Abstract
The chiral dissymmetric tetradentate ligand SPhbipox (6’-(4-phenyloxazolin-2-yl)-2,2’-bipyridine-6-carboxylic acid) leads to the diastereoselective assembly of a homochiral Eu(III) triangle and of a highly emissive (QY=27%) heptanuclear wheel which is the largest example of chiral luminescent complex of Eu(III) reported to date. We show that the nuclearity of the assembly is controlled by the solvent and the europium cation. All the compounds show large circularly polarized luminescence with an activity which varies with the nature of the assembly (highest for the homochiral trimer).
Supramolecular chiral recognition processes play a key role in stereoselective catalytic and biological transformations. Chirality is also of high current interest in nanotechnology for the control of final architectures. The control of chirality around a metal center in molecular or supramolecular complexes is a key step for the development of enantioselective catalysts, and chiroptical probes, sensors. 1, 2 In particular the circularly polarized luminescence (CPL) observed in chiral lanthanide complexes is of high current interest in biological probing and display technologies1, 3-6 due to the attractive properties of lanthanide ions (large Stokes shift, long lifetimes, narrow emission bands, high quantum yields).7 However, enantiopure lanthanide complexes are mostly limited to few examples of mononuclear compounds1, 8,3, 9,4, 5, 10, 11 and dinuclear helicates12-14 Although the supramolecular chemistry of lanthanide ions is less developed with respect to d-block metal ions, reports of lanthanide based polynuclear assemblies15-20 which have pre-programmed new original structures21-23 and of nanosized complexes that show fascinating optical,24 and magnetic properties25 are rapidly increasing. However, the high kinetic lability and lack of stereochemical preferences of lanthanide ions renders very difficult the control of their coordination sphere. Thus only very few enantiopure polynuclear lanthanide assemblies have been reported.26-30
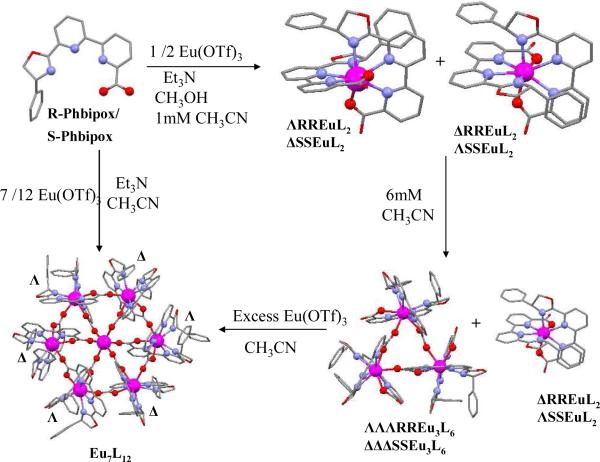
We have recently reported the diastereoselective self-assembly of an enantiopure trinuclear europium triangle ΔΔΔ-[Eu(S-Phbipox)2]3+3 promoted by the asymmetric ligand S-PhbipoxH (6’-(4-phenyloxazolin-2-yl)-2,2’-bipyridine-6-carboxylic acid).30 The trimetallic enantiopure complex ΔΔΔ-[Eu(S-Phbipox)2]3+3 self-assembles from concentrated acetonitrile solutions, while at lower concentrations only the diastereomeric mononuclear complexes Δ-[Eu(S-Phbipox)2]+ and Λ-[Eu(S-Phbipox)2]+ form with partial stereoselectivity (Λ/Δ ratio of ~1.8) (Scheme 1).
Scheme 1.
Synthesis of the enantiopure wheels 1, 2. (Excess Eu(OTf)3 ranges from 0.06 to 0.12 equiv per ligand).
Here we show that the controlled addition of europium cation to the mixture of diastereomeric Δ- and Λ-[Eu(R-Phbipox)2]+ complexes leads to the diastereoselective self assembly of the heptameric enantiopure europium wheel [Eu⊂(Λ-Eu(R- Phbipox)2Δ-Eu(R-Phbipox)2)3](OTf)9, 1. Starting from a mixture of Eu(III) complexes of S-Phbipox the isostructural [Eu⊂(Δ-Eu(S-Phbipox)2Λ-Eu(S-Phbipox)2)3](OTf)9, 2 was also isolated. These complexes are a very rare example of large chiral enantiopure assembly of an f element and the first chiral luminescent wheels. Moreover we show that the Δ-Eu and Λ-Eu monomers, ΛΛΛ-Eu3 and ΔΔΔ-Eu3 trinuclear and [Eu⊂(ΛΔΛΔΛΔ-Eu6)] heptanuclear assemblies give rise to Eu(III)-centered circularly polarized luminescence (CPL) upon excitation of the Phpibox ligand with one of the highest reported efficiencies shown by the triangular complex.
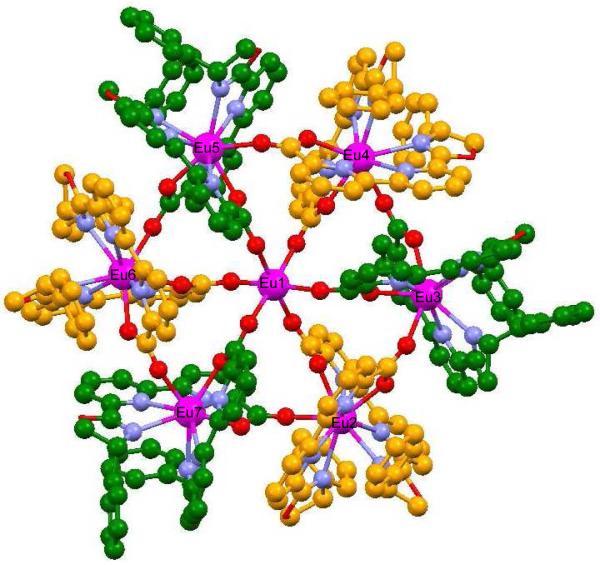
Compounds 1 and 2 were prepared in 81% yield from the reaction of R- and S-PhbipoxNEt3 with Eu(OTf)3 in 12:7 ratio. X-ray diffraction studies were carried out on crystals prepared by slow diffusion of hexane into a benzonitrile solution of the complex [Eu⊂(Λ-Eu(R-Phbipox)2Δ-Eu(R-Phbipox)2)3](OTf)9, 1. The isostructural nature of 2 was confirmed by the unit cell measurement on X-ray quality crystals. In the cation [Eu⊂(Λ-Eu(R-Phbipox)2Δ-Eu(R-Phbipox)2)3]9+ (Figure 1) three [Λ-Eu(R-Phbipox)2]+ and three [Δ-Eu(R-Phbipox)2]+ complexes are connected alternatively through a bridging carboxylate oxygen from one of the R-Phbipox ligands to form the heterochiral cyclic structure. A seventh europium ion is encapsulated in the ring center and has a regular octahedral coordination sphere composed of six carboxylate oxygens from the coordinated Phbipox carboxylate ligands not involved in the ring formation. The overall structure is similar to the structure of the heptameric europium wheel [Eu⊂(Eu(terpya)2)6](OTf)9 obtained from the cation promoted self-assembly of six europium complexes of the achiral dissymmetric terpyridine-monocarboxylate ligand (terpya).18
Figure 1.
Crystal structure of the cation [Eu⊂(Λ-Eu(R-Phbipox)2ΔEu(R-Phbipox)2)3]9+ in 1. Oxygen: red; europium: pink; carbons of [Λ-Eu(R-Phbipox)2]+: green; carbons of [Δ-Eu(R-Phbipox)2]+:orange.
In 1 the external europium ions are nine coordinated by six nitrogens and two oxygens from the two Phbipox ligands and one oxygen from a bridging Phbipox ligand of the adjacent mononuclear complex. The Eu(III) coordination sphere is best described as a distorted tricapped trigonal prism (Figure S1). The six crystallographically inequivalent lanthanide ions of the non-centrosymmetric cation [Eu⊂(Λ-Eu(R-Phbipox)2Δ-Eu(R-Phbipox)2)3]9+ are located in the corners of a pseudo-hexagon. The diameter of the hexagon, defined as the distance of the two opposite europium ions, is 12.6 Ǻ (Figure S2). In each [Eu(R-Phbipox)2]+ unit of 1 strong π-π interactions are found between the oxazoline phenyl rings of each tetradentate ligand and one pyridine ring of the other ligand (Table S1).
The crystal structure of 1 shows that Δ and Λ complexes alternate to yield the final chiral assembly. Mercury models of the possible alternative diastereoisomers (all involving the proximity of complexes with the same chirality) suggest the presence of important steric constraints arising from the orientation of the phenyl substituents on the oxazoline ring when complexes of the same Δ or Λ chirality are brought together. Similar steric constraints were shown to be at the origin of the diastereoselective assembly of the ΔΔΔ-[Eu(S-Phbipox)2]3+3 trimer.30 Such sterical constraints should play an important role in the diastereoselectivity of the self-assembly process which was confirmed by NMR studies. The proton NMR of an acetonitrile solution of [Eu(R-Phbipox)2]+ and Eu(OTf)3 in a 6:1 ratio (Figure 2c) shows the presence of one major set of 48 signals assigned to the protons of the four different R-Phbipox ligands from two different (Δ and Λ) mononuclear complexes present in 1 in agreement with the pseudo-C3 symmetry of the solid state structure of 1. The same 1H NMR spectrum was measured for an acetonitrile solution of R-PhbipoxH and Eu(OTf)3 in a 12:7 ratio in the presence of 12 equivalents of triethylamine suggesting that self assembly can also occur directly from the mixture of ligand and metal at the correct stoichiometric ratio. Again the same 1H NMR spectrum was observed for the isolated heptanuclear complex 1 in anhydrous acetonitrile (Figure S4). This indicates that the chiral assembly is stable in acetonitrile solution. In contrast, 1H NMR studies show that dissolution of the heptanuclear complex 1 (1 mM) in methanol or pyridine slowly (over a day) leads to the disruption of the cyclic structure to afford the corresponding mononuclear complex as indicated by the presence of the 24 signals assigned to the two mononuclear Λ and Δ species. This probably results from the displacement of the bridging carboxylate by the solvent molecules and was already observed for the trinuclear europium triangle (ΔΔΔ)-[Eu(S-Phbipox)2]3+3.30
Figure 2.
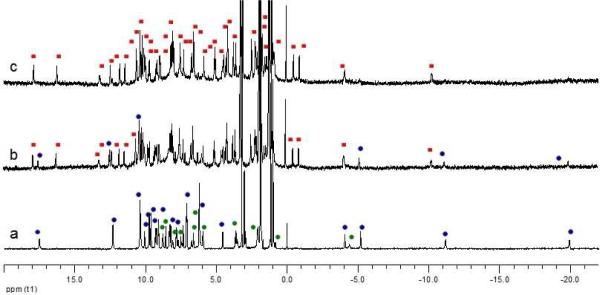
1H NMR titration by addition of excess 1/8 (b), 1/6(c) equivalent of Eu(OTf)3 to a 6 mM solution of [Eu(R-Phbipox)2)](OTf) in anhydrous acetonitrile (a) at 298 K and 500 MHz. Blue, green dots and red squares: trinuclear, mononuclear and heptanuclear (1) complexes, respectively.
The formation of 1 can be followed by 1HNMR titration of a 6 mM solution of [Eu(R-Phbipox)2]+ in acetonitrile with 0.12- 0.25 equiv of Eu(OTf)3. The 1HNMR of the 6 mM solution [Eu(R-Phbipox)2]OTf in acetonitrile (Figure 2a) shows the presence of the trinuclear ΛΛΛ-[Eu(R-Phbipox)2]3+3 complex as previously reported for the S-Phbipox enantiomer.30 The structure of this assembly has been confirmed by X-ray diffraction on crystals isolated from a concentrated acetonitrile solution of [Eu(R-Phbipox)2]OTf. X-ray diffraction analysis revealed the presence of two distinct co-crystallized molecules in the compound {ΛΛΛ-[Eu(R-Phbipox)2]]3.Δ-[Eu(R-Phbipox)2]}OTf4, 3, (Figure S3). Addition of 0.12- 0.17 equiv of Eu(OTf)3 to the 6 mM solution of [Eu(R-Phbipox)2]OTf in acetonitrile results in the progressive disappearance of the NMR signals assigned to the monomeric and trimeric complexes and to the appearance of the signals assigned to 1 (Figure 2b). Addition of 0.25 equiv of Eu(OTf)3 did not lead to the formation of additional species suggesting that the heptanuclear wheel is the only thermodynamically favored assembly in solution. These results confirm the diastereoselectivity of the cation promoted assembly of the heptanuclear wheel from three delta and three lambda diastereomers. Moreover they show that the excess cation promotes the transformation of the trinuclear assembly into the heptanuclear assembly.
In order to confirm the nuclearity of the oligomeric assembly in solution, Pulsed-Field Gradient STimulated Echo (PFGSTE) diffusion NMR31 was used to measure the diffusion coefficients (D) of a mixture of trinuclear ΛΛΛ-[Eu(R-Phbipox)2]3+3 and heptanuclear [Eu⊂(Λ-Eu(R-Phbipox)2Δ-Eu(R-Phbipox)2)3](OTf)9 complexes in anhydrous deuterated acetonitrile. The diffusion coefficient is a function of the molecular weight and can been conveniently used to discriminate metallosupramolecular architectures in solution.32 The values measured in acetonitrile (M1/MΛΛΛ= (DΛΛΛ/D1)3= 2.2) are in agreement with the presence of trinuclear and heptanuclear complexes. Stokes-Einstein equation has been applied to calculate the spherical hydrodynamic radius of the complexes as they have both globular shapes. The calculated values (Table S2) of 8.0 Å for the trimeric species and 10.3 Å for the heptanuclear ones in acetonitrile compare very well with the values estimated from the crystal structures (7.730 and 10.1 Å respectively).
Preliminary studies (Figure S5) show that the analogous heptanuclear assembly [Nd⊂(Λ-Nd(R-Phbipox)2Δ-Nd(R-Phbipox)2)3](OTf)9 can be obtained following the procedure shown in Scheme 1. X-ray diffraction studies of the obtained crystals were not of sufficient quality to obtain a high quality structure, but the unit cell parameters confirmed the isostructural nature of the isolated complex to the Eu wheel 1. Preliminary NMR studies also show that in contrast to Eu and Nd wheel, the addition of an excess of Yb(III) ion to a solution of the [Yb(R-Phpybox)2]+ complexes does not result in the formation of well defined heptanuclear solution species, but only broad NMR signals are observed suggesting that the diastereoselective assembly of 1 and 2 is controlled by the size of the central cations.
The luminescence emission spectra of 1 at room temperature and upon broad band excitation in acetonitrile and solid state are given in Figure S6 while luminescence quantum yields of 1 and of the mononuclear analogue [Eu(R-Phbipox)2](OTf) in acetonitrile solution and solid state are given in Table 1. The analysis of the crystal field splittings in the emission spectra of both complexes is consistent with their X-ray structure. They are highly luminescent with a quantum yield of the metal centered luminescence slightly higher for the heptanuclear complex (27 to 37%) than the mononuclear complex (21 and 25%). This suggests that the cyclic arrangement of six Eu components does not lead to intramolecular quenching effects as observed in the trimetallic helicates,33 despite the shorter distance between neighboring EuL2 units (6.34 Ǻ) with respect to the intermetallic distance in the trimetallic helicates (9-9.3Ǻ). Indeed, the sensitization efficiency ηsens of the mononuclear and heptanuclear complexes are in the same range with relatively small variations (average of 55 %) due to their similar coordination environment. In view of the strong luminescence emission of these Eu(III) complexes we have decided to use CPL, a technique allying sensitivity and specificity of the signal for chiral environments, to monitor the assembly of trinuclear and heptanuclear assemblies from the mononuclear Phbipox complexes of europium.
Table 1.
Absolute quantum yields and luminescence dissymmetry factors of the Eu (5D0) excited level in [Eu(R-Phbipox)2](OTf), [Eu(S-Phbipox)2](OTf) and [Eu⊂(Eu(S-Phbipox)2)6](OTf)9 complexes in acetonitrile solutions at 298K with λex = 339-340 nm.
| Complex in CH3CN |
glum (λ/nm)[a] |
||
|---|---|---|---|
| 5D0→7F1 | 5D0→7F2 | ||
| [Eu(R-Phbipox)2]+ (6 mM) | 25 (1) | -0.45 | +0.02 |
| [Eu(S-Phbipox)2]+ (6 mM) | +0.45 | ||
| [Eu(S-Phbipox)2]+ (2 mM) | +0.15 | -0.005 | |
| [Eu(R-Phbipox)2]+ (1 mM) | -0.04 | +0.007 | |
| [Eu(S-Phbipox)2]+ (1 mM) | +0.06 | -0.003 | |
| [Eu⊂(Eu(S-Phbipox)2)6]9+(1 mM) | 27 (1) | +0.1 | -0.01 |
Obtained by the CPL measurements at the 5D0→7F1 (magnetic dipole, λ = 590.6-592.8 nm) and 5D0→7F2 (electric dipole, λ = 613-614 nm) transitions; All the glum values reported in this study have been measured with a standard deviation, σd, of ±0.01 and ±0.001 for the CPL spectra of the 5D0→7F1 and 7F2 transitions, respectively.
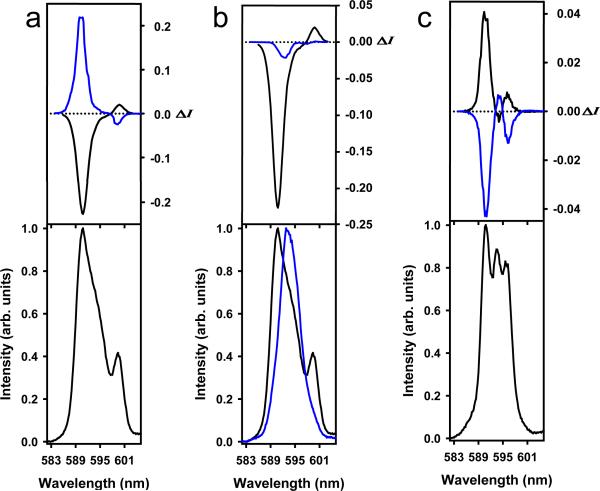
A strong CPL signal was measured upon UV excitation of the mononuclear complexes [Eu(S-Phbipox)2](OTf), [Eu(R-Phbipox)2](OTf) in 1 and 6 mM solutions in anhydrous acetonitrile. The complexes ([Eu(S-Phbipox)2](OTf) and [Eu(R-Phbipox)2](OTf) give rise to mirror image 5D0→7F1 transition CPL spectra at 6 mM (Figure 3a). The CPL spectra of the complex [Eu(R-Phbipox)2](OTf) in CH3CN show important differences in the magnitude, sign, and shape of the signals depending on the concentration (Figure 3b). Similarly the luminescence dissymmetry factor glum (Table 1, S4 and S5) increases with concentration reaching a high glum value of -0.45 for [Eu(R-Phbipox)2](OTf) and +0.45 for [Eu(S-Phbipox)2](OTf) at the Eu(5D0→7F1) transition at 6 mM which is among the highest reported for chiral complexes of europium.3,9,34 This increase parallels the formation of the homochiral trinuclear assemblies ΛΛΛ-[Eu(R-Phbipox)2]3+3 ΔΔΔ-[Eu(S-Phbipox)2]3+3 at high concentrations of the [Eu(R-Phbipox)2](OTf) and [Eu(S-Phbipox)2](OTf) complexes in acetonitrile.30 In 1 mM acetonitrile solutions, where only the monomeric complexes are present, a glum value of less than 0.10 was measured. CPL spectra of the 5D0→7F1 and 7F2 transitions of the heptanuclear wheels 1 and 2 were also recorded at a concentration of 2 mM in anhydrous acetonitrile and give rise to mirror images (Figure 3c and S7).
Figure 3.
Circularly polarized luminescence (upper curves) and total luminescence (lower curves) spectra of the 5D0→7F1 transition in anhydrous CH3CN solutions at 295 K of a) [Eu(S-Phbipox)2](OTf) (blue) and [Eu(R-Phbipox)2](OTf) (black) (6 mM, λex = 335-338 nm, respectively) and of b) [Eu(R-Phbipox)2](OTf), in 1 mM (blue) and 6mM (black) (λex = 331-335nm, respectively) c) [Eu⊂(Eu(S-Phbipox)2)6](OTf)9 (black) and [Eu⊂ (Eu(R-Phbipox)2)6](OTf)9 (blue) (2 mM, λex = 347-348 nm, respectively).
The CPL activity measured for the heptanuclear assembly 2 with a glum value of ~ 0.10 (λ = 590.6 nm) is similar to that found for the mononuclear complex (measured glum~ 0.06). This is in agreement with the heterochiral nature of the heptanuclear wheel 1 which retains the same mixture of Λ and Δ isomers found in solutions of the mononuclear complexes [Eu(S-Phbipox)2)](OTf).
In conclusion we have shown that a chiral dissymmetric tetradentate ligand can be used to promote the selective assembly of a new chiral heptanuclear wheel which is the largest example of chiral complexes of Eu(III) reported to date. The nuclearity of the assembly of europium polynuclear complexes promoted by the chiral Phbipox ligand can be controlled by the lanthanide cation. The cation controlled self-assembly of the wheel from mono and trinuclear species is diastereoselective and only one of the 13 possible isomers forms. The CPL studies show that the formation of the different species can be followed by CPL and while the CPL activity of the mononuclear species is retained in the heptanuclear wheel, the trimeric complex shows one of the highest CPL activity reported to date. The cation directed assembly presented here is also well adapted for the synthesis of chiral heteropolymetallic species and work in this direction is in progress.
Supplementary Material
Acknowledgement
We thank Colette Lebrun and Pierre A. Bayle for their help with the spectroscopic characterizations. We thank Lydia Plassais for the ligand synthesis. We thank SNBL for the in-house beam time allocation. G.M. thanks the National Institute of Health, Minority Biomedical Research Support (1 SC3 GM089589–03 and 3 S06 GM008192–27S1) and the Henry Dreyfus Teacher–Scholar Award for financial support, F.M. thanks the Howard Hughes Medical Institute Award (52006312) for a research fellowship.
Footnotes
Supporting Information Available: Complete synthetic and experimental details; selected 1H NMR and Fluorescence spectra; coefficient diffusion and CPL data; X-ray crystallographic data and files in CIF format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Montgomery CP, Murray BS, New EJ, Pal R, Parker D. Acc. Chem. Res. 2009;42:925–937. doi: 10.1021/ar800174z. [DOI] [PubMed] [Google Scholar]; Muller G. Dalton Trans. 2009:9692–9707. doi: 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crassous J. Chem. Soc. Rev. 2009;38:830–845. doi: 10.1039/b806203j. [DOI] [PubMed] [Google Scholar]
- 3.Seitz M, Moore EG, Ingrarn AJ, Muller G, Raymond KN. J. Am. Chem. Soc. 2007;129:15468–15470. doi: 10.1021/ja076005e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton JW, Di Bari L, Parker D, Pescitelli G, Puschmann H, Yufit DS. Chem. Commun. 2011;47:12289–12291. doi: 10.1039/c1cc14904k. [DOI] [PubMed] [Google Scholar]
- 5.Bruce JI, Dickins RS, Govenlock LJ, Gunnlaugsson T, Lopinski S, Lowe MP, Parker D, Peacock RD, Perry JJB, Aime S, Botta M. J. Am. Chem. Soc. 2000;122:9674–9684. [Google Scholar]
- 6.Tsukube H, Shinoda S. Chem. Rev. 2002;102:2389–2403. doi: 10.1021/cr010450p. [DOI] [PubMed] [Google Scholar]
- 7.Bunzli JCG, Piguet C. Chem. Soc. Rev. 2005;34:1048–1077. doi: 10.1039/b406082m. [DOI] [PubMed] [Google Scholar]
- 8.Gregolinski J, Starynowicz P, Hua KT, Lunkley JL, Muller G, Lisowski J. J. Am. Chem. Soc. 2008;130:17761–17773. doi: 10.1021/ja805033j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petoud S, Muller G, Moore EG, Xu JD, Sokolnicki J, Riehl JP, Le UN, Cohen SM, Raymond KN. J. Am. Chem. Soc. 2007;129:77–83. doi: 10.1021/ja064902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parker D. Coord. Chem. Rev. 2000;205:109–130. [Google Scholar]; Di Bari L, Salvadori P. Coord. Chem. Rev. 2005;249:2854–2879. [Google Scholar]
- 11.Yu JH, Parker D, Pal R, Poole RA, Cann MJ. J. Am. Chem. Soc. 2006;128:2294–2299. doi: 10.1021/ja056303g. [DOI] [PubMed] [Google Scholar]; Di Bari L, Lelli M, Pintacuda G, Pescitelli G, Marchetti F, Salvadori P. J. Am. Chem. Soc. 2003;125:5549–5558. doi: 10.1021/ja0297640. [DOI] [PubMed] [Google Scholar]
- 12.Cantuel M, Bernardinelli G, Muller G, Riehl JP, Piguet C. Inorg. Chem. 2004;43:1840–1849. doi: 10.1021/ic035292u. [DOI] [PubMed] [Google Scholar]
- 13.Stomeo F, Lincheneau C, Leonard JP, O'Brien JE, Peacock RD, McCoy CP, Gunnlaugsson T. J. Am. Chem. Soc. 2009;131:9636–9637. doi: 10.1021/ja9032204. [DOI] [PubMed] [Google Scholar]
- 14.Yuasa J, Ohno T, Miyata K, Tsumatori H, Hasegawa Y, Kawai T. J. Am. Chem. Soc. 2011;133:9892–9902. doi: 10.1021/ja201984u. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Raymond KN. Angewandte Chemie. 2000;39:2745–2747. doi: 10.1002/1521-3773(20000804)39:15<2745::aid-anie2745>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Bunzli JCG, Piguet C. Chem. Rev. 2002;102:1897–1928. doi: 10.1021/cr010299j. [DOI] [PubMed] [Google Scholar]
- 17.Senegas JM, Koeller S, Bernardinelli G, Piguet C. Chem. Commun. 2005:2235–2237. doi: 10.1039/b501399b. [DOI] [PubMed] [Google Scholar]
- 18.Bretonniere Y, Mazzanti M, Pecaut J, Olmstead MM. J. Am. Chem. Soc. 2002;124:9012–9013. doi: 10.1021/ja012177e. [DOI] [PubMed] [Google Scholar]
- 19.Bretonnière Y, Mazzanti M, Wietzke R, Pécaut J. Chem. Commun. 2000:1543–1544. doi: 10.1021/ic991115a. [DOI] [PubMed] [Google Scholar]
- 20.Chen XY, Bretonniere Y, Pecaut J, Imbert D, Bunzli JC, Mazzanti M. Inorg. Chem. 2007;46:625–637. doi: 10.1021/ic061806o. [DOI] [PubMed] [Google Scholar]
- 21.El Aroussi B, Zebret S, Besnard C, Perrottet P, Hamacek J. J. Am. Chem. Soc. 2011;133:10764–10767. doi: 10.1021/ja204474v. [DOI] [PubMed] [Google Scholar]; Zeckert K, Hamacek J, Senegas JM, Dalla-Favera N, Floquet S, Bernardinelli G, Piguet C. Angew. Chem. Int. Ed. 2005;44:7954–7958. doi: 10.1002/anie.200503040. [DOI] [PubMed] [Google Scholar]
- 22.Placidi MP, Villaraza AJL, Natrajan LS, Sykes D, Kenwright AM, Faulkner S. J. Am. Chem. Soc. 2009;131:9916–9917. doi: 10.1021/ja904362f. [DOI] [PubMed] [Google Scholar]; Natrajan LS, Villaraza AJL, Kenwright AM, Faulkner S. Chem. Commun. 2009:6020–6022. doi: 10.1039/b913702e. [DOI] [PubMed] [Google Scholar]; Faulkner S, Pope SJA. J. Am. Chem. Soc. 2003;125:10526–10527. doi: 10.1021/ja035634v. [DOI] [PubMed] [Google Scholar]
- 23.Hamacek J, Poggiali D, Zebret S, El Aroussi B, Schneider MW, Mastalerz M. Chem. Commun. 2012;48:1281–1283. doi: 10.1039/c2cc17322k. [DOI] [PubMed] [Google Scholar]; Zebret S, Dupont N, Bernardinelli G, Hamacek J. Chem. Eur. J. 2009;15:3355–3358. doi: 10.1002/chem.200802676. [DOI] [PubMed] [Google Scholar]; Hamacek J, Blanc S, Elhabiri M, Leize E, Van Dorsselaer A, Piguet C, Albrecht-Gary AM. J. Am. Chem. Soc. 2003;125:1541–1550. doi: 10.1021/ja028861q. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee S, Huebner L, Romanelli MD, Kumar GA, Riman RE, Emge TJ, Brennan JG. J. Am. Chem. Soc. 2005;127:15900–15906. doi: 10.1021/ja054261q. [DOI] [PubMed] [Google Scholar]
- 25.Blagg RJ, Muryn CA, McInnes EJL, Tuna F, Winpenny RE. P. Angew. Chem. Int. Ed. 2011;50:6530–6533. doi: 10.1002/anie.201101932. [DOI] [PubMed] [Google Scholar]
- 26.Mamula O, Lama M, Telfer SG, Nakamura A, Kuroda R, Stoeckli-Evans H, Scopelitti R. Angew. Chem. Int. Ed. 2005;44:2527–2531. doi: 10.1002/anie.200500094. [DOI] [PubMed] [Google Scholar]
- 27.Lama M, Mamula O, Kottas GS, Rizzo F, De Cola L, Nakamura A, Kuroda R, Stoeckli-Evans H. Chem. Eur. J. 2007;13:7358–7373. doi: 10.1002/chem.200700324. [DOI] [PubMed] [Google Scholar]
- 28.Tang XL, Wang WH, Dou W, Jiang J, Liu WS, Qin WW, Zhang GL, Zhang HR, Yu KB, Zheng LM. Angew. Chem. Int. Ed. 2009;48:3499–3502. doi: 10.1002/anie.200900838. [DOI] [PubMed] [Google Scholar]
- 29.Jeong KS, Kim YS, Kim YJ, Lee E, Yoon JH, Park WH, Park YW, Jeon SJ, Kim ZH, Kim J, Jeong N. Angew. Chem. Int. Ed. 2006;45:8134–8138. doi: 10.1002/anie.200603622. [DOI] [PubMed] [Google Scholar]
- 30.Bozoklu G, Marchal C, Gateau C, Pecaut J, Imbert D, Mazzanti M. Chem. Eur. J. 2010;16:6159–6163. doi: 10.1002/chem.201000572. [DOI] [PubMed] [Google Scholar]
- 31.Edward JT. J. Chem. Ed. 1970;47:261–269. [Google Scholar]
- 32.Allouche L, Marquis A, Lehn J-M. Chem. Eur. J. 2006:7520–7525. doi: 10.1002/chem.200600552. [DOI] [PubMed] [Google Scholar]
- 33.Floquet S, Ouali N, Bocquet B, Bernardinelli G, Imbert D, Bunzli JCG, Hopfgartner G, Piguet C. Chem. Eur. J. 2003;9:1860–1875. doi: 10.1002/chem.200390214. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery CP, New EJ, Parker D, Peacock RD. Chem. Commun. 2008:4261–4263. doi: 10.1039/b810978h. [DOI] [PubMed] [Google Scholar]; Lunkley JL, Shirotani D, Yamanari K, Kaizaki S, Muller G. Inorg. Chem. 2011;50:12724–12732. doi: 10.1021/ic201851r. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lunkley JL, Shirotani D, Yamanari K, Kaizaki S, Muller G. J. Am. Chem. Soc. 2008;130:13814–13815. doi: 10.1021/ja805681w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.