI] [PubMed] [Google Scholar]
- GLASGOW L. A., HABEL K. Interferon production by mouse leukocytes in vitro and in vivo. J Exp Med. 1963 Jan 1;117:149–160. doi: 10.1084/jem.117.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow L. A. Transfer of interferon-producing macrophages: new approach to viral chemotherapy. Science. 1970 Nov 20;170(3960):854–856. doi: 10.1126/science.170.3960.854. [DOI] [PubMed] [Google Scholar]
- Golstein P., Blomgren H. Further evidence for autonomy of T cells mediating specific in vitro cytotoxicity: efficiency of very small amounts of highly purified T cells. Cell Immunol. 1973 Oct;9(1):127–141. doi: 10.1016/0008-8749(73)90174-3. [DOI] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Gresser I., Lindenmann J. Virus-specific interferon action. Protection of newborn Mx carriers against lethal infection with influenza virus. J Exp Med. 1981 Jul 1;154(1):199–203. doi: 10.1084/jem.154.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A., Martin D. H., Wopschall L. J. The influence of x-irradiation on survival and interferon levels in viral infected mice. Proc Soc Exp Biol Med. 1968 Jun;128(2):455–458. doi: 10.3181/00379727-128-33036. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Huang K. Y., Donahoe R. M., Gordon F. B., Dressler H. R. Enhancement of phagocytosis by interferon-containing preparations. Infect Immun. 1971 Nov;4(5):581–588. doi: 10.1128/iai.4.5.581-588.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A. INTERFERON. Adv Virus Res. 1963;10:1–38. [PubMed] [Google Scholar]
- Iwasaki T., Nozima T. Defense mechanisms against primary influenza virus infection in mice. I. The roles of interferon and neutralizing antibodies and thymus dependence of interferon and antibody production. J Immunol. 1977 Jan;118(1):256–263. [PubMed] [Google Scholar]
- KONO Y., HO M. THE ROLE OF THE RETICULOENDOTHELIAL SYSTEM IN INTERFERON FORMATION IN THE RABBIT. Virology. 1965 Jan;25:163–166. doi: 10.1016/0042-6822(65)90268-0. [DOI] [PubMed] [Google Scholar]
- Kolot F. B., Baron S., Yeager H., Jr, Schwartz S. L. Comparative production of interferon by explanted lymphoreticular tissue and alveolar macrophages from rabbits and humans. Infect Immun. 1976 Jan;13(1):63–68. doi: 10.1128/iai.13.1.63-68.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Induction of natural killer cells during murine influenza virus infection. Immunobiology. 1981;160(3-4):352–366. doi: 10.1016/s0171-2985(81)80061-7. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Oldstone M. B., Welsh R. M. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature. 1977 Jul 7;268(5615):67–68. doi: 10.1038/268067a0. [DOI] [PubMed] [Google Scholar]
- Pantelouris E. M., Pringle C. R. Interferon production in athymic nude mice. J Gen Virol. 1976 Jul;32(1):149–152. doi: 10.1099/0022-1317-32-1-149. [DOI] [PubMed] [Google Scholar]
- Postic B., DeAngelis C., Breinig M. K., Ho M. Effects of cortisol and adrenalectomy on induction of interferon by endotoxin. Proc Soc Exp Biol Med. 1967 May;125(1):89–92. doi: 10.3181/00379727-125-32021. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. E., Jordan G. W., Stevens D. A., Merigan T. C. Lymphocyte interferon production and transformation after Herpes simplex infections in humans. J Immunol. 1974 Feb;112(2):728–736. [PubMed] [Google Scholar]
- Roberts N. J., Jr, Douglas R. G., Jr, Simons R. M., Diamond M. E. Virus-induced interferon production by human macrophages. J Immunol. 1979 Jul;123(1):365–369. [PubMed] [Google Scholar]
- Smith T. J., Wagner R. R. Rabbit macrophage interferons. I. Conditions for biosynthesis by virus-infected and uninfected cells. J Exp Med. 1967 Apr 1;125(4):559–577. doi: 10.1084/jem.125.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gresser I., Tovey M. G., Bandu M., Le Goff S. Identification of the cell multiplication inhibitory factors in interferon preparations as interferons. Nature. 1976 Jul 22;262(5566):300–302. doi: 10.1038/262300a0. [DOI] [PubMed] [Google Scholar]
- Subrahmanyan T. P., Mims C. A. Fate of intravenously administered interferon and the distribution of interferon during virus infections in mice. Br J Exp Pathol. 1966 Apr;47(2):168–176. [PMC free article] [PubMed] [Google Scholar]
- Tsukui K., Iwasaki T., Kawade Y. Heterogeneity of mouse lymphocytes in interferon production upon influenza virus challenge in culture. Cell Immunol. 1978 Oct;40(2):451–456. doi: 10.1016/0008-8749(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Winchester R. J., Fu S. M., Hoffman T., Kunkel H. G. IgG on lymphocyte surfaces; technical problems and the significance of a third cell population. J Immunol. 1975 Apr;114(4):1210–1212. [PubMed] [Google Scholar]
- Wyde P. R., Cate T. R. Cellular changes in lungs of mice infected with influenza virus: characterization of the cytotoxic responses. Infect Immun. 1978 Nov;22(2):423–429. doi: 10.1128/iai.22.2.423-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Couch R. B., Mackler B. F., Cate T. R., Levy B. M. Effects of low- and high-passage influenza virus infection in normal and nude mice. Infect Immun. 1977 Jan;15(1):221–229. doi: 10.1128/iai.15.1.221-229.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Peavy D. L., Cate T. R. Morphological and cytochemical characterization of cells infiltrating mouse lungs after influenza infection. Infect Immun. 1978 Jul;21(1):140–146. doi: 10.1128/iai.21.1.140-146.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Salvin S. B. Production and properties of migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. J Immunol. 1973 Dec;111(6):1914–1922. [PubMed] [Google Scholar]
- Zee Y. C., Osebold J. W., Dotson W. M. Antibody responses and interferon titers in the respiratory tracts of mice after aerosolized exposure to influenza virus. Infect Immun. 1979 Jul;25(1):202–207. doi: 10.1128/iai.25.1.202-207.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



