Abstract
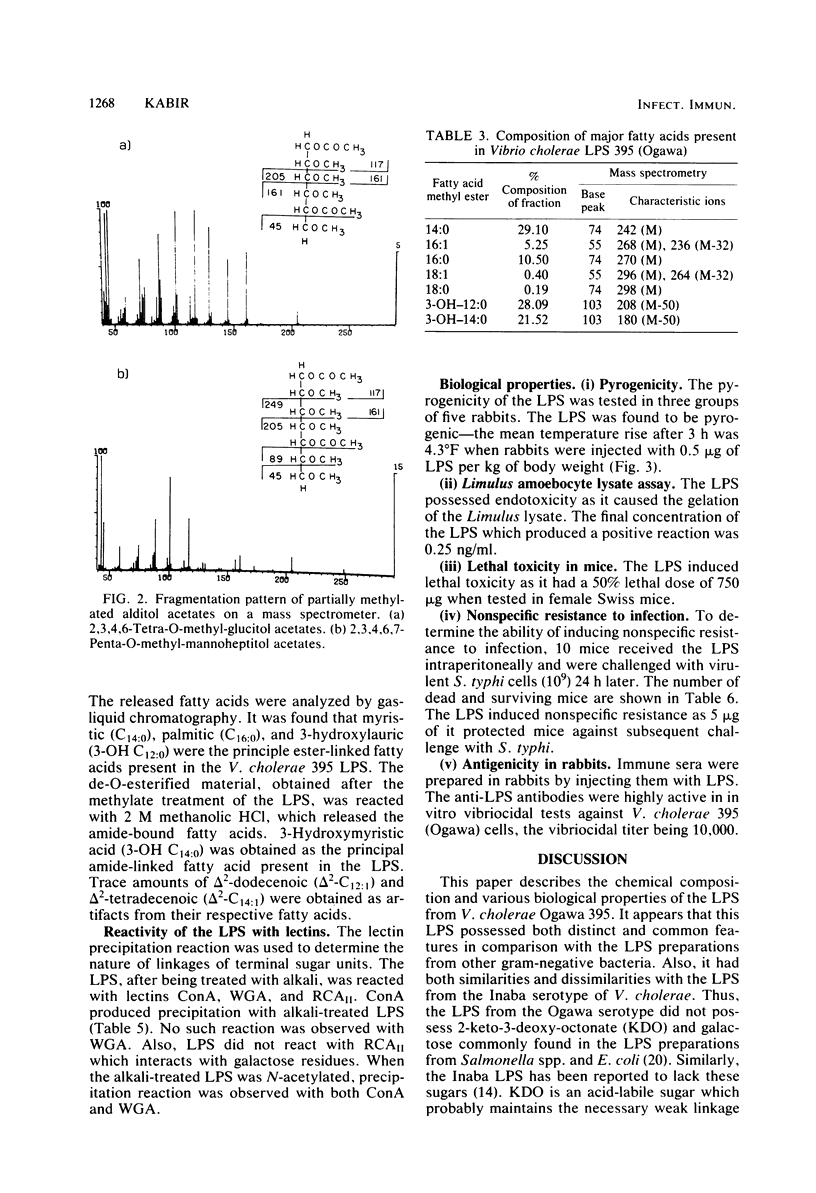
The chemical structure and biological properties of the lipopolysaccharide (LPS) from Vibrio cholerae 395 (Ogawa), isolated by the phenol-water procedure, were studied. Upon acid hydrolysis, the LPS was split into its polysaccharide and lipid A moieties. The polysaccharide contained both neutral (glucose, heptose, fructose) and amino (glucosamine, quinovosamine) sugars. The LPS contained the acid-labile amino sugar, 4-amino-arabinose, which was absent in the Inaba serotype of V. cholerae. The LPS differed from the LPSs of Enterobacteriaceae by the absence of 2-keto-3-deoxyoctonate and the presence of fructose. Analysis of the methylated polysaccharide by gas-liquid chromatography and mass spectrometry showed that it had a branched structure with glucose and heptose residues primarily appearing at the nonreducing-end groups. Interactions with lectins, concanavalin A. and wheat germ agglutinin suggested that terminal glucose residues were alpha linked, whereas terminal glucosamine residues were connected by alpha-1,3 linkages. The major fatty acids of the LPS were C14:0, C16:0, C12h:0, and C14h:0 compounds, of which only the C14h:0 were amide linked, the remainder being ester linked to the backbone. Biological studies showed that the LPS possessed endotoxic properties such as lethality, pyrogenicity, limulus lysate gelation, and ability to induce non-specific resistance to infection. Thus, the LPS from V. cholerae 395 (Ogawa) possessed both common and distinct features as compared with the LPSs from the Enterobacteriaceae.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahamed N. M., Radziejewska-Lebrecht J., Widemann C., Mayer H. Reactivity of isolated lipopolysaccharides of enterobacterial R mutants with complete or incomplete core structures with lectins. Zentralbl Bakteriol A. 1980;247(4):468–482. [PubMed] [Google Scholar]
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Armstrong I. L., Redmond J. W. The fatty acids present in the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1974 May 29;348(2):302–305. doi: 10.1016/0005-2760(74)90242-2. [DOI] [PubMed] [Google Scholar]
- BHASKARAN K., GORRILL R. H. A study of antigenic variation in Vibrio cholerae. J Gen Microbiol. 1957 Jun;16(3):721–729. doi: 10.1099/00221287-16-3-721. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Atthasampunna P., Chulasamaya M., Charunmethee P. Pathogenesis of experimental cholera: biologic ativities of purified procholeragen A. J Immunol. 1966 Mar;96(3):440–449. [PubMed] [Google Scholar]
- Gangarosa E. J., Sanati A., Saghari H., Feeley J. C. Multiple serotypes of vibrio cholerae isolated from a case of cholera. Evidence suggesting in-vivo mutation. Lancet. 1967 Mar 25;1(7491):646–648. doi: 10.1016/s0140-6736(67)92542-1. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Haeffner N., Chaby R., Szabó L. Identification of 2-methyl-3-hydroxydecanoic and 2-methyl-3-hydroxytetradecanoic acids in the 'lipid X' fraction of the Bordetella pertussis endotoxin. Eur J Biochem. 1977 Aug 1;77(3):535–544. doi: 10.1111/j.1432-1033.1977.tb11696.x. [DOI] [PubMed] [Google Scholar]
- Hisatsune K., Kondo S., Kawata T., Kishimoto Y. Fatty acid composition of lipopolysaccharides of Vibrio cholerae 35A3 (Inaba), NIB 90 (Ogawa), and 4715 (Nag). J Bacteriol. 1979 Apr;138(1):288–290. doi: 10.1128/jb.138.1.288-290.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Jann K., Beyaert G. O. 2-Amino-2,6-dideoxy-d-glucose (D-quinovosamine): a constituent of the lipopolysaccharides of Vibrio cholerae. Eur J Biochem. 1973 Sep 3;37(3):531–534. doi: 10.1111/j.1432-1033.1973.tb03015.x. [DOI] [PubMed] [Google Scholar]
- Kabir S. Composition and immunochemical properties of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1980 Oct;144(1):382–389. doi: 10.1128/jb.144.1.382-389.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S., Mann P. Immunological properties of the cell envelope components of Vibrio cholerae. J Gen Microbiol. 1980 Aug;119(2):517–525. doi: 10.1099/00221287-119-2-517. [DOI] [PubMed] [Google Scholar]
- Kennedy J. R., Richardson S. H. Fine structure of Vibrio cholerae during toxin production. J Bacteriol. 1969 Dec;100(3):1393–1401. doi: 10.1128/jb.100.3.1393-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Parker R. B. Isolation of a phenol-soluble endotoxin from Leptotrichia buccalis. Arch Oral Biol. 1973 Jan;18(1):85–93. doi: 10.1016/0003-9969(73)90023-x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Lehmann V., Rietschel E. T. Recent findings on the chemical structure and biological activity of bacterial lipopolysaccharides. J Hyg Epidemiol Microbiol Immunol. 1974;18(4):381–390. [PubMed] [Google Scholar]
- Majumdar A. S., Dutta P., Dutta D., Ghose A. C. Antibacterial and antitoxin responses in the serum and milk of cholera patients. Infect Immun. 1981 Apr;32(1):1–8. doi: 10.1128/iai.32.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley W. H., Aziz K. M., Rahman A. S., Chowdhury A. K., Ahmed A. Field trials of monovalent Ogawa and Inaba cholera vaccines in rural Bangladesh--three years of observation. Bull World Health Organ. 1973;49(4):381–387. [PMC free article] [PubMed] [Google Scholar]
- Mosley W. H., Woodward W. E., Aziz K. M., Rahman A. S., Chowdhury A. K., Ahmed A., Feeley J. C. The 1968-1969 cholera-vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. J Infect Dis. 1970 May;121(Suppl):1–9. doi: 10.1093/infdis/121.supplement.s1. [DOI] [PubMed] [Google Scholar]
- Raziuddin S. Immunochemical studies of the lipopolysaccharides of Vibrio cholerae: constitution of O specific side chain and core polysaccharide. Infect Immun. 1980 Jan;27(1):211–215. doi: 10.1128/iai.27.1.211-215.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziuddin S., Kawasaki T. Biochemical studies on the cell wall lipopolysaccharides (O-antigens) of Vibrio cholerae 569 B (Inaba) and El-tor (Inaba). Biochim Biophys Acta. 1976 Apr 22;431(1):116–126. doi: 10.1016/0005-2760(76)90265-4. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. The 4-amino sugars present in the lipopolysaccharides of vibro cholerae and related vibrios. Biochim Biophys Acta. 1978 Sep 6;542(3):378–384. doi: 10.1016/0304-4165(78)90369-0. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Kim Y. B., Watson D. W., Galanos C., Lüderitz O., Westphal O. Pyrogenicity and immunogenicity of lipid A complexed with bovine serum albumin or human serum albumin. Infect Immun. 1973 Aug;8(2):173–177. doi: 10.1128/iai.8.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Palin W. J., Watson D. W. Nature and linkages of the fatty acids present in lipopolysaccharides from Vibrio metchnikovii and Vibrio parahemolyticus. Eur J Biochem. 1973 Aug 1;37(1):116–120. doi: 10.1111/j.1432-1033.1973.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakazaki R., Tamura K. Somatic antigen variation in Vibrio cholerae. Jpn J Med Sci Biol. 1971 Apr;24(2):93–100. doi: 10.7883/yoken1952.24.93. [DOI] [PubMed] [Google Scholar]
- Sen A. K., Mukherjee A. K., Guhathakurta B., Dutta A., Sasmal D. Structural investigations of the lipopolysaccharide isolated from Vibrio cholera, Inaba 569 B. Carbohydr Res. 1979 Jul;72:191–199. doi: 10.1016/s0008-6215(00)83935-x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Volk W. A., Galanos C., Lüderitz O. The occurrence of 4-amino-4-deoxy-L-arabinose as a constituent in Salmonella lipopolysaccharide preparations. Eur J Biochem. 1970 Dec;17(2):223–229. doi: 10.1111/j.1432-1033.1970.tb01157.x. [DOI] [PubMed] [Google Scholar]
- WATSON D. W., KIM Y. B. MODIFICATION OF HOST RESPONSES TO BACTERIAL ENDOTOXINS. I. SPECIFICITY OF PYROGENIC TOLERANCE AND THE ROLE OF HYPERSENSITIVITY IN PYROGENICITY, LETHALITY, AND SKIN REACTIVITY. J Exp Med. 1963 Sep 1;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbraith L., Lightfoot G. A. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur J Biochem. 1973 Feb 15;33(1):158–174. doi: 10.1111/j.1432-1033.1973.tb02666.x. [DOI] [PubMed] [Google Scholar]




