Abstract
Neuroinflammation plays a critical role in the progression of many neurodegenerative diseases and neuropsychiatric illnesses. It is evident that microglia in particular are central to mediating the effects of neuroinflammation. Activated microglia release a number of cytokines and chemokines, which in turn activate many signal transduction pathways. For instance, interleukin-1 beta and tumor necrosis factor alpha regulate transcription of a number of genes within the brain including proinflammatory products of the arachidonic acid (AA) cascade. Co-activation of pro-inflammatory markers and associated cytotoxic products during neuroinflammation process are detrimental to neurons by altering the synaptic proteins. In this review, we discuss both neuroinflammation as well as excitotoxicity insults reduce synaptic markers such as synaptophysin and drebrin in rat brain. Further we discuss here, neurodegenerative and neuropsychiatric illness are associated with increased neuroinflammatory and excitotoxicity markers as well as upregulated brain arachidonic acid markers and the loss of synaptic markers. The decrease in synaptic markers might contribute to reported cognitive defects in neurodegenerative and neuropsychiatric illnesses.
Introduction
It is becoming increasingly clear that neuroinflammation plays a crucial role in the development and progression of many neurodegenerative and psychiatric illnesses including Alzheimer’s, Parkinson’s, Huntington’s disease, bipolar disorder, schizophrenia and depression (Bales et al, 2000; Dobos et al, 2010; Doorduin et al, 2009; Hunot and Hirsch, 2003; Rao et al, 2010; Silvestroni et al, 2009). Neuroinflammation is a complex combination of the responses of all cell types present within the central nervous system (CNS), including neurons, macroglia, microglia and infiltrating leukocytes. Infection, trauma, and toxins are capable of producing an immediate short lived induction of innate immune response within the CNS (Crutcher et al, 2006; Popovich and Longbrake, 2008). Acute neuroinflammation triggers activation of resident microglia and the release of inflammatory mediators such as cytokines and chemokines (Tansey et al, 2007). Acute insult is typically short-lived and unlikely to be harmful to long term neuronal survival. It is believed that an acute neuroinflammatory response is generally beneficial to the CNS, since it tends to minimize further injury and contributes to repair of damaged tissue. On the other hand chronic inflammation produces long lasting and self perpetuating neuroinflammatory mediators that remain after the initial neuroinflammatory insult.
Neuroinflammation: induction of proinflammatory cytokines and arachidonic acid cascade enzymes
During neuroinflammation, proinflammatory cytokines such as interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNFα), IL-6 and chemokines including interferon gamma, macrophage inflammatory protein and inducible protein (IP)-10 are released by activated microglia that promote neuroinflammatory state. IL-1β and TNFα regulate expression of many genes, including gene transcription for arachidonic acid (AA) cascade enzymes in various cell types via nuclear kappa B (NF-κB) or AP-2 (Acarin et al, 2002; Bauer et al, 1997; Hoeck et al, 1993; Jupp et al, 2003; Spriggs et al, 1990). In the brain, AA and its metabolites influence signal transduction, gene transcription, neuronal activity, apoptosis, and other processes (Kam and See, 2000; Leslie and Watkins, 1985; O’Banion, 1999). AA released from membrane phospholipids by Ca2+-dependent cytosolic phospholipase A2 (cPLA2-IVA), secretory (sPLA2 IIA), or Ca2+- independent (iPLA2 VIA), which differ in their calcium requirement, phosphorylation, and substrate specificities (Akiba et al, 1999; Murakami et al, 1999; Murakami and Kudo, 2002; Murakami et al, 1998; Yang et al, 1999). The released AA can be metabolized to prostaglandin (PG)H2 by cyclooxygenase (COX)-1 or COX-2, converted to cytoprotective epoxyeicosatrienoic acids by cytochrome p450 epoxygenase or to cytotoxic leukotrienes by 5, −12 or −15 lipoxygenase (LOX) (Funk, 2001). PGH2 is converted to PGE2 by membrane prostaglandin E synthase (mPGES) or cytosolic PGES (cPGES), or by thromboxane synthase (TXS) to TXA2. COX-1 and cPGES are expressed constitutively in the brain, whereas COX-2 and mPGES are inducible (Pepicelli et al, 2002; Seibert et al, 1995) (Figure-1).
Figure 1.
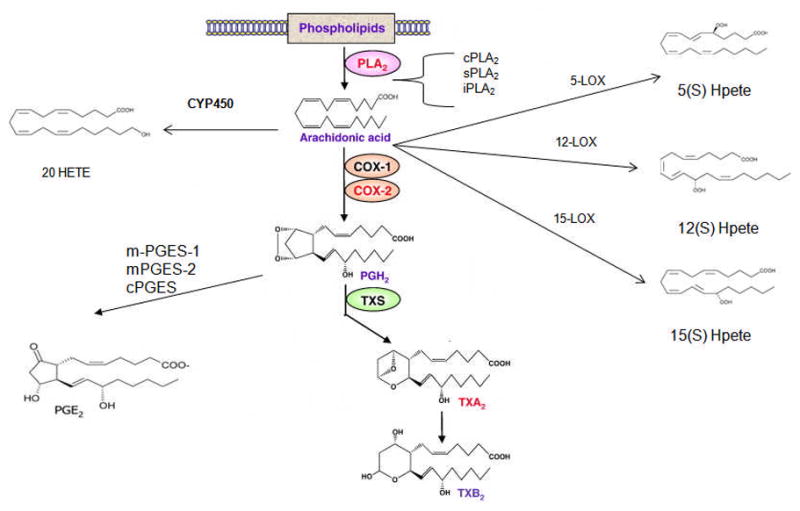
Schematic representation of arachidonic acid cascade
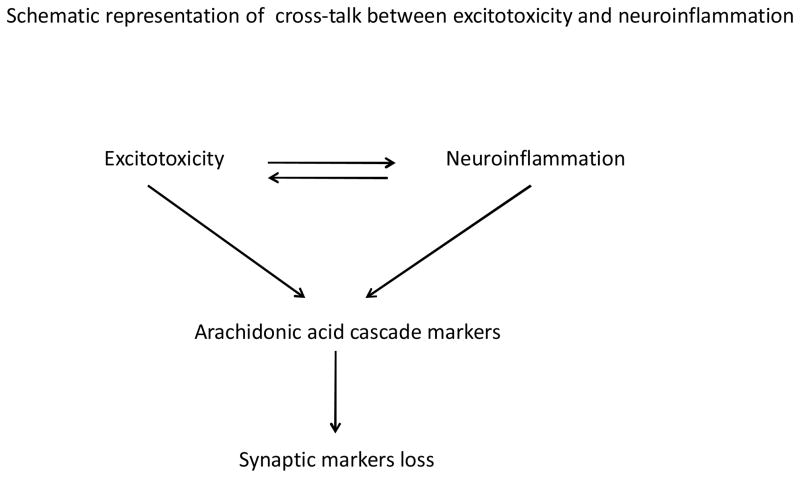
Cross-talk between neuroinflammation and excitotoxicity
Neuroinflammatory markers and AA cascade markers are elevated by excitotoxicity. For instance, activated microglia release nitric oxide which blocks reuptake of glutamate at the presynaptic site, which results in excessive glutamate at the synaptic cleft and activation of NMDA receptors. It is well known that excessive glutamate levels causes excitotoxicity. Activation of NMDA receptors activates AA turnover via upregulation of cPLA2 activity, protein and mRNA levels in an AP-2 dependent manner (Rao et al, 2007b). In addition, chronic NMDA administration to rats upregulates levels of proinflammatory IL-1β, TNFα, glial fibrillary acidic protein (GFAP) and inducible nitric oxide synthase (iNOS) in rat brains (Chang et al, 2008b). Altogether finding suggests that there is cross-talk between neuroinflammation and excitotoxicity that involves release of AA products in the brain. Upregulation of neuroinflammatory and AA cascade markers in chronic NMDA administrated rat has been shown to cause neuronal loss (Kim et al, 2009a). This suggests that both neuroinflammation and associated elevated AA cascade are detrimental to neuronal survival. In those conditions synaptic markers (synaptophysin and drebrin) are also prone to damage.
Synaptic proteins
Drebrin is an actin-binding protein neuron-specific isoform (Kojima et al, 1988), abundantly found within dendritic spines that are located at postsynaptic excitatory synapses (Aoki et al, 2005). Drebrin expression was found to be maximal during embryogenesis and decreases thereafter (Shirao, 1995). The over expression of drebrin provokes the elongation of spines in mature neurons (Hayashi and Shirao, 1999) and the change of dendritic filopodia into aberrantly enlarged megapodia in immature neurons (Mizui et al, 2005). Conversely, the suppression of drebrin expression reduces spine density and results in the formation of thin immature spines (Takahashi et al, 2006). These findings support the idea that the drebrin–actin complex plays a crucial role in the regulation of dendritic spine morphology.
Synaptophysin is a 38-kd glycoprotein localized in synaptic vesicle membranes. The main functions of synaptophysin include docking, fusion, and endocytosis, otherwise known as membrane trafficking (Evans and Cousin, 2005).
Neuroinflammation: synaptic protein loss
Neurodegenerative disease
Hatanpaa and others reported decreased cortical drebrin in Alzheimer’s as well as normal aging (Hatanpaa et al, 1999). Studies also report that drebrin is decreased in postmortem hippocampal (Harigaya et al, 1996) and temporal (Counts et al, 2006) regions obtained from severe and mildly cognitively impaired patients. In Alzheimer disease (AD), neuroinflammation plays a role in altering the neuronal synaptic proteins. In recent year’s decreased drebrin have been shown to correlate with cognitive impairment in patients with Alzheimer disease (AD) (Counts et al, 2006; Kobayashi et al, 2004; Kojima and Shirao, 2007; Zhao et al, 2006). The decreased drebrin in AD might be due to upregulated neuroinflammatory and AA cascade markers, since an excess of neuroinflammatory markers such as TNFα, IL-1β and AA have been shown to damage neurons by increasing pro-apoptotic marker and caspase 3 activities (Fang et al, 2008; Gibson et al, 2004; Zhu et al). It is possible that elevated AA alone or in combination with neuroinflammation might be involved in reducing the synaptic proteins. Upregulated neuroinflammation, AA cascades and reduced drebrin can occur in bipolar disorder (Kim et al, 2009b; Rao et al), HIV-1 transgenic rat (Basselin et al), schizophrenia (Rao et al upublished data) and in n-3 polyunsaturated fatty acid deprived animals (Rao et al, 2007a).
Synaptophysin has been shown to be reduce during aging (Haley et al) as well as in neurodegenerative disease including AD (Hamos et al, 1989).
Neuropsychiatric illness
Postmortem brains from bipolar disorder (BD) and schizophrenic patients showed upregulated neuroinflammatory markers and AA cascade markers as well as decreased synaptic markers (drebrin and synaptophysin) (Glantz and Lewis, 1997a, b; Kim et al, 2009b; Rao et al). Cognitive impairment has been reported in BD and SZ patients (Wingo et al, 2009; Wobrock et al, 2009).
Animal models of neuroinflammation
Similar to clinical studies, in animal models of neuroinflammation such as a non-infectious rat model of HIV-1 and rats treated with a high dose of lipopolysaccharide (LPS) (200 ng/hr) infusion for six days both exhibited upregulated neuroinflammatory markers TNFα, and cd11b (figure-1 and 2). In both models there are upregulated AA cascade markers (Rao et al 2009; Kellom et al unpublished data). However, low dose infusion of LPS (0.5ng/hr) for six days increased TNF alpha protein level without a significant change in cPLA2 transcription or drebrin protein in rat brain. This suggests that TNFα alone cannot decrease drebrin levels in rat brain. In combination, these findings suggest neuroinflammation associated with AA signaling could downregulate drebrin levels in rat brain.
Figure 2.
Representation of cross-talk between neuroinflammation and excitotoxicity involving arachidonic acid cascade.
Excitotoxicity
In a model of excitotoxicity, an intense stimulation of NMDA results in drebrin loss in cultured hippocampal neurons (Halpain et al, 1998). Chronic NMDA administration to rats upregulates brain AA turnover with increased cPLA2 activity and cPLA2 transcription in rat brain. Chronic NMDA exposure in rats result in upregulated protein and mRNA levels of neuroinflammatory markers such as IL-1β, TNF α, GFAP and iNOS in rat frontal cortex (Chang et al, 2008a). These studies imply that excitotoxicity and neuroinflammation signaling pathways cross-talk with each other and involve AA signaling. Further, chronic NMDA administration to rats, results in upregulation of pro-apoptotic factors Bad and Bax which causes neuronal loss (Kim et al, 2009a). The combination of neuroinflammation and AA signaling could influence the synaptic loss.
N-3 polyunsaturated dietary deprivation model
Clinical and pre-clinical studies support the idea that neuroinflammation associated AA cascade signaling results in synaptic loss. A recent study of triple transgenic AD mice has shows that n-3 polyunsaturated fatty acid(PUFA) dietary deprivation in mice reduces brain drebrin levels (Julien et al, 2008). Dietary n-3 deprivation in rats shows upregulated brain AA signaling with increased activity and transcription of both cPLA2 and sPLA2 (Rao et al, 2007a). This finding suggests either neuroinflammation or AA cascade increase could influence the reduction of the synaptic proteins in brain. The role of neuroinflammation or AA influence on synaptic proteins loss is not clear. It is apparent that drebrin is regulated by the transcriptional factor NXF and is modulated by DHA via phosphotidyl inositol kinase (Calon et al, 2004; Ooe et al, 2004). It perhaps AA may be act on this kinase and transcription factor of drebrin which could result in the reduction of drebrin transcription. A recent study demonstrate that cPLA2 inhibitor protects against prion and amyloid beta 1-42 induced synaptic loss in cultured rat cortical and hippocampal neurons (Bate et al). Further detailed molecular studies are needed to understand the role of proinflammatory AA and its metabolites effects on the drebrin and synaptophysin transcription factors.
Drugs
The classical antidepressant fluoxetine, upon chronic administration to rats, upregulates hippocampal drebrin level compared with the chronically stressed group (Yang et al, 2003). Unlike antidepressants, antipsychotic drugs, olanzapine and haloperidol did not significantly change the hippocampal drebrin level in monkeys (Hill et al, 2006). Thus suggests drebrin is modulated by various factors including neuroinflammatory markers. The influence of AA on drebrin transcription is not clear. Further studies are warranted to understand the role of neuroinflammation and AA on drebrin regulation.
Conclusions
Neuroinflammatory and arachidonic acid markers are associated with synaptic protein loss of drebrin and synaptophysin. These changes could contribute to cognitive impairments in neurodegenerative and neuropsychiatric illnesses.
Acknowledgments
This research was entirely supported by the Intramural Research Programs of the National Institute on Aging, National Institutes of Health, Bethesda, MD 20892. We thank the National Cancer Institute (NCI), Center for Cancer Research (CCR) Fellows Editorial Board and Dr. Ed Reese, for proofreading the manuscript.
Abbreviations
- AA
arachidonic acid
- AD
Alzheimer disease
- BD
bipolar disorder
- cPGES
cytosolic prostaglandin E synthase
- cPLA2
calcium-dependent phospholipase A2
- COX
cyclooxygenase
- GFAP
glial fibrillary acidic protein
- gp120
glycoprotein 120
- HIV
human immunodeficiency virus
- HAD
HIV-associated dementia
- IL-1β
interleukin-1β
- iPLA2
calcium-independent phospholipase A2
- mPGES
membrane prostaglandin E synthase
- LOX
lipoxygenase
- NF-κB
nuclear factor-kappa B
- PG
prostaglandin
- sPLA2
secretory phospholipase A2
- TNFα
tumor necrosis factor α
- Tg
transgenic
- TX
thromboxane
- TXS
thromboxane synthase
Footnotes
Conflict of interest: No
References
- Acarin L, Peluffo H, Gonzalez B, Castellano B. Expression of inducible nitric oxide synthase and cyclooxygenase-2 after excitotoxic damage to the immature rat brain. J Neurosci Res. 2002;68(6):745–754. doi: 10.1002/jnr.10261. [DOI] [PubMed] [Google Scholar]
- Akiba S, Mizunaga S, Kume K, Hayama M, Sato T. Involvement of group VI Ca2+-independent phospholipase A2 in protein kinase C-dependent arachidonic acid liberation in zymosan-stimulated macrophage-like P388D1 cells. J Biol Chem. 1999;274(28):19906–19912. doi: 10.1074/jbc.274.28.19906. [DOI] [PubMed] [Google Scholar]
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, et al. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483(4):383–402. doi: 10.1002/cne.20449. [DOI] [PubMed] [Google Scholar]
- Bales KR, Du Y, Holtzman D, Cordell B, Paul SM. Neuroinflammation and Alzheimer’s disease: critical roles for cytokine/Abeta-induced glial activation, NF-kappaB, and apolipoprotein E. Neurobiol Aging. 2000;21(3):427–432. doi: 10.1016/s0197-4580(00)00143-3. discussion 451–423. [DOI] [PubMed] [Google Scholar]
- Basselin M, Ramadan E, Igarashi M, Chang L, Chen M, Kraft AD, et al. Imaging upregulated brain arachidonic acid metabolism in HIV-1 transgenic rats. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bate C, Tayebi M, Williams A. Phospholipase A2 inhibitors protect against prion and Abeta mediated synapse degeneration. Mol Neurodegener. 5:13. doi: 10.1186/1750-1326-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MK, Lieb K, Schulze-Osthoff K, Berger M, Gebicke-Haerter PJ, Bauer J, et al. Expression and regulation of cyclooxygenase-2 in rat microglia. Eur J Biochem. 1997;243(3):726–731. doi: 10.1111/j.1432-1033.1997.00726.x. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA Administration Increases Neuroinflammatory Markers in Rat Frontal Cortex: Cross-Talk Between Excitotoxicity and Neuroinflammation. Neurochem Res. 2008a doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Kim HW, Rapoport SI, Rao JS. Chronic NMDA administration increases neuroinflammatory markers in rat frontal cortex: cross-talk between excitotoxicity and neuroinflammation. Neurochem Res. 2008b;33(11):2318–2323. doi: 10.1007/s11064-008-9731-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65(6):592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Gendelman HE, Kipnis J, Perez-Polo JR, Perry VH, Popovich PG, et al. Debate: “is increasing neuroinflammation beneficial for neural repair?”. J Neuroimmune Pharmacol. 2006;1(3):195–211. doi: 10.1007/s11481-006-9021-7. [DOI] [PubMed] [Google Scholar]
- Dobos N, Korf J, Luiten PG, Eisel UL. Neuroinflammation in Alzheimer’s disease and major depression. Biol Psychiatry. 2010;67(6):503–504. doi: 10.1016/j.biopsych.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50(11):1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Tyrosine phosphorylation of synaptophysin in synaptic vesicle recycling. Biochem Soc Trans. 2005;33(Pt 6):1350–1353. doi: 10.1042/BST20051350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. Journal of neurochemistry. 2008;104(5):1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Gibson RM, Rothwell NJ, Le Feuvre RA. CNS injury: the role of the cytokine IL-1. Vet J. 2004;168(3):230–237. doi: 10.1016/j.tvjl.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997a;54(7):660–669. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997b;54(10):943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- Haley GE, Kohama SG, Urbanski HF, Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Dordr) doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18(23):9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, DeGennaro LJ, Drachman DA. Synaptic loss in Alzheimer’s disease and other dementias. Neurology. 1989;39(3):355–361. doi: 10.1212/wnl.39.3.355. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J Neurosci Res. 1996;43(1):87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(6):637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999;19(10):3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11(6):557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Hoeck WG, Ramesha CS, Chang DJ, Fan N, Heller RA. Cytoplasmic phospholipase A2 activity and gene expression are stimulated by tumor necrosis factor: dexamethasone blocks the induced synthesis. Proc Natl Acad Sci U S A. 1993;90(10):4475–4479. doi: 10.1073/pnas.90.10.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S49–58. doi: 10.1002/ana.10481. discussion S58–60. [DOI] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, et al. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Jupp OJ, Vandenabeele P, MacEwan DJ. Distinct regulation of cytosolic phospholipase A2 phosphorylation, translocation, proteolysis and activation by tumour necrosis factor-receptor subtypes. The Biochemical journal. 2003;374(Pt 2):453–461. doi: 10.1042/BJ20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam PC, See AU. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000;55(5):442–449. doi: 10.1046/j.1365-2044.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- Kim HW, Chang YC, Chen M, Rapoport SI, Rao JS. Chronic NMDA administration to rats increases brain pro-apoptotic factors while decreasing anti-Apoptotic factors and causes cell death. BMC Neurosci. 2009a;10:123. doi: 10.1186/1471-2202-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 37(3):596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2009b doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Sekino Y, Shirao T, Tanaka S, Ogura T, Inada K, et al. Antisense knockdown of drebrin A, a dendritic spine protein, causes stronger preference, impaired pre-pulse inhibition, and an increased sensitivity to psychostimulant. Neurosci Res. 2004;49(2):205–217. doi: 10.1016/j.neures.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Kojima N, Kato Y, Shirao T, Obata K. Nucleotide sequences of two embryonic drebrins, developmentally regulated brain proteins, and developmental change in their mRNAs. Brain Res. 1988;464(3):207–215. doi: 10.1016/0169-328x(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Kojima N, Shirao T. Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. Neurosci Res. 2007;58(1):1–5. doi: 10.1016/j.neures.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Leslie JB, Watkins WD. Eicosanoids in the central nervous system. J Neurosurg. 1985;63(5):659–668. doi: 10.3171/jns.1985.63.5.0659. [DOI] [PubMed] [Google Scholar]
- Mizui T, Takahashi H, Sekino Y, Shirao T. Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol Cell Neurosci. 2005;30(1):149–157. doi: 10.1016/j.mcn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kambe T, Shimbara S, Kudo I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J Biol Chem. 1999;274(5):3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Phospholipase A2. J Biochem (Tokyo) 2002;131(3):285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, et al. The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2. J Biol Chem. 1998;273(23):14411–14423. doi: 10.1074/jbc.273.23.14411. [DOI] [PubMed] [Google Scholar]
- O’Banion MK. Cyclooxygenase-2: molecular biology, pharmacology, and neurobiology. Crit Rev Neurobiol. 1999;13(1):45–82. doi: 10.1615/critrevneurobiol.v13.i1.30. [DOI] [PubMed] [Google Scholar]
- Ooe N, Saito K, Mikami N, Nakatuka I, Kaneko H. Identification of a novel basic helix-loop-helix-PAS factor, NXF, reveals a Sim2 competitive, positive regulatory role in dendritic-cytoskeleton modulator drebrin gene expression. Mol Cell Biol. 2004;24(2):608–616. doi: 10.1128/MCB.24.2.608-616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Bonanno G, Raiteri M, Ajmone-Cat MA, Greco A, et al. In vivo activation of N-methyl-D-aspartate receptors in the rat hippocampus increases prostaglandin E(2) extracellular levels and triggers lipid peroxidation through cyclooxygenase-mediated mechanisms. Journal of neurochemistry. 2002;81(5):1028–1034. doi: 10.1046/j.1471-4159.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9(6):481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007a;12(2):151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Rapoport SI, Bazinet RP, Lee HJ. Chronic NMDA administration to rats up-regulates frontal cortex cytosolic phospholipase A2 and its transcription factor, activator protein-2. J Neurochem. 2007b;102(6):1918–1927. doi: 10.1111/j.1471-4159.2007.04648.x. [DOI] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 15(4):384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15(4):384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert K, Masferrer J, Zhang Y, Gregory S, Olson G, Hauser S, et al. Mediation of inflammation by cyclooxygenase-2. Agents and actions. 1995;46:41–50. doi: 10.1007/978-3-0348-7276-8_5. [DOI] [PubMed] [Google Scholar]
- Shirao T. The roles of microfilament-associated proteins, drebrins, in brain morphogenesis: a review. J Biochem. 1995;117(2):231–236. doi: 10.1093/jb/117.2.231. [DOI] [PubMed] [Google Scholar]
- Silvestroni A, Faull RL, Strand AD, Moller T. Distinct neuroinflammatory profile in post-mortem human Huntington’s disease. Neuroreport. 2009;20(12):1098–1103. doi: 10.1097/WNR.0b013e32832e34ee. [DOI] [PubMed] [Google Scholar]
- Spriggs DR, Sherman ML, Imamura K, Mohri M, Rodriguez C, Robbins G, et al. Phospholipase A2 activation and autoinduction of tumor necrosis factor gene expression by tumor necrosis factor. Cancer Res. 1990;50(22):7101–7107. [PubMed] [Google Scholar]
- Takahashi H, Mizui T, Shirao T. Down-regulation of drebrin A expression suppresses synaptic targeting of NMDA receptors in developing hippocampal neurones. J Neurochem. 2006;97(Suppl 1):110–115. doi: 10.1111/j.1471-4159.2005.03536.x. [DOI] [PubMed] [Google Scholar]
- Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208(1):1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo AP, Harvey PD, Baldessarini RJ. Neurocognitive impairment in bipolar disorder patients: functional implications. Bipolar Disord. 2009;11(2):113–125. doi: 10.1111/j.1399-5618.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Ecker UK, Scherk H, Schneider-Axmann T, Falkai P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World J Biol Psychiatry. 2009;10(4 Pt 2):442–451. doi: 10.1080/15622970701849986. [DOI] [PubMed] [Google Scholar]
- Yang HC, Mosior M, Johnson CA, Chen Y, Dennis EA. Group-specific assays that distinguish between the four major types of mammalian phospholipase A2. Anal Biochem. 1999;269(2):278–288. doi: 10.1006/abio.1999.4053. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Li YF, Zhang HT, Zhang FQ, Zhao N, Gong ZH, et al. Up-regulation of microtubule-associated protein 4 and drebrin A mRNA expression by antidepressants in rat hippocampus following chronic stress. Neurosci Lett. 2003;351(3):206–208. doi: 10.1016/j.neulet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9(2):234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zheng H, Shao X, Wang W, Yao Q, Li Z. Excitotoxicity of TNFalpha derived from KA activated microglia on hippocampal neurons in vitro and in vivo. Journal of neurochemistry. doi: 10.1111/j.1471-4159.2010.06763.x. [DOI] [PubMed] [Google Scholar]