Abstract
Breast milk is the ideal nutrition for term infants but must be supplemented to provide adequate growth for most premature infants. Human milk oligosaccharides (HMOs) are remarkably abundant and diverse in breast milk and yet provide no nutritive value to the infant. HMOs appear to have at least two major functions: prebiotic activity (stimulation of the growth of commensal bacteria in the gut) and protection against pathogens. Investigations of HMOs in milk from women delivering preterm have been limited. We present the first detailed mass spectrometric analysis of the fucosylation and sialylation in HMOs in serial specimens of milk from fifteen women delivering preterm and seven women delivering at term using nano-high performance liquid chromatography chip/time-of-flight mass spectrometry. A mixed-effects model with Levene’s test was used for the statistical analyses. We find that lacto-N-tetraose, a core HMO, is both more abundant and more highly variable in the milk of women delivering preterm. Furthermore, fucosylation in preterm milk is not as well regulated as in term milk, resulting in higher within and between mother variation in women delivering preterm vs. term. Of particular clinical interest, the α1,2-linked fucosylated oligosaccharide 2′-fucosyllactose, an indicator of secretor status, is not consistently present across lactation of several mothers that delivered preterm. The immaturity of HMO production does not appear to resolve over the time of lactation and may have relevance to the susceptibility of premature infants to necrotizing enterocolitis, late onset sepsis, and related neurodevelopmental impairments.
Keywords: HMO, LNT, mass spectrometry, sialic acid, sialylation, fucosyllactose, premature infant
INTRODUCTION
Breast milk is the ideal nutrition for term infants for growth, development, and protection from infections. Human milk from women delivering preterm is not nutritionally adequate for small premature infants and must be fortified to ensure adequate growth in this evolutionarily “new” population 1. Preterm milk is initially higher in protein, carbohydrate, and sodium than term milk, but the protein content decreases over time and is not adequate for the rapid growth requirements of infants born before 32 weeks gestation 2. Current human milk fortifiers provide additional protein, fat, vitamins, and a variety of electrolytes and minerals. The fortification of preterm milk with concentrated pooled donor human milk is an intriguing option with potential benefits for growth and protection from infections 3. The wide variation of preterm milk composition between women and in a given woman over time has prompted proposals to individualize human milk fortification for this population 4.
Human milk oligosaccharides (HMOs) are unbound sugars with diverse and complicated structures. HMOs commonly have a lactose core (Galβ-1,4Glc) at the reducing end that can be elongated with up to 25 N-acetyllactosamine repeat units (Galβ1 3/4GlcNAc). The oligosaccharide backbone can be sialylated in α2-3 or α2-6 linkages and/or fucosylated in α1-2, α1-3, or α1-4 linkages 5, 6. Humans produce greater numbers and more complex structures of oligosaccharides than any other mammal with many women producing more than 100 different structures 7. A survey of nine small HMOs in over 500 milk samples from 435 donors in 10 countries demonstrated regional differences in several HMOs and striking similarities in others 8 Because the human gut lacks the enzymes to deconstruct these compounds, they do not provide any nutritive value for the infant and yet they are the third most abundant solid component in human milk 5. This raises the question of HMO function given that significant energy is required for the mammary gland to synthesize these molecules. Evidence suggests at least two major functions.
First, HMOs promote growth of specific strains of beneficial bacteria such as bifidobacteria 9, 10. The ability of bacteria to deconstruct HMOs is encoded in the bacterial genome and varies among bifidobacterial strains suggesting the co-evolution of human lactation and specific commensal organisms 10. In other words, the primary function of HMOs appears to be to nourish specific strains of bacteria allowing them to flourish. In this way the mother’s milk shapes the intestinal microbiota of her infant.
Second, HMOs (especially fucosylated species) act as free floating receptor analogs, competing for bacterial binding in the intestinal lumen to prevent intestinal pathogen adhesion to epithelial surfaces 11. HMOs and cell surface glycoforms are synthesized by similar glycosyltransferases and thus have common epitopes. Inhibition of binding to cell surfaces by HMOs has been demonstrated for organisms that commonly cause sepsis (e.g. Streptococcus pneumonia 12 and Listeria monocytogenes 13) and diarrhea (e.g. enteropathogenic E. coli 14, Campylobacter jejuni 15, and Norovirus 16) in infants. HMOs have been shown in animal models to decrease toxin induced diarrhea 17, 18.
In addition to these major functions, a role for sialylated oligosaccharides, i.e. N-acetylneuraminic acid containing oligosaccharides, in early brain growth has been postulated from animal studies 19,20. Sialic acids increase the production of gangliosides, which are important components of membrane receptors and cell surfaces of the nervous system 21. The precise role of dietary sialic acid, if any, in human brain development is unclear. Studies of 13C labeled galactose ingested by mothers during lactation suggest that some HMOs are absorbed intact from the intestinal tract with limited data suggesting a role for HMOs in leukocyte adhesion and platelet-neutrophil interactions 22.
Proportions of fucosylated and sialylated HMOs in human term milk are generally 60 80% and 10 15%, respectively 5, and do not vary significantly at different stages of lactation 23. Analysis of HMOs in preterm milk has been limited. Early studies suggested colostral preterm milk contains a higher amount of oligosaccharides than term milk with the amount of oligosaccharides decreasing during the course of lactation in preterm milk 24, 25. Studies of limited numbers of HMOs have shown wide variation between individuals with no significant differences in ten neutral oligosaccharides between preterm and term milk 26, higher sialylated HMO content in preterm milk than term milk that persists over time 27, and significant variation among premature infants in 23 HMOs based on secretor status and Lewis antigen status 28. In milk from mothers delivering at term, monitoring of hundreds of structures indicated that the amount of oligosaccharides and particularly those important for beneficial bacteria remain relatively constant over the lactation period 23.
In this study, we use mass spectrometry to profile the temporal and individual variations of overall abundance as well as fucosylation and sialylation of HMOs in preterm and term milk. Specifically, we use matrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (MALDI FT ICR MS) to profile the preterm and term milk samples and nano-high performance liquid chip/time-of-flight (nano-HPLC Chip/TOF) MS to investigate the temporal and individual variations of fucosylation and sialylation in preterm and term milk. While both instruments provide high sensitivity and high mass accuracy (<5ppm), the latter provides an added dimension of reproducible retention times for each oligosaccharide, allowing the monitoring of hundreds of structures 5, 23. HMOs were characterized using an in house library for neutral 6 and sialylated 29 HMOs based on mass-to-charge ratios (m/z) and retention time values.
MATERIALS AND METHODS
Milk Sample Collection and Processing
Milk samples from fifteen mothers delivering prematurely were collected in the Neonatal Intensive Care Unit at UC Davis Children’s Hospital. Milk samples were also collected from seven mothers of healthy term infants following discharge from the hospital. The UC Davis Institutional Review Board approved all aspects of the study and informed consent was obtained from all subjects. The samples were collected in standard collection tubes and frozen at −80°C prior to extraction. Samples were labeled with randomly generated numbers at the time of collection to protect patient privacy and ensure blinding during analysis.
Extraction and Purification of Human Milk Oligosaccharides (HMOs)
HMOs were extracted and purified as described previously 5, 23. Briefly, 500 uL of thawed milk was centrifuged for 30 min at 4°C. The top fat layer was removed and to the decanted skimmed milk were added four volumes of chloroform/methanol (2:1 by volume). The mixture was then centrifuged at 4,000 g for 30 min at 4°C and the upper layer was carefully transferred to another vial. Two volumes of ethanol were added to the upper layer and the mixture was left at 4°C overnight, then centrifuged for 30 min at 4°C. The supernatant solution was evaporated to dryness using a centrifugal evaporator (Savant AES 2010).
To prevent double peaks due to the beta and alpha anomers of a single HMO, the HMOs were reduced to alditol form by adding 1.0 M sodium borohydride and incubating at 65°C for 1 hr (or 42°C overnight). HMOs were desalted and purified by graphitized carbon solid-phase extraction using 20% acetonitrile (ACN) and 40% ACN with 0.05% trifluoroacetic acid (TFA). Cartridges were cleaned and conditioned using nanopure water, 80% ACN, and nanopure water. Samples were then loaded to the cartridges, desalted with water, and eluted with 20 and 40% ACN. Eluted ACN fractions were evaporated to dryness. Samples were reconstituted with nanopure water prior to mass spectrometry analysis.
MALDI-FTICR MS Analysis
Mass spectra were recorded on an FT ICR MS with an external source ProMALDI (Varian, Palo Alto, CA) equipped with a 7.0 Tesla magnet, as described previously 30, 31. External calibration was performed using maltooligosaccharides 32, allowing mass accuracy of 10ppm or better. 2,5-dihydroxy-benzoic acid was used as a matrix (5 mg/100 IL in 50% ACN:H2O) for both positive and negative modes. Sodium chloride (0.01M in 50% ACN:H2O) was used as a cation dopant for the positive ion mode. The HMOs in the 20% fraction were detected as [M+Na]+ ions in the positive ionization mode while the HMOs in the 40% aqueous ACN fraction were analyzed in the negative ionization mode as [M−H]− ions.
Milk samples were also analyzed by nano LC Chip/TOF MS, which adds another dimension of separation based on how the oligosaccharides are retained in the porous graphitized carbon column. For example, a single HMO peak at m/z 1243 [M+Na]+ (neutral mass of 1220) on MALDI FT ICR MS analysis separates into five isomeric peaks with distinct retention times on nano LC Chip/TOF MS analysis: MFLNH I (monofucosyllacto-N-hexaose) at 17.70 min, MFLNH III at 17.29 min, MFpLNH IV (monofucosyl paralacto N hexaose) at 15.64 min, IFLNH I (fucosyl paralacto N hexaose) at 21.55 min, and IFLNH III at 18.44 min 6.
Nano-HPLC Chip/TOF MS Analysis
HMO fractions were pooled and analyzed using an Agilent 6200 Series HPLC Chip/TOF MS system as described previously 23. Briefly, separation was performed using a binary gradient solvent system consisting of A: 3% ACN in 0.1% formic acid solution, and B: 90% ACN in 0.1% formic acid solution. The column was initially equilibrated and eluted at a flow rate of 0.4 IL for nanopump and 4 IL for capillary pump. The gradient ran for 65 min and was programmed as follows: 2.5 20min: 0% 16% B; 20 30 min: 16% 44%B; 30 35min: B increased to 100%; 35 45 min: continue at 100% B; and 45 65 min: 0% B to equilibrate the chip column before next sample injection.
Data Preparation and Statistical Analysis
Identification of HMOs was performed based on accurate masses and retention times using the HMO library developed by Wu et al. 6, 29 Fucosylation and sialylation percentage values were calculated by adding abundances of all fucosylated and sialylated oligosaccharide species, respectively, and normalizing the values with the total HMO abundance per sample. Mono-, di-, tri- and tetrafucosylation was determined by adding the abundances of oligosaccharides containing 1, 2, 3, and 4 fucose residues, respectively, and normalizing the values either with the total HMO abundance or total HMO fucosylation abundance. Percentages of 2′ fucosyllactose and 3 fucosyllactose were determined by normalizing against the total HMO abundance. Percentages of 3 fucosyllactose below the detection limit of 0.0010% were replaced with a value of 0.00005%. Reported results were substantively the same as results from alternative imputation procedures, including replacing nondetectable values with (i) the minimum (0.000%) or (ii) maximum (0.0010%) possible nondetectable value or with (iii) randomly imputed values within the nondetectable range.
Due to the small sample size and variable number of samples from different mothers, we used heterogenous variance mixed effects models to model the mean levels and variances in HMO abundance values over time in mothers delivering at term and preterm 33. Mean levels were modeled with fixed effects for gestation group (full term vs. preterm) and post menstrual age (PMA) at collection (specified as a linear term to assess over time changes and centered at 40 weeks to aid in interpretation) and with interactions of the fixed effects included if statistical significance testing indicated that over time changes in mean levels varied by group. The variation of the marginal residuals (deviations of the actual observations from the fixed effects mean model) was modeled as the sum of mother specific random effects (to account for between mother differences in mean marginal residual levels) and conditional residual errors (to account for within mother, over time variations). Models were specified with gestation group specific variance components and estimated by restricted maximum likelihood estimation using Version 9.2 of the SAS System 34. Levene’s testing procedure was used to compare full and preterm mothers on between mother and within mother, over time variance components, with the procedure applied to absolute values of empirical best linear unbiased predictions of the random effects and to absolute values of estimated conditional residuals, respectively 33. To aid interpretation of the amount of between mother and within mother variation, the group specific mean absolute deviations (MAD) compared by Levene’s test are reported for select outcomes. Group specific adjusted standard deviations represent the square root of the sum of the variance components estimated in mixed effects model.
RESULTS
Preterm Milk and Term Milk HMO Profiling
Table 1 summarizes preterm milk samples obtained from fifteen mothers who delivered infants from 23 to 30 weeks of gestation. Some mothers provided several samples across lactation, giving a total of 41 milk samples. Seven mothers delivered preterm infants with birth weight <1000 grams and eight mothers delivered infants with birth weight 1000 1500 grams. Table 2 presents similar data for 23 human milk samples obtained from seven mothers who delivered full term (after 37 weeks).
Table 1. Percentage of LNT, fucosylation and sialylation in the free HMOs in milk samples collected from fifteen mothers who delivered pre-term.
ID = premature mother number, GA = gestational age at birth, PMA = postmenstrual age (the gestational age at birth plus the chronological age in weeks at the time of milk collection). %Fuc and %Sia are the percent fucosylation and sialylation, respectively, normalized to the total HMO abundance per sample. Percent monofucosylation, difucosylation, trifucosylation and tetrafucosylation are in columns marked as %Mono Fuc, %Di Fuc, %Tri Fuc, %Tetra Fuc correspondingly and refer to the percentages of HMOs with one, two, three and four fucoses, respectively. The percent abundance of 2′-fucosyllactose, an oligosaccharide secretor status marker bearing the alpha-1,2-fucose, is shown in the column %2′-FL, along with its observed neutral mass from nano-LC Chip/TOF MS and mass error in parts per million (ppm). ND=not detected.
| ID | BW (GM) |
GA (WEEKS) |
PMA (WEEKS) |
% SIA |
% FUC |
% MONO FUC |
% DI FUC |
% TRI FUC |
% TETRA FUC |
% 2′FL |
2′ FL MASS |
2′ FL MASS ERROR, PPM |
3-FL | LNT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3P | 570 | 25 | 32 | 26.1% | 58.0% | 71.4% | 25.2% | 3.4% | 0.0% | 0.1% | 490.1898 | 0.204 | 0.14% | 21.2% |
| 4P | 1120 | 27 | 28 | 23.7% | 32.9% | 80.2% | 16.5% | 3.1% | 0.1% | 0.0% | 490.1899 | 0.408 | 0.03% | 43.1% |
| 31 | 27.9% | 26.2% | 83.4% | 14.8% | 1.8% | 0.0% | 0.1% | 490.1897 | 0.000 | 0.03% | 48.8% | |||
| 6P | 1170 | 27 | 31 | 8.4% | 52.8% | 84.0% | 14.8% | 1.2% | 0.0% | 5.6% | 490.1915 | 3.672 | ND | 35.0% |
| 33 | 7.6% | 61.2% | 75.9% | 21.3% | 2.5% | 0.3% | 7.4% | 490.1912 | 3.060 | ND | 26.0% | |||
| 34 | 6.2% | 71.6% | 79.8% | 16.6% | 3.3% | 0.3% | 19.1% | 490.1901 | 0.816 | 0.06% | 19.3% | |||
| 7P | 1450 | 30 | 31 | 3.6% | 30.0% | 87.3% | 12.5% | 0.3% | 0.0% | ND | ND | ND | 0.01% | 52.5% |
| 32 | 9.9% | 58.0% | 76.6% | 21.0% | 2.3% | 0.0% | 0.3% | 490.1891 | 1.224 | ND | 33.9% | |||
| 34 | 8.3% | 36.6% | 67.9% | 30.1% | 2.0% | 0.0% | 0.3% | 490.1883 | 2.856 | 0.06% | 51.5% | |||
| 36 | 17.3% | 34.1% | 75.6% | 20.3% | 4.1% | 0.0% | 0.7% | 490.1900 | 0.612 | ND | 35.9% | |||
| 9P | 1030 | 26 | 31 | 21.7% | 58.6% | 63.6% | 34.4% | 1.9% | 0.1% | 16.1% | 490.1903 | 1.224 | 0.04% | 13.1% |
| 10P | 920 | 28 | 30 | 16.3% | 26.1% | 67.1% | 30.0% | 2.4% | 0.1% | 0.6% | 490.1907 | 2.040 | ND | 28.9% |
| 32 | 6.2% | 62.5% | 87.6% | 10.9% | 1.5% | 0.1% | 16.3% | 490.1895 | 0.408 | ND | 26.9% | |||
| 34 | 3.9% | 72.5% | 86.3% | 12.5% | 1.1% | 0.1% | 28.4% | 490.1893 | 0.816 | ND | 20.6% | |||
| 36 | 6.5% | 30.7% | 60.0% | 37.7% | 2.2% | 0.1% | 3.9% | 490.1895 | 0.408 | ND | 46.3% | |||
| 13P | 570 | 24 | 28 | 17.9% | 49.5% | 55.0% | 41.2% | 3.5% | 0.3% | 7.9% | 490.1906 | 1.836 | ND | 23.6% |
| 30 | 10.3% | 54.0% | 58.3% | 35.8% | 4.5% | 0.7% | 5.4% | 490.1903 | 1.224 | ND | 20.1% | |||
| 14P | 1105 | 28 | 30 | 22.0% | 33.1% | 80.8% | 16.8% | 2.3% | 0.1% | 0.1% | 490.1902 | 1.020 | ND | 29.1% |
| 31 | 13.8% | 45.6% | 84.0% | 15.4% | 0.6% | 0.0% | 2.6% | 490.1883 | 2.856 | ND | 28.7% | |||
| 32 | 22.7% | 37.1% | 72.5% | 21.9% | 4.4% | 0.2% | 0.3% | 490.1908 | 2.244 | ND | 29.7% | |||
| 16P | 660 | 25 | 29 | 12.7% | 26.6% | 90.1% | 9.2% | 0.7% | 0.0% | 0.1% | 490.1908 | 2.244 | ND | 48.3% |
| 31 | 6.4% | 56.0% | 80.5% | 18.2% | 1.4% | 0.0% | 0.2% | 490.1892 | 1.020 | ND | 32.5% | |||
| 33 | 15.6% | 35.1% | 50.4% | 41.8% | 7.6% | 0.1% | 0.1% | 490.1900 | 0.612 | ND | 39.8% | |||
| 35 | 13.3% | 31.6% | 62.9% | 31.7% | 5.4% | 0.0% | 0.2% | 490.1902 | 1.020 | ND | 42.3% | |||
| 18P | 930 | 25 | 26 | 25.1% | 36.7% | 81.4% | 14.6% | 3.8% | 0.2% | 0.2% | 490.1904 | 1.428 | ND | 31.1% |
| 28 | 21.2% | 25.3% | 84.0% | 14.5% | 1.6% | 0.0% | 0.2% | 490.1899 | 0.408 | ND | 37.6% | |||
| 20P | 900 | 29 | 31 | 6.1% | 80.8% | 81.4% | 16.3% | 2.0% | 0.3% | 40.4% | 490.1894 | 0.612 | ND | 11.7% |
| 37 | 5.0% | 84.2% | 78.9% | 19.2% | 1.7% | 0.2% | 40.7% | 490.1898 | 0.204 | ND | 10.1% | |||
| 21P | 1060 | 29 | 31 | 10.5% | 67.9% | 86.0% | 11.8% | 2.0% | 0.1% | 29.8% | 490.1898 | 0.204 | 0.09% | 14.9% |
| 33 | 12.8% | 68.5% | 82.1% | 14.8% | 3.0% | 0.2% | 27.1% | 490.1882 | 3.060 | 0.04% | 14.7% | |||
| 22P | 490 | 23 | 27 | 11.7% | 70.0% | 71.4% | 26.1% | 2.2% | 0.3% | 34.3% | 490.1879 | 3.672 | ND | 13.3% |
| 26P | 1070 | 28 | 31 | 11.4% | 67.6% | 77.0% | 18.4% | 4.2% | 0.4% | 25.8% | 490.1899 | 0.408 | 0.05% | 16.7% |
| 33 | 9.8% | 70.8% | 76.1% | 18.3% | 5.0% | 0.5% | 23.5% | 490.1897 | 0.000 | 0.02% | 14.3% | |||
| 35 | 8.9% | 78.0% | 63.4% | 29.9% | 5.6% | 1.1% | 28.5% | 490.1908 | 2.244 | 0.11% | 12.8% | |||
| 36 | 9.1% | 62.4% | 69.8% | 27.0% | 3.2% | 0.0% | 0.2% | 490.1897 | 0.000 | 0.51% | 23.4% | |||
| 44 | 8.8% | 83.8% | 62.5% | 31.1% | 5.3% | 1.1% | 34.0% | 490.1907 | 2.040 | 0.36% | 7.7% | |||
| 27P | 940 | 28 | 31 | 11.8% | 58.9% | 73.3% | 25.3% | 1.4% | 0.0% | 0.1% | 490.1889 | 1.632 | 0.16% | 23.2% |
| 33 | 11.1% | 58.6% | 73.8% | 24.1% | 2.1% | 0.0% | 0.4% | 490.1891 | 1.326 | 0.37% | 20.8% | |||
| 35 | 8.5% | 61.3% | 69.9% | 23.9% | 2.1% | 0.3% | 10.4% | 490.1913 | 3.264 | 0.09% | 12.3% | |||
| 38 | 12.4% | 53.6% | 63.8% | 32.3% | 3.9% | 0.1% | 0.0% | 490.1898 | 0.102 | 0.44% | 30.7% | |||
| 41 | 7.7% | 56.3% | 59.9% | 34.7% | 5.2% | 0.2% | 0.1% | 490.1893 | 0.816 | 0.71% | 30.2% |
Table 2. Percentage LNT, fucosylation and sialylation in the HMOs in milk samples from seven mothers who delivered at term.
Column labels are the same as in Table 1, except that age is the age of the infant at milk collection.
| ID | BW (GM) |
GA (WEEKS) |
AGE (WEEKS) |
% SIA |
% FUC |
% MONO FUC |
% DI FUC |
% TRI FUC |
% TETRA FUC |
% 2′FL |
2′FL MASS |
2′FL MASS ERROR, PPM |
3′-FL | LNT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2F | 3288 | 40 | 0 | 8.5% | 59.6% | 79.0% | 17.6% | 2.8% | 0.6% | 21.4% | 490.1881 | 3.264 | 0.10% | 19.2% |
| 1 | 20.1% | 57.3% | 74.3% | 20.4% | 4.5% | 0.8% | 14.0% | 490.1881 | 3.264 | 0.09% | 13.5% | |||
| 2 | 15.5% | 52.5% | 79.6% | 18.2% | 1.7% | 0.5% | 10.9% | 490.1864 | 6.732 | ND | 14.8% | |||
| 3 | 15.9% | 58.8% | 76.8% | 18.9% | 3.6% | 0.6% | 16.0% | 490.1884 | 2.652 | 0.16% | 13.6% | |||
| 9 | 10.3% | 62.1% | 80.8% | 18.0% | 1.2% | 0.0% | 23.6% | 490.1876 | 4.284 | 0.11% | 12.4% | |||
| 13 | 7.8% | 70.3% | 73.2% | 22.2% | 3.8% | 0.8% | 24.7% | 490.1886 | 2.244 | 0.75% | 15.5% | |||
| 35 | 10.8% | 67.1% | 75.1% | 21.9% | 2.7% | 0.4% | 23.3% | 490.1878 | 3.876 | 1.52% | 16.1% | |||
| 3F | >2500 | 39 | 0 | 19.3% | 53.6% | 79.0% | 17.6% | 2.8% | 0.6% | 21.4% | 490.1881 | 3.264 | 0.03% | 19.1% |
| 1 | 24.8% | 55.2% | 69.6% | 22.7% | 6.4% | 1.3% | 16.9% | 490.1878 | 3.876 | 0.04% | 16.8% | |||
| 2 | 20.8% | 60.2% | 74.7% | 19.4% | 4.9% | 0.9% | 16.7% | 490.1879 | 3.672 | 0.08% | 15.1% | |||
| 5 | 18.4% | 63.8% | 73.1% | 20.1% | 5.5% | 1.3% | 19.5% | 490.1877 | 4.080 | 0.12% | 14.7% | |||
| 9 | 21.5% | 66.5% | 69.4% | 23.9% | 5.1% | 1.6% | 21.3% | 490.1882 | 3.060 | 0.25% | 14.0% | |||
| 16 | 19.3% | 61.7% | 76.5% | 18.3% | 4.5% | 0.8% | 20.6% | 490.1881 | 3.264 | 0.21% | 15.1% | |||
| 4F | 3815 | 40 | 1 | 20.8% | 56.8% | 77.8% | 17.2% | 4.3% | 0.6% | 14.1% | 490.1875 | 4.488 | 0.04% | 15.8% |
| 2 | 18.3% | 62.7% | 79.0% | 17.6% | 2.8% | 0.6% | 17.0% | 490.1878 | 3.876 | 0.07% | 15.4% | |||
| 14 | 11.7% | 66.7% | 77.1% | 19.1% | 3.2% | 0.6% | 25.1% | 490.1884 | 2.652 | 0.33% | 15.7% | |||
| 21 | 13.4% | 68.3% | 74.1% | 21.4% | 3.8% | 0.7% | 29.1% | 490.1880 | 3.468 | 0.51% | 15.8% | |||
| 30 | 10.2% | 70.0% | 74.6% | 21.9% | 3.0% | 0.5% | 33.3% | 490.1881 | 3.264 | 0.90% | 16.3% | |||
| 39 | 11.7% | 70.0% | 71.4% | 26.1% | 2.2% | 0.3% | 34.3% | 490.1879 | 3.672 | 0.82% | 15.3% | |||
| 10F | 3318 | 40 | 19 | 4.9% | 75.8% | 79.2% | 17.1% | 3.3% | 0.3% | 39.4% | 490.1898 | −0.204 | 0.25% | 14.6% |
| 11F | 4000 | 38 | 10 | 9.0% | 68.0% | 61.7% | 30.9% | 6.1% | 1.2% | 10.2% | 490.1900 | −0.612 | 0.55% | 24.8% |
| 12F | 4000 | 40 | >0 | 6.2% | 68.9% | 62.9% | 33.2% | 3.4% | 0.5% | 19.1% | 490.1904 | −1.428 | 0.93% | 21.5% |
| 13F | 3227 | 40 | >0 | 10.4% | 65.1% | 70.7% | 26.0% | 3.3% | 0.0% | 0.1% | 490.1898 | −0.204 | 1.16% | 24.0% |
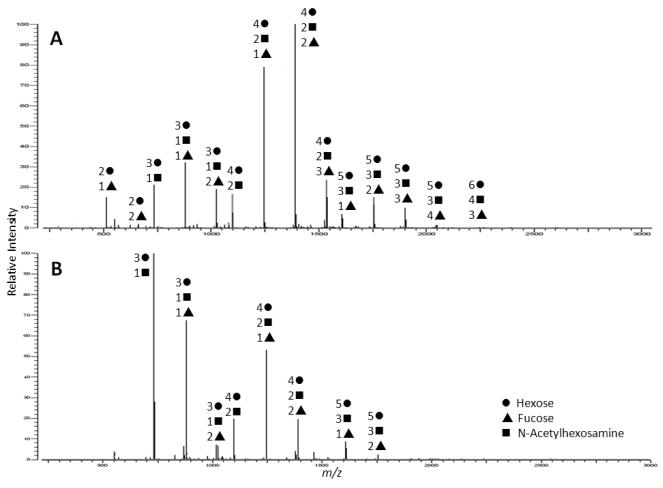
MALDI FT ICR MS analysis suggested that many of the preterm milk samples had less diversity (fewer HMO peaks) than the milk from term mothers. Figure 1 demonstrates two typical spectra for HMOs isolated from term (Fig 1A) and preterm (Fig 1B) milk. Nano LC Chip/TOF MS analysis demonstrated possible HMO peaks for 41 milk samples from women delivering preterm (mean 146 peaks; range 79 293) and 23 term milk samples (mean 200 peaks range 100 279). Adjusted mean levels of total number of HMOs at gestational age 40 were similar in both groups. One HMO, lacto N tetraose (LNT), was significantly less abundant in term milk than preterm milk (adjusted full term vs. preterm mean difference 7.25%, 95% CI: 14.45% to 0.04%). LNT is a core HMO structure and is metabolized by strains of bifidobacteria that are common in infants but not by strains found in adults35. A higher degree of variability for LNT was seen in the samples from mothers delivering preterm than at term. The mean absolute deviation (MAD) is a robust measure of this variability. The LNT MADs for between mother (7.8% vs 3.4%; p=0.005) and within mother errors (4.6% vs 1.1%; p<0.001) were statistically significantly higher in the preterm group compared to the full term group.
Figure 1.
MALDI FT ICR (+) MS profiles of neutral human milk oligosaccharides isolated from human milk of mothers who delivered A) at term and B) pre term. Samples were analyzed in the positive ionization mode.
There were also differences between the two groups in the changes in number of HMOs over time. The milk samples from mothers delivering preterm increased by an average of 4 HMOs per week (pre term slope= 4.2, 95% confidence intervals (CI): 0.1 to 8.3), while the milk samples from mothers delivering at term decreased by an average of 2 HMOs per week (full term slope = 2.1, 95% CI: 3.9 to – 0.3). This pattern may suggest that the time of term delivery is the acme of new HMO production, similar to fetal and placental processes influencing rate of growth (growth of the fetus slows and even regresses if delivery is delayed significantly-beyond the due date). The increase in the preterm group may have clinical relevance; for example, an infant born at 26 weeks and receiving her mother’s milk would potentially receive about 56 new HMOs by 40 weeks PMA that were not present at birth. Variation in number of HMOs within the two groups (pre vs. full term) was not significantly different either between mothers or within mothers over time.
Total Fucosylation and Sialylation in Preterm and Term Milk
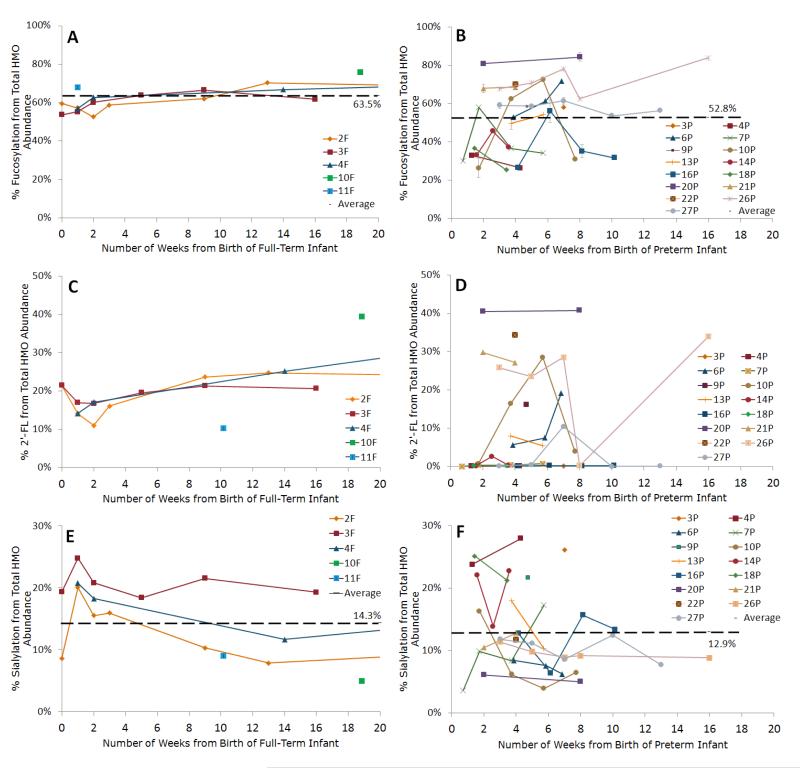
Figures 2 and 3 present the percentage of fucosylated HMOs for individual mothers delivering preterm and at term. There was no difference in percentage of fucosylated HMOs between term and preterm milk samples (adjusted term vs. preterm mean difference at 40 weeks PMA = 6.6%, 95% CI: 3.2% to 16%). For percentage fucosylation, the between mothers MAD was 10% for the preterm mothers and 2.7% for the term mothers (p < 0.001), and the within mothers MAD (individual variation over time) was 7.3% and 2.7% respectively (p < 0.001).
Figure 2.
Line graphs of the percent fucosylation of human milk oligosaccharides (HMOs) in (A) term and (B) preterm milk during the course of lactation; percent abundance of 2′ fucosyllactose, an HMO containing an α 1,2 fucose in (C) term and (D) preterm milk; and percent sialylation of HMOs in (E) term and (F) preterm milk. Y values are expressed as percentage from the total HMO abundance normalized per sample. Each color represents a different mother. Averages are shown as black broken lines. Average percent values for preterm milk shown in B, D, and F include all preterm milk samples (N=41) listed in Table 1. Average percent values for term milk shown in A, C, and E include all term milk samples (N=23) listed in Table 2. Error bars are expressed as standard error of the mean (SEM).
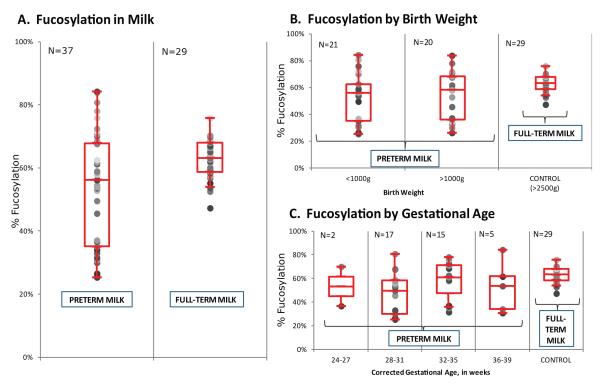
Figure 3.
Fucosylation in human milk of mothers who delivered preterm and full term infants. Preterm milk transitioning into full term milk, i.e. preterm milk collected after 36 weeks of corrected gestation were not included in the plot. Box whisker plots of fucosylation in HMOs by A) gestational age, B) birth weight, and C) corrected gestational age at the time of milk collection. N = number of samples. Error bars are expressed as standard error of the mean (SEM).
Percentage of sialylated HMOs in milk from women delivering at term and preterm and changes in individual mothers over time are presented in Figure 2E and 2F. The percentage of sialylated HMOs did not differ between term and preterm milk (adjusted full term vs. preterm mean difference at 40 weeks PMA = 0.8%, 95% CI: 5.4% to 6.9%), nor was there a significant difference in variability (MADs 4.6% and 3.6% for between mother premature and term milk and 2.4% and 2.3% for within mother premature and term milk respectively).
Mono-, Di-, Tri-, and Tetrafucosylation in Preterm and Term Milk
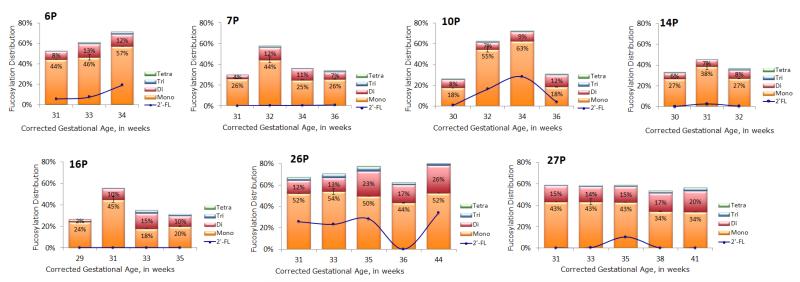
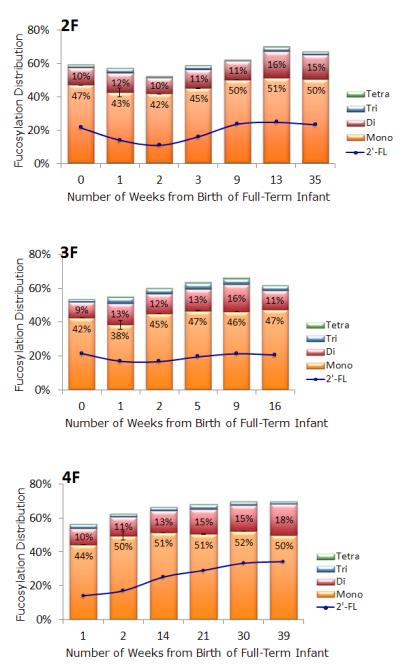
Fucosylation was further investigated to determine the differences in abundance of mono, di, tri, and tetrafucosylation (based on the number of fucose residues), as shown in Tables 1 and 2. Abundance of mono, di, tri, and tetrafucosylation was similar for both preterm and full term milk. Changes in degree of fucosylation over time for individual mothers providing multiple specimens are demonstrated in Figure 4 (preterm) and Figure 5 (term).
Figure 4.
Bar graphs of mono, di, tri and tetrafucosylation of free HMOs in human milk of seven mothers who delivered prematurely. The line graph inset in each bar graph is the percent abundance of 2′ fucosyllactose present in the sample. Only mothers who had three or more samples were graphed. The height of each bar represents the total fucosylation in the sample. Error bars are expressed as standard error of the mean (SEM).
Figure 5.
Bar graphs of mono, di, tri and tetrafucosylation of free HMOs in human milk of three mothers who delivered at term. The line graph inset in each bar graph is the percent abundance of 2′ fucosyllactose present in the sample. Only mothers who had three or more samples were graphed. The height of each bar represents the total fucosylation in the sample. Error bars are expressed as standard error of the mean (SEM).
Secretor status (2′-Fucosyllactose) in Preterm and Term Milk
To determine “milk secretor status” we monitored an oligosaccharide containing an α 1,2 fucose, 2′ fucosyllactose (2′ FL), with a calculated neutral mass of 490.1897 (less than 4ppm error) and retention time of 11.720 ± 0.75 minutes across the lactation samples. In Figures 4 and 5, the line represents percentage of 2′ FL.
The percentage of HMOs that were 2′ FL did not differ significantly between milk from mothers delivering preterm and term at 40 weeks PMA (adjusted term vs. preterm mean difference = 0.1%, 95% CI: 10% to 12%). For 2′ FL, the between mothers MADs were not significantly different, but the within mothers MADs were 4.2% for the preterm group and 2.6% for the term group (p < 0.05), suggesting fluctuation in the secretor status of preterm milk.
Twenty two of twenty three samples from women delivering at term gave an intensity of 2′ FL greater than 5% (mean 20.5%, SD 9.7%) consistent with secretor status. One full term mother 13F had 0.1% of 2′ FL in her free milk oligosaccharides, suggesting that she is a non secretor.
The preterm milk samples showed a predominance of low abundances for 2′ FL. Six of the fifteen mothers delivering preterm had a consistently low 2′ FL abundance of <5% across lactation. Another set of six mothers delivering preterm had 2′ FL abundance greater than 5% at all time points, ranging from 5.4% to 40.7%. Three preterm mothers (10P, 26P, and 27P) had both low (<5%) and high percent abundances of 2′ FL at different time points.
The secretor status of two preterm mothers, 26P and 27P, was verified using their saliva and blood type. Mother 26P was found to be a Group O Secretor. The unexpectedly low value obtained at week 36 was re analyzed to confirm the veracity of 0.2% abundance of 2′ FL and the same value was found. Such a low abundance in the one specimen is unexpected in a secretor mother. Mother 27P was found to be a Group AB non secretor. Four out of five samples gave low abundances of 2′ FL, as expected for mothers who are non secretors; the single elevated value was unexpected. Variation over time in individual mothers is presented in Figure 2C and D.
These results suggest that “milk secretor status” is not consistent in women delivering preterm. In contrast, 3 FL which is not influenced by secretor status, did not differ significantly between milk from mothers delivering preterm and term at 40 weeks PMA. For 3 FL, within mothers MADs were not significantly different; the between mother MADs were low in both groups, but differed significantly (0.38% for term and 0.04% for preterm, p=0.01).
DISCUSSION
Preterm vs. Term Milk
Milk from mothers delivering preterm differs from that of mothers delivering at term in content of protein2 , fat 36, lactose 37, calcium 38, and a variety of bioactive molecules 39. Many of these differences persist as long as 8 weeks after birth suggesting that premature delivery significantly alters lactation. We have demonstrated more abundant LNT and higher variability in LNT production in preterm milk. LNT is highly abundant in human milk and one of the primary drivers of colonization with infant strains of bifidobacteria. These data also show a higher degree of variation in percentage of fucosylated HMOs, both between women and over time, in women delivering preterm compared to women delivering full term. Premature infants are at increased risk for infections due to immaturity of many facets of innate and adaptive immunity. Given the postulated importance of fucosylated HMOs in pathogen binding, fluctuations in fucosylated HMOs in mothers’ milk may further increase this risk. This high degree of variability in HMO fucosylation is further evidence of dysregulation in the “premature breast.”
On the contrary, differences in the amount of between mother and within mother, over time variation in sialylation of HMOs between women delivering at term and preterm were not statistically significant. This differs from previous observations 27; the small number of samples in the current study raises the possibility of a type 2 error. Given the postulated role of sialic acid in neurodevelopment 27, further exploration of immaturity in regulation of HMO sialylation is warranted.
Secretor Status and Preterm Milk
Secretor status varies with racial and ethnic background with Japanese and western European women about 80% secretors 22 and African and Bangladeshi women about 60% secretors 40. The presence of an α 1,2 linked fucosylated HMO, 2′ fucosyllactose (2′ FL), in human milk indicates that the mother is a secretor 41, 42. A large study of human milk as a marker of secretor status showed 100% of samples from Mexico and Sweden and 46% of samples from the Philippines suggestive of maternal secretor status 8. The race/ethnicity of the women in this study were as follows: term 6 white non Hispanic, 1 white Hispanic; preterm 10 white non Hispanic, 3 white Hispanic, 2 black non Hispanic. Our sample size is too small to confirm any racial associations with secretor status. In the present study nano HPLC Chip/TOF MS provided an easy method to distinguish 2′ FL from its isomer 3 fucosyllactose (bearing an α 1,3 linked fucose) since their retention times differ by at least ten minutes. The unexpectedly high number of apparent non secretors among women delivering preterm and the lack of consistency in “milk secretor status” over time in patients 10P, 26P, and 27P support the hypothesis that fucosylation of HMOs is inconsistent in women delivering preterm. The alternative hypothesis (that non secretor status increases a woman’s risk of delivering preterm) seems less likely given the inconsistency in the three patients noted.
The addition of fucose residues in oligosaccharides relies on the genetically determined activities of three or more distinct fucosyltransferases 22. One of the fucosyltransferases, the α-1,2-fucosyltransferase, is found only in secretors, i.e. people who secrete soluble blood group substances (A, B, and O(H)) that match their specific blood group type in their body fluids such as tears, milk or saliva 41. Mothers who are non secretors typically do not express (or express in minute amounts) α-1,2-fucosyloligosaccharides in their milk or other body secretions 43.
Secretor status in premature infants has recently been described as a risk factor for late onset sepsis, necrotizing enterocolitis, and death 44. The mechanism by which secretor status is protective in this high risk population is not clear, but the recent findings that secretor status affects the bifidobacterial composition of the intestinal microbiota in adults 45, that premature infants generally have very low numbers of fecal bifidobacteria 46, and that probiotic bifidobacteria appear to be protective against necrotizing enterocolitis in premature infants 47 suggest the hypothesis that non secretor premature infants enrich a less protective constellation of gut microbes than secretor premature infants. In term infants, milk from secretor mothers is protective against diarrhea, including that caused by campylobacter and calicivirus 48. Non secretor adults have been found to be more susceptible to bacterial infections 49, 50, fungal infections 51, and autoimmune diseases 50, and to be protected against norovirus 52 (though the associations with norovirus gastroenteritis 53, 54 and ankylosing spondylitis 55 have been questioned).
SIGNIFICANCE
Preterm infants have the disadvantages of having an immature immune system, a leaky gut, and an intestinal microbiota that differs markedly from that of the term infant 46. They are denied the protective effects of placental transfer of maternal antibodies and of swallowed amniotic fluid that occurs mostly during the third semester of pregnancy 56. Thus, they are prone to opportunistic infections and nutrient deficiencies at the time of maximal growth and development. Necrotizing enterocolitis is a common and devastating disease in this population; the risk of developing this disease is increased by formula feeding 57 and by an intestinal microbiota that is dominated by proteobacteria 58, 59. Given the postulated protective effects of fucosylated HMOs, hypofucosylation may increase risk of infection as well. In the case of preterm delivery, the immature breast appears unable to effectively regulate the expression of fucosylated HMOs. Correlation of degree of fucosylation with the infant’s fecal microbiota would be a challenging but helpful analysis.
Donor human milk is mostly provided by women who delivered at term. Provision of pasteurized donor human milk decreases the risk of necrotizing enterocolitis compared to formula 60,3. Pasteurization decreases human milk B cell and T cell numbers, soluble CD14, immunoglobulins, lactoferrin iron binding activity, and lysozyme activity, but not oligosaccharide composition or quantity 61. The observation that fucosylation in HMOs of preterm milk is highly variable raises the provocative question of whether the more consistent HMO composition of donor human milk from mothers who delivered at term may be one of the mechanisms of benefit to premature infants. Perhaps even more compelling would be to identify non secretor premature infants so that these infants of exceptionally high risk could be provided with highly fucosylated HMOs with the explicit goal of preventing necrotizing enterocolitis, late onset sepsis and death.
ACKNOWLEDGEMENTS
This work was supported in part by grants from the Eunice K. Shriver National Institute of Child Health and Human Development Grant HD059127, the National Center for Research Resources, a component of the National Institutes of Health, Grant UL1 RR024146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The infants of mothers delivering at term were enrolled in observational studies funded by the US Department of Agriculture NRI CSREES Award 2008 35200 18776 and supported by the UC Davis Foods for Health Institute.
ABBREVIATIONS
- 2′-FL
2′-fucosyllactose
- 3-FL
3-fucosyllactose
- CI
confidence intervals
- ELISA
enzyme linked immunosorbent assay
- HMO
human milk oligosaccharide
- LC
liquid chromatography
- LNT
lacto N tetraose
- MAD
mean absolute deviation
- MALDI FT ICR
matrix assisted laser desorption/ionization Fourier transform ion cyclotron resonance
- MS
mass spectrometry
- m/z
mass to charge ratios
- PMA
post menstrual age
- TOF
time of flight
Footnotes
Notes The authors declare no competing financial interest.
REFERENCES
- 1.Ziegler EE. Meeting the Nutritional Needs of the Low Birth Weight Infant. Annals of Nutrition and Metabolism. 2011;58:8–18. doi: 10.1159/000323381. [DOI] [PubMed] [Google Scholar]
- 2.Bauer J, Gerss J. Longitudinal analysis of macronutrients and minerals in human milk produced by mothers of preterm infants. Clin Nutr. 2011;30(2):215–20. doi: 10.1016/j.clnu.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kohlendorfer U. Kiechl, Chan GM, Blanco CL, Abrams S, Cotten CM, Laroia N, Ehrenkranz RA, Dudell G, Cristofalo EA, Meier P, Lee ML, Rechtman DJ, Lucas A. An exclusively human milk based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk based products. J Pediatr. 2010;156(4):562–7. e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 4.Arslanoglu S, Moro GE, Ziegler EE. The Wapm Working Group On, N., Optimization of human milk fortification for preterm infants: new concepts and recommendations. J Perinat Med. 2010;38(3):233–8. doi: 10.1515/jpm.2010.073. [DOI] [PubMed] [Google Scholar]
- 5.Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH, German JB, Freeman SL, Killeen K, Grimm R, Lebrilla CB. A strategy for annotating the human milk glycome. J Agric Food Chem. 2006;54(20):7471–80. doi: 10.1021/jf0615810. [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Tao N, German JB, Grimm R, Lebrilla CB. Development of an annotated library of neutral human milk oligosaccharides. J Proteome Res. 2010;9(8):4138–51. doi: 10.1021/pr100362f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao N, Wu S, Kim J, An HJ, Hinde K, Power ML, Gagneux P, German JB, Lebrilla CB. Evolutionary glycomics: characterization of milk oligosaccharides in primates. J Proteome Res. 2011;10(4):1548–57. doi: 10.1021/pr1009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erney RM, Malone WT, Skelding MB, Marcon AA, Leyer K. M. Kleman, O’Ryan ML, Palacios G. Ruiz, Hilty MD, Pickering LK, Prieto PA. Variability of human milk neutral oligosaccharides in a diverse population. Journal of Pediatric Gastroenterology and Nutrition. 2000;30(2):181–192. doi: 10.1097/00005176-200002000-00016. [DOI] [PubMed] [Google Scholar]
- 9.LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB, Mills DA, German JB. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem. 2007;55(22):8914–9. doi: 10.1021/jf0710480. [DOI] [PubMed] [Google Scholar]
- 10.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18(7):298–307. doi: 10.1016/j.tim.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newburg DS, Palacios G. M. Ruiz, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 12.Andersson B, Porras O, Hanson LA, Lagergard T, Eden C. Svanborg. Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153(2):232–7. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 13.Coppa GV, Bruni S, Zampini L, Galeazzi T, Facinelli B, Capretti R, Carlucci A, Gabrielli O. Oligosaccharides of human milk inhibit the adhesion of Listeria monocytogenes to Caco 2 cells. Ital. J. Pediatr. 2003;29:61–68. [Google Scholar]
- 14.Cravioto A, Tello A, Villafan H, Ruiz J, del Vedovo S, Neeser JR. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp 2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163(6):1247–55. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- 15.Palacios G. M. Ruiz, Cervantes LE, Ramos P, Munguia B. Chavez, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. J Biol Chem. 2003;278(16):14112–20. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Huang P, Zhong W, Tan M, Farkas T, Morrow AL, Newburg DS, Palacios G. M. Ruiz, Pickering LK. Human milk contains elements that block binding of noroviruses to human histo blood group antigens in saliva. J Infect Dis. 2004;190(10):1850–9. doi: 10.1086/425159. [DOI] [PubMed] [Google Scholar]
- 17.Crane JK, Azar SS, Stam A, Newburg DS. Oligosaccharides from human milk block binding and activity of the Escherichia coli heat stable enterotoxin (STa) in T84 intestinal cells. J Nutr. 1994;124(12):2358–64. doi: 10.1093/jn/124.12.358. [DOI] [PubMed] [Google Scholar]
- 18.Otnaess AB, Laegreid A, Ertresvag K. Inhibition of enterotoxin from Escherichia coli and Vibrio cholerae by gangliosides from human milk. Infect Immun. 1983;40(2):563–9. doi: 10.1128/iai.40.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Yu B, Karim M, Hu H, Sun Y, McGreevy P, Petocz P, Held S, Miller J. Brand. Dietary sialic acid supplementation improves learning and memory in piglets. Am J Clin Nutr. 2007;85(2):561–9. doi: 10.1093/ajcn/85.2.561. [DOI] [PubMed] [Google Scholar]
- 20.Wang B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. 2009;29:177–222. doi: 10.1146/annurev.nutr.28.061807.155515. [DOI] [PubMed] [Google Scholar]
- 21.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14(8):351–60. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr. 2000;20:699–722. doi: 10.1146/annurev.nutr.20.1.699. [DOI] [PubMed] [Google Scholar]
- 23.Ninonuevo MR, Perkins PD, Francis J, Lamotte LM, LoCascio RG, Freeman SL, Mills DA, German JB, Grimm R, Lebrilla CB. Daily variations in oligosaccharides of human milk determined by microfluidic chips and mass spectrometry. J Agric Food Chem. 2008;56(2):618–26. doi: 10.1021/jf071972u. [DOI] [PubMed] [Google Scholar]
- 24.Coppa GV, Pierani P, Zampini L, Gabrielli O, Carlucci A, Catassi C, Giorgi PL. Lactose, oligosaccharide and monosaccharide content of milk from mothers delivering preterm newborns over the first month of lactation. Minerva Pediatr. 1997;49(10):471–5. [PubMed] [Google Scholar]
- 25.Coppa GV, Pierani P, Zampini L, Gabrielli O, Catassi C, Carlucci A, Giorgi PL. Carbohydrate content of milk from mothers delivering preterm newborns: Preliminary results. Rivista Italiana Di Pediatria-Italian Journal of Pediatrics. 1996;22(3):357–359. [Google Scholar]
- 26.Nakhla T, Fu D, Zopf D, Brodsky NL, Hurt H. Neutral oligosaccharide content of preterm human milk. Br J Nutr. 1999;82(5):361–7. doi: 10.1017/s0007114599001609. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Miller J.Brand, McVeagh P, Petocz P. Concentration and distribution of sialic acid in human milk and infant formulas. Am J Clin Nutr. 2001;74(4):510–5. doi: 10.1093/ajcn/74.4.510. [DOI] [PubMed] [Google Scholar]
- 28.Gabrielli O, Zampini L, Galeazzi T, Padella L, Santoro L, Peila C, Giuliani F, Bertino E, Fabris C, Coppa GV. Preterm Milk Oligosaccharides During the First Month of Lactation. Pediatrics. 2011;128(6):E1520–E1531. doi: 10.1542/peds.2011-1206. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Grimm R, German JB, Lebrilla CB. Annotation and structural analysis of sialylated human milk oligosaccharides. J Proteome Res. 2011;10(2):856–68. doi: 10.1021/pr101006u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Leoz ML, Young LJ, An HJ, Kronewitter SR, Kim J, Miyamoto S, Borowsky AD, Chew HK, Lebrilla CB. High mannose glycans are elevated during breast cancer progression. Mol Cell Proteomics. 2011;10(1):M110–002717. doi: 10.1074/mcp.M110.002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Leoz ML, An HJ, Kronewitter S, Kim J, Beecroft S, Vinall R, Miyamoto S, de Vere White R, Lam KS, Lebrilla C. Glycomic approach for potential biomarkers on prostate cancer: profiling of N linked glycans in human sera and pRNS cell lines. Dis Markers. 2008;25(4-5):243–58. doi: 10.1155/2008/515318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clowers BH, Dodds ED, Seipert RR, Lebrilla CB. Dual polarity accurate mass calibration for electrospray ionization and matrix assisted laser desorption/ionization mass spectrometry using maltooligosaccharides. Anal Biochem. 2008;381(2):205–13. doi: 10.1016/j.ab.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models. Second Edition SAS Institute; Cary, NC, USA: 2006. [Google Scholar]
- 34.SAS Institute Inc. Version 9.2 of the SAS System for Windows. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. SAS Institute Inc.; Cary, NC: 2008. Copyright © 2002. [Google Scholar]
- 35.Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. In vitro fermentation of breast milk oligosaccharides by Bifidobacterium infantis and Lactobacillus gasseri. Appl Environ Microbiol. 2006;72(6):4497–9. doi: 10.1128/AEM.02515-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puigmarti C. Molto, Castellote AI, Estrany X. Carbonell, Sabater M. C. Lopez. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clinical Nutrition. 2011;30(1):116–123. doi: 10.1016/j.clnu.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Coppa GV, Gabrielli O, Pierani P, Catassi C, Carlucci A, Giorgi PL. Changes in Carbohydrate Composition in Human Milk over 4 Months of Lactation. Pediatrics. 1993;91(3):637–641. [PubMed] [Google Scholar]
- 38.Friel JK, Andrews WL, Jackson SE, Longerich HP, Mercer C, McDonald A, Dawson B, Sutradhar B. Elemental composition of human milk from mothers of premature and full term infants during the first 3 months of lactation. Biological Trace Element Research. 1999;67(3):225–247. doi: 10.1007/BF02784423. [DOI] [PubMed] [Google Scholar]
- 39.Castellote C, Casillas R, Santana C. Ramirez, Cano F. J. Perez, Castell M, Moretones M. Gloria, Sabater M. C. Lopez, Franch A. Premature Delivery Influences the Immunological Composition of Colostrum and Transitional and Mature Human Milk. Journal of Nutrition. 2011;141(6):1181–1187. doi: 10.3945/jn.110.133652. [DOI] [PubMed] [Google Scholar]
- 40.Akhter S, Kibria G, Akhter N, Habibullah M, Islam S, Zakariah M. ABO and Lewis Blood Grouping with ABH Secretor and Non secretor Status: A Cross Sectional Study in Dhaka. Faridpur Med Coll J. 2011;6(1):38–40. [Google Scholar]
- 41.Grollman EF, Ginsburg V. Correlation between secretor status and the occurrence of 2′ fucosyllactose in human milk. Biochem Biophys Res Commun. 1967;28(1):50–3. doi: 10.1016/0006-291x(67)90404-4. [DOI] [PubMed] [Google Scholar]
- 42.Shen L, Grollman EF, Ginsburg V. An enzymatic basis for secretor status and blood group substance specificity in humans. Proc Natl Acad Sci U S A. 1968;59(1):224–30. doi: 10.1073/pnas.59.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newburg DS, Palacios G. M. Ruiz, Altaye M, Chaturvedi P, Derr J. Meinzen, Mde L. Guerrero, Morrow AL. Innate protection conferred by fucosylated oligosaccharides of human milk against diarrhea in breastfed infants. Glycobiology. 2004;14(3):253–63. doi: 10.1093/glycob/cwh020. [DOI] [PubMed] [Google Scholar]
- 44.Morrow AL, Derr J. Meinzen, Huang P, Schibler KR, Cahill T, Keddache M, Kallapur SG, Newburg DS, Tabangin M, Warner BB, Jiang X. Fucosyltransferase 2 Non Secretor and Low Secretor Status Predicts Severe Outcomes in Premature Infants. The Journal of Pediatrics. 2011;158(5):745–751. doi: 10.1016/j.jpeds.2010.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, Partanen J, Aranko K, Matto J. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 2011;6(5):e20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westerbeek EA, van den Berg A, Lafeber HN, Knol J, Fetter WP, van Elburg RM. The intestinal bacterial colonisation in preterm infants: a review of the literature. Clin Nutr. 2006;25(3):361–8. doi: 10.1016/j.clnu.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Alfaleh K, Anabrees J, Bassler D, Al Kharfi T. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011;(3):CD005496. doi: 10.1002/14651858.CD005496.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Morrow AL, Palacios G. M. Ruiz, Altaye M, Jiang X, Guerrero ML, Derr J. K. Meinzen, Farkas T, Chaturvedi P, Pickering LK, Newburg DS. Human milk oligosaccharides are associated with protection against diarrhea in breast fed infants. Journal of Pediatrics. 2004;145(3):297–303. doi: 10.1016/j.jpeds.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 49.Raza MW, Blackwell CC, Molyneaux P, James VS, Ogilvie MM, Inglis JM, Weir DM. Association between secretor status and respiratory viral illness. BMJ. 1991;303(6806):815–8. doi: 10.1136/bmj.303.6806.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackwell CC. The role of ABO blood groups and secretor status in host defences. FEMS Microbiol Immunol. 1989;1(6-7):341–9. doi: 10.1111/j.1574-6968.1989.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 51.Aly FZ, Blackwell CC, MacKenzie DA, Weir DM, Elton RA, Cumming CG, Sofaer JA, Clarke BF. Chronic atrophic oral candidiasis among patients with diabetes mellitus role of secretor status. Epidemiol Infect. 1991;106(2):355–63. doi: 10.1017/s0950268800048500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan M, Jin M, Xie H, Duan Z, Jiang X, Fang Z. Outbreak studies of a GII 3 and a GII 4 norovirus revealed an association between HBGA phenotypes and viral infection. J Med Virol. 2008;80(7):1296–301. doi: 10.1002/jmv.21200. [DOI] [PubMed] [Google Scholar]
- 53.Nordgren J, Kindberg E, Lindgren PE, Matussek A, Svensson L. Norovirus gastroenteritis outbreak with a secretor independent susceptibility pattern, Sweden. Emerg Infect Dis. 2010;16(1):81–7. doi: 10.3201/eid1601.090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bucardo F, Nordgren J, Carlsson B, Kindberg E, Paniagua M, Mollby R, Svensson L. Asymptomatic norovirus infections in Nicaraguan children and its association with viral properties and histo blood group antigens. Pediatr Infect Dis J. 2010;29(10):934–9. doi: 10.1097/INF.0b013e3181ed9f2f. [DOI] [PubMed] [Google Scholar]
- 55.Smith GW, James V, Mackenzie DA, Stewart J, Blackwell CC, Elton RA, Nuki G. Ankylosing spondylitis and secretor status: a re evaluation. Br J Rheumatol. 1997;36(7):778–80. doi: 10.1093/rheumatology/36.7.778. [DOI] [PubMed] [Google Scholar]
- 56.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–8. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 57.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–23. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. doi: 10.1038/ismej.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quigley MA, Henderson G, Anthony MY, McGuire W. Formula milk versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2007;(4):CD002971. doi: 10.1002/14651858.CD002971.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Ewaschuk JB, Unger S, Harvey S, O’Connor DL, Field CJ. Effect of pasteurization on immune components of milk: implications for feeding preterm infants. Appl Physiol Nutr Metab. 2011;36(2):175–82. doi: 10.1139/h11-008. [DOI] [PubMed] [Google Scholar]