Abstract
Purpose
To determine the feasibility and potential efficacy of a self-management program that combines cognitive-behavioral strategies with exercise for use by seniors with chronic back pain, and to assess for possible race/ethnicity differences in program impact.
Design/Methods
Sixty-nine participants attending senior centers in New York City enrolled in the 8-week group-based program, with approximately equal numbers of African Americans (n=24), Hispanics (n=25), and non-Hispanic whites (n=20) enrolling. Participants provided weekly input on their perceived understanding and usefulness of program components. Efficacy outcomes included pain-related disability, as measured by the Roland Morris Disability Questionnaire (RMDQ), pain intensity, pain self-efficacy, depressive symptom score, social activity, and functional status.
Results
Eighty percent of enrollees completed the program, and 84% of program participants indicated they did the weekly practice/homework exercises. Program content was rated as understandable and highly useful to participants. Significant decreases in RMDQ scores were found for non-Hispanic white (adjusted change score −3.53), African American (−3.89), and Hispanic (−8.45), participants. Significant improvements in all other efficacy outcomes (pain intensity, social activity, activities of daily living, depressive symptoms) were observed, but only for Hispanic participants.
Conclusions
These results confirm that implementation of the protocol in urban senior centers is feasible, and the program shows potential efficacy in affecting pain-related disability among a diverse population of older adults. The race/ethnicity differences observed in the current study merit further investigation.
INTRODUCTION
Chronic back pain (CBP) is a common health problem among older persons1 that is often associated with substantial disability and healthcare costs.2–5 Despite the prevalence of, and disability associated with CBP in older populations, effective treatment strategies remain inadequately defined. Analgesic medications are commonly used to treat CBP,6, 7 but this approach has significant limitations among older individuals because of the high prevalence of co-occurring comorbidities, as well as medication-related costs, side-effects, and risks.8–10 Developing effective nonpharmacologic treatments could possibly provide substantial benefit to many older persons with CBP.
A growing body of research11–15 has focused on the use of nonpharmacologic therapies for the treatment of chronic pain disorders including13 psychological (e.g., cognitive-behavioral) and physical therapy (e.g., exercise) interventions. Cognitive-behavioral therapy (CBT) is an intervention that seeks to enhance affected individuals’ control over pain using diverse psychological techniques.16 Standard CBT pain protocols teach individuals specific cognitive and behavioral skills to manage pain better; inform individuals regarding the effects that specific cognitions, emotions, and behaviors can have on pain; and emphasize the primary role that individuals can play in controlling their own pain and their adaptations to pain. Although CBT has proven efficacy for reducing pain and disability among persons with diverse chronic pain disorders17, 18 few older adults use cognitive-behavioral techniques for managing pain.6, 19, 20 Exercise therapy (ET) has the potential to reverse muscle atrophy and improve spinal flexibility, improve aerobic fitness, and reduce pain among older persons with CBP.21–23 One systematic review found strong evidence that ET (versus usual care) is effective for reducing pain and improving physical function among persons with CBP.21 Despite this evidence, relatively few older persons with CBP use exercise as a means of managing pain.6, 20, 24
In response to the above findings, the investigators developed an intervention that includes instruction in the use of both cognitive-behavioral (CB) and exercise techniques (ET) to manage CBP. The combined CBET protocol includes a discrete number of techniques that can be feasibly performed by most older, ambulatory adults (Table 1). As both protocol components encourage use of similar behavioral and cognitive pain coping skills, including behavioral activation, perceptions of self-efficacy, and personal mastery with regard to the management of pain, instructing individuals in the simultaneous use of CB and ET techniques should be mutually reinforcing.
Table 1.
Cognitive-behavioral & exercise techniques presented at each class.
| Session | Cognitive-behavioral content | Exercise/other content |
|---|---|---|
| 1 | Pain theories; Introduction to goal setting | Importance of exercise overview; Learn warm-up stretches |
| 2 | Goal setting – making specific plans to achieve goals Relaxation – Diaphragmatic breathing | Practice stretch exercises; Learn new set of stretches |
| 3 | Recognizing automatic thoughts and emotions | Review importance of body posture, Learn walking exercises, Learn technique(s) to monitor exercise intensity |
| 4 | Evaluating automatic thoughts; Use of positive thoughts; Visual imagery | Review body mechanics, Review and practice above exercises, introduce strength/balance exercises |
| 5 | Pleasant activity scheduling | Practice exercises listed above |
| 6 | Time-based pacing, Progressive muscle relaxation | Learn about importance of adequate hydration, Practice exercises listed above |
| 7 | Sleep hygiene | Practice exercises listed above |
| 8 | Overall review; Present strategies for program maintenance | Relapse prevention, Managing future pain flares, Practice exercises listed above |
In prior studies19, 25 we have demonstrated that older adults are very willing to engage in self-management programs for chronic pain that include both cognitive and exercise components. In the current study, we sought to establish the feasibility and potential efficacy of the CBET protocol among community-dwelling older adults with CBP. Because prior research has demonstrated race/ethnicity differences in types of self-care strategies used to manage pain,25–31 as well as varying levels of exposure to self-management programs for pain,25 we sought to determine whether treatment outcomes would vary as a function of participants’ race/ethnicity status.
METHODS
Study Setting
We partnered with six senior centers in New York City to assemble a race/ethnicity-stratified sample of older adults with CBP. All six centers were multi-purpose elder services agencies that provide members daily lunches and offer a wide range of services, including health promotion and disease prevention seminars, exercise classes, and social services. Two centers provided services to predominantly African American seniors; two served mainly older Hispanics; and the remaining two provided services to largely older non-Hispanic whites.
Sample Assembly
Because the protocol was developed for use as a group-based intervention, our target was to enroll approximately 12 participants from each center. This class size was selected because of concern that any larger number would compromise the instructor’s ability to address individual participants’ issues and concerns. Recruitment method varied by site. At all sites, senior center staff members posted flyers and made announcements about the program during regularly scheduled lunches. Investigators provided formal presentations to senior center clientele at 2 of the centers (at the recommendation of center directors). Seniors who responded to these recruitment methods were screened by research staff for eligibility status. Individuals were eligible to participate if they: 1) were 60 years of age or older; 2) spoke English or Spanish; 3) experienced chronic, non-cancer-related back pain, defined as providing an affirmative answer to the question “During the past three months, have you been bothered by pain/discomfort in your back on most days of every month”; 4) were cognitively intact, defined as a score of 5 or greater on a six-item cognitive screen;32 ; and 5) were approved for participation in the program by their primary physician. Individuals who answered ‘yes’ to item 3 were asked whether their pain was due to cancer. Individuals were not asked about the specific etiology or location of their CBP, so the sample consisted of individuals with CBP in various locations of the spine and of undetermined etiology.
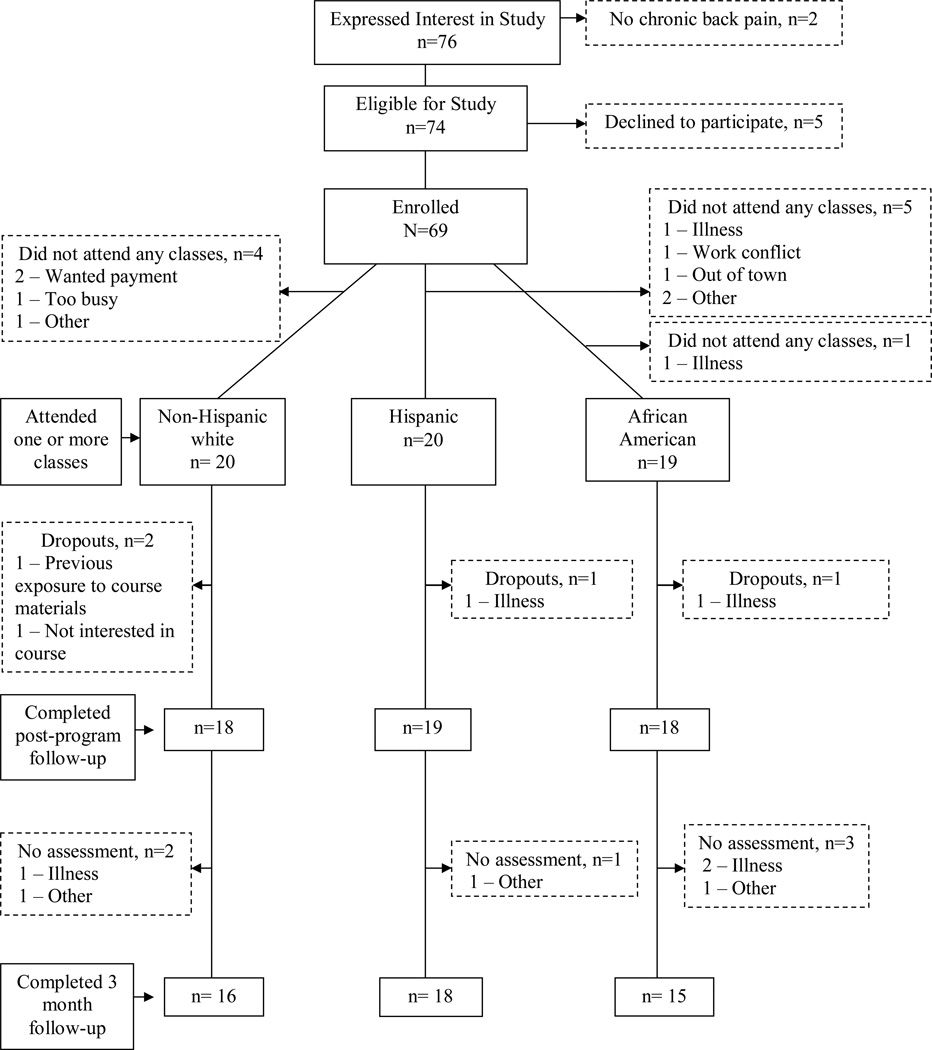
Seventy-six participants expressed interest in the study and were screened for eligibility; 2 did not have CBP and were excluded. Of the 74 eligible individuals, 69 (90%) provided written consent and enrolled in the study. Of these, 10 (4 non-Hispanic white, 5 Hispanic, and 1 African American) did not attend any classes (2 expected payment, 2 were ill, 1 was too busy, and 5 did not attend for other reasons), leaving 59 participants (18 non-Hispanic whites, 20 Hispanics, and 20 African Americans) who attended one or more of the classes.
Data Collection
Participants completed a face-to-face interview with trained research assistants to collect baseline data one week prior to the start of the program. A research assistant fluent in Spanish conducted interviews with Spanish-speaking participants using a professionally translated version of the instrument that was piloted for comprehension with Spanish speaking older adults and elder service providers (and forward-and-back translated prior to use). Face-to-face interviews were also conducted at week 9 (one week after the final class). Brief telephone interviews occurred weekly during the 8-week program and 3 months after the last class, as described below. The Weill Cornell Medical College Institutional Review Board approved the study.
Information on participants’ demographic status was obtained during the initial interview. We assessed for 17 self-reported physician-diagnosed chronic conditions. Participants were asked to estimate the number of years (or months) of CBP, number of days of restricted activity due to back pain in the past 30 days,29 and describe all pharmacologic and nonpharmacologic strategies they currently employed for pain-reduction purposes.
Primary Outcomes
The following measures were administered at the baseline assessment and again at week 9. Pain-related disability was assessed using the 24-item Roland-Morris Disability Questionnaire.33 Average pain intensity was ascertained using a 0 to 10 point numeric rating scale. Participants’ level of pain-self efficacy was assessed using the Pain Self-Efficacy Questionnaire (PSEQ), which asks participants to rate confidence in their ability to perform 10 activities (e.g., household chores, social activities) despite pain.34 Level of social activity during the previous month was ascertained using a previously validated protocol.35 The frequency with which participants engaged in 9 social activities (e.g., visiting friends, attending religious services) was determined, and a total social activity score (0–27) was calculated. Depressive symptomatology was measured using the 9-item Patient Health Questionnaire.36 Functional status was assessed by inquiring about participants’ self-reported ability to perform 4 basic (bathing, dressing, grooming, and walking) and four instrumental (getting to places out of walking distance, shopping, preparing meals, and housework) activities of daily living (ADL).25 A summary ADL score (0–16) was calculated for each participant.
Weekly phone calls
Research assistants contacted participants weekly by telephone (3–6 days after each session) to assess their perceived usefulness of each class using a 0 to 10 scale (0 = not useful at all and 10 = most useful.) Participants were also asked to report whether they 1) understood the materials presented during the class (yes/no), 2) had practiced the CB and ET techniques learned in each class (yes/no), and 3) experienced any problems practicing them (yes/no).
Three month follow-up assessments
Research assistants contacted participants by telephone and asked individuals whether they were 1) walking more than before they enrolled in the program (yes/no), 2) doing other physical exercises besides walking (yes/no), and 3) practicing the cognitive strategies (yes/no) and/or breathing exercises (yes/no) presented in the program.
Intervention
The protocol, entitled Moving Past the Pain (MPP), consists of 8 weekly sessions lasting approximately 90 minutes each, and was developed by an interdisciplinary team consisting of a physician with expertise in geriatric medicine, an exercise expert with masters-level training in exercise physiology and substantial prior experience working with group-based programs for older adults, and a physical therapist with expertise in geriatrics and instructional design.
The cognitive-behavioral component of the program was based upon the therapist manual from a previous CBT study.17 Since the program was designed to be delivered by an exercise expert rather than a psychologist and in a group setting rather than in one-to-one sessions, adaptation of the content was required. One topic, anger management, was excluded and the remaining eight content areas (e.g., goal setting, relaxation, activity pacing) were developed for presentation using lay language in a group setting. The instructor’s manual and participant handouts for the cognitive-behavioral content of the new program were reviewed by a psychologist with expertise in CBT. The psychologist reviewed the materials for accuracy and comprehensiveness. Based upon suggestions made by this reviewer, adjustments were made to the language and sequencing of content. The instructor’s manual and participant handouts were translated into Spanish by a professional translator. Program content for each session is shown in Table 1. The program’s exercise component was designed to promote overall fitness, while considering the limitations and precautions associated with CBP. The program included a brief warm up, stretching major muscle groups, mild resistance exercises, walking, and a brief cool down. The program was developed so that in early sessions the exercises were introduced gradually, with an emphasis on correct form. After an initial set of exercises was selected by the research team, a draft outline of the eight sessions was developed. Two physical therapists with expertise in geriatric physical therapy reviewed the exercise portion of the program and provided comments about its comprehensiveness, shared concerns and suggestions about the exercises selected, and made recommendations given the constraints of the program (e.g., limited access to equipment). Based upon this feedback, the program was modified to drop some exercises and provide options for others (e.g., a seated version of the program was developed).
Additional content important for general back care (e.g., body mechanics) and exercise (e.g., hydration) was interspersed throughout the 8 sessions. Handout materials were developed to highlight key take-home points and present homework exercises for the subsequent week. Homework consisting of specific exercises and practice of the pain self-management strategies was developed based upon content taught each week. To encourage homework completion, log sheets were provided for participants to record days that the exercises and self-management activities were completed.
The instructor, a bilingual exercise expert with experience working with older adults, was trained by the researchers to administer the protocol, and met weekly with a program developer (KB) to address concerns raised during sessions and to review content for upcoming sessions. While the content for each week varied, the overall format was the same. After the initial session, each session began with a return of the prior week’s homework log sheets, collection of the current homework log sheets, a brief review of the material covered in the prior week, and a discussion of the challenges and successes experienced by participants doing the homework exercises. Next, an overview of new content was provided, followed by a period of direct instruction on these topics. Instruction was followed by practice and/or discussion of the new content, and guiding questions were employed by the instructor to facilitate active participation in each session. After 20 to 30 minutes of direct instruction, the instructor led the group in exercises, correcting form and providing feedback as appropriate. After the exercise cool-down, there was another period of instruction with group participation, a review of homework for the following week, and closing comments. All of the sessions took place in space provided by the centers, and the instructor took attendance at each session.
Data Analysis
Descriptive statistics were calculated for each feasibility and efficacy outcome. Efficacy outcomes were analyzed in a general linear mixed model that included fixed classification factors for race/ethnicity (non-Hispanic white, Hispanic, African American), time (baseline versus follow-up), the interaction of these factors, and individuals as levels of a random classification factor. The model also included 5 additional variables specified a priori: age, sex, education, co-morbidity score, and number of pain co-therapies employed at baseline.
In specifying the race/ethnicity factor, we had a choice of using individual ethnicity or that of the center participants attended. Three participants whose ethnicity did not match the predominant ethnicity of their center were enrolled (all attended a center that provides services to mostly non-Hispanic whites). The analyses presented in this paper are based on the individual criterion (i.e., by race/ethnicity status). We also carried out analyses based on the center criterion; these did not differ substantively. Because race/ethnicity status and center are almost completely confounded, a fixed factor for center could not be included in addition to the race/ethnicity variable. (There were too few centers to include centers as levels of a random factor.)
Two race/ethnicity contrasts (non-Hispanic white vs. African American and non-Hispanic white vs. Hispanic) were specified a priori, given prior research showing substantial disparities in pain management as a function of race/ethnicity status37–39 A third contrast (Hispanic versus African American) is presented as additional information of potential interest.
RESULTS
Table 1 shows that Hispanic participants reported significantly fewer years of higher education, as compared to African American (p=0.008) and non-Hispanic white (p<0.001) participants. Although the non-Hispanic white (p=0.078) and African American (p=0.095) groups reported substantially longer durations of back pain relative to the Hispanic group; these differences approached, but did not achieve statistical significance. Although some race/ethnicity differences were noted in the relative proportions of each group that reported use of specific pain-reduction techniques (e.g., opioids), significant group differences were only found for the use of prayer (non-Hispanic white vs. Hispanic, p=0.001, and Hispanic vs. African American, p=0.001).
Of the 59 participants who attended at least one class, 4 (2 non-Hispanic white, 1 Hispanic, and 1 African American) dropped out (Figure 1). Overall attendance, defined as the total number of session attended by the 59 participants divided by the total number of possible sessions (59 × 8 = 472), was 82%. Thirty seven percent attended all 8 sessions, 46% attended 6 or 7 sessions, while the remaining 17% attended 5 or fewer sessions. Attendance did not vary significantly by center or by race/ethnicity status. Participants who dropped out (n=15) did not differ from program completers (n=54) with respect to baseline demographic, pain-related characteristics, and baseline scores on the efficacy outcomes.
Figure 1.
Study Sample Assembly and Follow-Up Data
Overall mean perceived usefulness scores (generated by averaging scores from all of the weekly phone calls) ranged from 8.1 (range, 6.8–9.4) for the non-Hispanic white to 8.9 (8.0–9.8) for the African American to 9.0 (8.3–9.8) for the Hispanic group. Ninety seven percent reported that they understood the materials presented at each class, and 84% reported doing the weekly practice and homework exercises. Few problems were reported practicing the exercise and cognitive techniques at home, with only 9% indicating any difficulty over the 8-week program.
Table 3 shows that all three groups experienced significant reductions in pain-related disability. Average pain scores decreased for non-Hispanic white (p=0.065) and Hispanic (p<0.001) participants, while significant improvements were also found in the areas of pain self-efficacy (p<0.001), social activities (p=0.041), functional status (p=0.002), and depressive symptomatology (p=0.004) for Hispanic participants.
Table 3.
Results stratified by race/ethnicity.
| Non-Hispanic White (n=16) |
Hispanic (n= 17) |
African American (n=19) |
All Participants (n=52) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | Δ Score |
T1 | T2 | Δ Score |
T1 | T2 | Δ Score |
T1 | T2 | Δ Score |
|
| Pain-related disability (0–24) | 12.15 (1.16) | 8.62 (1.29) | −3.53* | 14.00 (1.23) | 5.55 (1.35) | −8.45* | 13.21 (1.11) | 9.32 (1.14) | −3.89* | 13.12 (0.72) | 7.83 (0.77) | −5.29* |
| Pain intensity (0–10) | 5.82 (0.57) | 4.38 (0.66) | −1.44 | 7.41 (0.59) | 3.84 (0.68) | −3.57* | 5.54 (0.55) | 5.56 (0.57) | 0.02 | 6.26 (0.35) | 4.59 (0.39) | −1.67* |
| Pain self-efficacy (0–60) | 44.64 (2.80) | 47.81 (3.19) | 3.17 | 45.43 (2.96) | 58.02 (3.31) | 12.59* | 49.49 (2.70) | 47.53 (2.78) | −1.96 | 46.52 (1.74) | 51.12 (1.88) | 4.6‡ |
| Social activities (0–27) | 15.32 (1.19) | 15.61 (1.32) | 0.29 | 18.62 (1.27) | 20.91 (1.39) | 2.29‡ | 16.11 (1.15) | 17.00 (1.17) | 0.89 | 16.68 (0.75) | 17.84 (0.80) | 1.16 |
| Depressive symptoms (0–27) | 4.29 (0.87) | 3.08 (0.99) | −1.21 | 4.38 (0.92) | 1.53 (1.03) | −2.85† | 3.04 (0.84) | 4.88 (0.87) | 1.84‡ | 3.91 (0.54) | 3.16 (0.59) | −0.75 |
| Functional status (0–16) | 14.28 (0.48) | 14.66 (0.52) | 0.38 | 14.16 (0.52) | 15.46 (0.55) | 1.30† | 14.62 (0.46) | 14.83 (0.47) | 0.21 | 14.35 (0.30) | 14.98 (0.32) | 0.63* |
Results are reported as mean (standard deviation) values at baseline (T1) and at the 9 week follow-up (T2) for each race/ethnicity group and for the entire sample (N=69). Change (Δ) scores represent either an increase or a decrease in score from baseline to follow-up. Negative change scores for the back pain-related disability (Roland Morris), pain intensity, and depressive symptom (PHQ-9) measures reflect improved outcomes. Positive change scores for the remaining measures indicate improved outcomes. All results are adjusted for patient age, sex, education, total number of co-morbidities, and the number of pain co-therapies employed at baseline.
P < 0.001;
p <0.01;
p< 0.05.
When comparing outcomes across the 3 race/ethnicity groups, the results show that Hispanic participants evidenced significantly greater reductions in pain-related disability relative to both non-Hispanic white (p=0.004) and African American (p=0.004) participants. Reductions in pain intensity were also more pronounced among Hispanics versus non-Hispanic whites (p=0.051) and African Americans (p=0.001). Race/ethnicity effects were observed for pain self-efficacy, with Hispanic participants experiencing greater improvement relative to the other two groups (p’s=0.037 and 0.001, respectively). Finally, significant race/ethnicity differences were also found for depressive symptomatology (Hispanic vs. African American, p=0.001) and ADL function (Hispanic vs. African American, p=0.046), with Hispanic participants manifesting greater improvements in both outcomes.
Finally, no interactions (e.g., race/ethnicity and years of pain) were significant at the 0.05 level and the total number of classes attended was not associated with treatment outcomes (most likely due to the fact that the vast majority of subjects participated in 6 or more classes). Of the 52 participants interviewed at week 9, 45 (87%) were successfully contacted by phone 3 months later. Rates of follow-up did not vary significantly as a function of race/ethnicity status. Participants reported sustained practice of physical activities including walking more (endorsed by 66%) than they did before participating in the program and doing some type of exercise other than walking (80%). Approximately one in three (35%) reported continued use of the cognitive techniques, and 66% stated that they were continuing to do the breathing exercises learned in the class. The 3-month follow-up results did vary by race/ethnicity status: All of the Hispanic participants reported walking more, as compared to 58% of non-Hispanic white and 47% of the African American participants.
DISCUSSION
The results of this pilot study confirm the feasibility of implementing a combined cognitive-behavioral plus exercise therapy program for use by seniors with CBP attending urban senior centers. Eighty percent of enrollees completed the program, a completion rate comparable to that reported in other health-related programs offered at senior centers.40–42 Telephone survey data indicate that participants felt they understood and perceived the components of the program to be useful, and three month follow-up data show sustained utilization of several programmatic components. The most commonly sustained components were physical strategies (exercises and breathing), with lower utilization of the cognitive strategies (e.g., activity pacing, imagery, attention to thoughts and emotions). Physical exercises (stretching, strengthening, endurance) in the MPP program were introduced gradually, and reviewed in each subsequent session, with the instructor providing feedback on correct form. This consistent repetition may explain the high level of post-program adherence to the physical exercise component of self management. The cognitive strategies were each introduced once and reinforced only in the next session. Perhaps greater attentiveness to practicing/reinforcing each of the cognitive strategies would enhance adherence to those aspects of the program.
This study also provides preliminary data on the program’s effectiveness. Reductions in pain-related disability, as measured by the Roland Morris Disability Questionnaire (RMDQ), ranged from 3.53 to 8.45 points. Prior research indicates that RMDQ change scores of 2.5 or greater constitute clinically meaningful reductions in pain-related disability.43 Participants in all three race/ethnicity groups achieved outcomes well above this threshold level, indicating that the program may confer substantial benefits with respect to this outcome.
The most striking finding from our study is the difference in program outcomes as a function of participants’ race/ethnicity status. While all subgroups showed improvement in pain-related disability scores, Hispanic participants achieved the greatest program benefit, with significant improvements noted across all 6 efficacy outcomes. Because of the community-based nature of the program there are a myriad of factors that could have influenced program outcomes. Key factors are briefly discussed below.
Program Variables
The MPP program was intentionally delivered at senior centers with relatively homogenous clientele to assess for potential race/ethnicity differences in program uptake and impact. To control for program variability, the same instructor taught the course at all six locations. Aside from delivery in Spanish for the Hispanic sample, and the selection of music requested by participants (and employed during the exercise portions of the class), the program’s structure and content were the same across all six sites. However, the senior centers in which these programs were implemented varied in terms of the space and time available for the program, and the types of other health promotion programs offered. It is possible that these organizational factors could have impacted program outcomes, but were not measured in the current study.
Participant Factors
Physical Status
All participants had the same general diagnosis (chronic back pain) and we gathered information on number of comorbid conditions. However, we did not account for differences in physical abilities (strength, flexibility, endurance) that may have influenced an individual’s ability to participate in and benefit from a program like MPP.
Cultural Issues
A growing body of literature addresses the racial and ethnic differences in the reporting and impact of pain.37, 44–47 There is wide acceptance that cultural issues impact both the expression and impact of pain,48–51 but we could find no published research on the differential impact of a pain self-management program based upon racial/ethnic groups. Of particular interest in the present study is the high program impact on Hispanic participants. Traditional Hispanic values (e.g., simpatia, familism, allocentrism) may impact individual receptivity to health behavior changes required by self-management programs, affect interactions with program providers, and may contribute to resiliency in the face of health challenges.48 However, the extent to which these and other traditional values influenced participants’ health behaviors and health promotion outcomes is not clear. Hispanic participants were predominantly from the Dominican Republic and Puerto Rico, but we did not distinguish among the various Hispanic ethnicities. Due to wide diversity in the strength of traditional values and the degree of acculturation,48 future research focusing on an examination of intra-cultural differences and their impact on health outcomes is warranted.
Preferred Coping Strategies
Pre-program coping strategies may also have had an impact on program outcomes. Consistent with prior research, we found racial/ethnic differences in the coping strategies used.26–28, 46, 52, 53 Most notably, Hispanic participants were much more likely to report the use of prayer as a means of coping with pain relative to non-Hispanic white and African American participants. While prayer/hoping is perceived to be a helpful coping strategy,28, 52 other research has shown its use to be associated with higher levels of disability.53 In prior research conducted at the same senior centers, we found that Hispanics and African Americans had lower prior exposure to pain self-management programs than non-Hispanic whites.25 It is conceivable that Hispanic participants learned more new coping strategies than non-Hispanic whites, or that these strategies were more consistent with their preferences and lifestyle than participants in other the racial/ethnic groups.
Psychological Attributes
Persistent pain is associated with heightened psychological distress and depression,54–60 and psychological factors are associated with negative treatment outcomes.61, 62 Our sample had relatively low levels of depressive symptoms, but we saw a worsening of depressive symptomology in African American participants, despite improvement in level of pain-related disability. This finding is troubling, particularly because it is in conflict with findings for the other 2 groups. Mean scores for Hispanic and non-Hispanic whites were lower at follow-up, with Hispanic subjects showing a significant decrease in depressive symptoms.
Socioeconomic Status (SES)
Lower socioeconomic status is associated with diminished health status,63–65 and has been found to partially explain racial/ethnic differences in pain severity.45, 46 We included only educational level as a measure of SES, and found differences in level of education amongst the 3 racial/ethnic groups. Hispanics had lower educational levels than the other groups, yet achieved the highest level of benefit from the program. Designed as a no-cost program accessible through senior centers (rather than medical settings) with educational handouts written at the 4th grade reading level, the program was designed to minimize barriers to participation associated with lower SES. However, the impact of SES on chronic pain self-management programming warrants further study.
As a pilot study, this work has several limitations. First, the lack of a control group limits our ability to firmly attribute the observed changes to the intervention. The small sample size limits our ability to assess for potentially important interactions that may help to interpret our results. In addition, our sample is comprised of older adults with CBP who were interested in the self-management program. This implies a level of motivation that may not be generalized to the larger population of community-dwelling older adults with CBP. We used broad categories of race/ethnicity that did not allow us to distinguish potential intra-group differences. Another important consideration when examining these results is the potential for measurement error. While we used standardized outcome measures, which were translated by a professional translator, there is potential for cross-cultural and intra-cultural differences in the interpretation of terms, which may have influenced study outcomes.66 Finally, while we gained input on post-program utilization of pain management strategies, we did not attempt to ascertain its long-term impact on pain, pain-related disability, and mood scores, as well as its effects on social and ADL functioning.
In conclusion, this study establishes the feasibility of implementing a pain self-management program for older adults with CBP in urban senior centers. Our data suggest that the MPP program may be particularly effective in reducing pain-related disability and warrants further study. Further research is also needed to investigate potential racial/ethnic difference with respect to program impact.
Table 2.
Characteristics of Study Sample.
| Non- Hispanic White (n=24) |
Hispanic (n= 25) |
African American (n=20) |
All participants (n=69) |
|
|---|---|---|---|---|
| Demographics | ||||
| Mean (sad) age in years | 77.68 (7.89) | 73.64 (8.23) | 75.67 (7.33) | 75.57 (7.92) |
| % Female | 77% | 88% | 76% | 81% |
| % > 12 yrs education | 68% | 12% | 48% | 41% |
| Medical | ||||
| Mean (sd) comorbidity score (0–17) | 3.09 (1.66) | 3.40 (1.50) | 3.71 (1.31) | 3.40 (1.50) |
| Days of restricted activity due to back pain in past 30 days | ||||
| % ≤ 7 days | 95% | 84% | 92% | 88% |
| % ≥ 8 days | 5% | 16% | 8% | 12% |
| Mean (sd) yrs of back pain | 15.66 (17.40) | 8.56 (7.62) | 15.29 (14.30) | 12.90 (13.64) |
| Pain therapies employed | ||||
| % Using heat | 47% | 39% | 72% | 53% |
| % Using an NSAID | 47% | 59% | 44% | 50% |
| % Using acetaminophen | 53% | 53% | 44% | 50% |
| % Using prayer | 10% | 56% | 11% | 25% |
| % Using exercise | 32% | 11% | 23% | 24% |
| % Using an opioid | 18% | 6% | 17% | 13% |
| Mean (sd) number of pain therapies | 3.45 (1.99) | 2.76 (2.58) | 3.19 (1.40) | 3.12 (2.08) |
Column entries for quantitative variables are means along with (standard deviations); for dichotomous variables column entries are proportions.
NSAID = Nonsteroidal anti-inflammatory drug.
ACKNOWLEDGEMENTS
This research project was supported by grants from the National Institute of Nursing Research (R03 NR010093-01), the National Institute on Aging (an Edward R. Roybal Center Grant: 1 P30 AG022845-02), and the John A. Hartford Foundation (Hartford Center of Excellence in Geriatric Medicine Award).
References
- 1.Edmond SL, Felson DT. Prevalence of back symptoms in elders. J Rheumatol. 2000;27:220–225. [PubMed] [Google Scholar]
- 2.Leveille SG, Guralnik JM, Hochberg M, Hirsch R, Ferrucci L, Langlois J, et al. Low back pain and disability in older women: independent association with difficulty but not inability to perform daily activities. J Gerontol A Biol Sci Med Sci. 1999;54:M487–M493. doi: 10.1093/gerona/54.10.m487. [DOI] [PubMed] [Google Scholar]
- 3.Edmond SL, Felson DT. Function and back symptoms in older adults. J Am Geriatr Soc. 2003;51:1702–1709. doi: 10.1046/j.1532-5415.2003.51553.x. [DOI] [PubMed] [Google Scholar]
- 4.Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- 6.Barry LC, Gill TM, Kerns RD, Reid MC. Identification of pain-reduction strategies used by community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 2005;60:1569–1575. doi: 10.1093/gerona/60.12.1569. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer P, Bodner EV, Ritchie CS, Allman RM. Pain and pain medication use in community-dwelling older adults. Am J Geriatr Pharmacother. 2006;4:316–324. doi: 10.1016/j.amjopharm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Hernández-Díaz S, García-Rodríguez LA. Epidemiologic assessment of the safety of conventional nonsteroidal anti-inflammatory drugs. Am J Med. 2001;110(Suppl 3A):20S–27S. doi: 10.1016/s0002-9343(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 9.Bell GM, Schnitzer TJ. Cox-2 inhibitors and other nonsteroidal anti-inflammatory drugs in the treatment of pain in the elderly. Clin Geriatr Med. 2001;17:489. doi: 10.1016/s0749-0690(05)70082-3. [DOI] [PubMed] [Google Scholar]
- 10.Shimp LA. Safety issues in the pharmacologic management of chronic pain in the elderly. Pharmacotherapy. 1998;18:1313–1322. [PubMed] [Google Scholar]
- 11.Berman RL, Iris MA, Bode R, Drengenberg C. The effectiveness of an online mind-body intervention for older adults with chronic pain. J Pain. 2009;10:68–79. doi: 10.1016/j.jpain.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Ersek M, Turner JA, Cain KC, Kemp CA. Results of a randomized controlled trial to examine the efficacy of a chronic pain self-management group for older adults [ISRCTN11899548] Pain. 2008;138:29–40. doi: 10.1016/j.pain.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cochrane T, Davey RC, Matthes Edwards SM. Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis. Health Technology Assessment. 2005;9(31):iii. doi: 10.3310/hta9310. [DOI] [PubMed] [Google Scholar]
- 15.Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217–228. doi: 10.1093/geront/44.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Kerns RD, Otis JD, Marcus KS. Cognitive-behavioral therapy for chronic pain in the elderly. Clin Geriatr Med. 2001;17:503. doi: 10.1016/s0749-0690(05)70083-5. [DOI] [PubMed] [Google Scholar]
- 17.Reid MC, Otis J, Barry LC, Kerns RD. Cognitive-behavioral therapy for chronic low back pain in older persons: a preliminary study. Pain Med. 2003;4:223–230. doi: 10.1046/j.1526-4637.2003.03030.x. [DOI] [PubMed] [Google Scholar]
- 18.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 19.Austrian JS, Kerns RD, Reid MC. Perceived barriers to trying self-management approaches for chronic pain in older persons. J Am Geriatr Soc. 2005;53:856–861. doi: 10.1111/j.1532-5415.2005.53268.x. 2005. [DOI] [PubMed] [Google Scholar]
- 20.Barry LC, Kerns RD, Guo Z, Duong BD, Iannone LP, Carrington Reid M. Identification of strategies used to cope with chronic pain in older persons receiving primary care from a Veterans Affairs Medical Center. J Am Geriatr Soc. 2004;52:950–956. doi: 10.1111/j.1532-5415.2004.52263.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayden JA, van Tulder MW, Tomlinson G. Systematic review: strategies for using exercise therapy to improve outcomes in chronic low back pain. Ann Intern Med. 2005;142:776–785. doi: 10.7326/0003-4819-142-9-200505030-00014. [DOI] [PubMed] [Google Scholar]
- 22.Smidt N, de Vet HCW, Bouter LM, Dekker J, Arendzen JH, de Bie RA, et al. Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust J Physiother. 2005;51:71–85. doi: 10.1016/s0004-9514(05)70036-2. [DOI] [PubMed] [Google Scholar]
- 23.Mannion AF, Muntener M, Taimela S, Dvorak J. Comparison of three active therapies for chronic low back pain: results of a randomized clinical trial with one-year follow-up. Rheumatology (Oxford, England) 2001;40:772–778. doi: 10.1093/rheumatology/40.7.772. [DOI] [PubMed] [Google Scholar]
- 24.Pitkala KH, Strandberg TE, Tilvis RS. Management of nonmalignant pain in home-dwelling older people: a population-based survey. J Am Geriatr Soc. 2002;50:1861–1865. doi: 10.1046/j.1532-5415.2002.50517.x. [DOI] [PubMed] [Google Scholar]
- 25.Townley S, Papaleontiou M, Amanfo L, Henderson CR, Jr, Pillemer K, Beissner K, et al. Preparing to implement a self-management program for back pain in New York City senior centers: what do prospective consumers think? Pain Med. 2010;11:405–415. doi: 10.1111/j.1526-4637.2009.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross-Cultural Gerontol. 2008;23(4):339–347. doi: 10.1007/s10823-008-9071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albert SM, Musa D, Kwoh K, Silverman M. Defining optimal self-management in osteoarthritis: racial differences in a population-based sample. J Cross-Cult Gerontol. 2008;23:349–360. doi: 10.1007/s10823-008-9085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bill-Harvey D, Rippey RM, Abeles M, Pfeiffer CA. Methods used by urban, low-income minorities to care for their arthritis. Arthritis Care % Research. 1989;2:60–64. doi: 10.1002/anr.1790020207. [DOI] [PubMed] [Google Scholar]
- 29.Deyo RA, Battie M, Beurskens AJ, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine. 1998;23:2003–2013. doi: 10.1097/00007632-199809150-00018. [DOI] [PubMed] [Google Scholar]
- 30.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Silverman M, Nutini J, Musa D, King J, Albert S. Daily temporal self-care responses to osteoarthritis symptoms by older African Americans and whites. J Cross-Cult Gerontol. 2008;23:319–337. doi: 10.1007/s10823-008-9082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11:153–163. doi: 10.1016/j.ejpain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Cornoni-Huntley J, Ostfeld AM, Taylor JO, Wallace RB, Blazer D, Berkman LF, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging. 1993;5:27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green CR, Baker TA, Smith EM, Sato Y. The effect of race in older adults presenting for chronic pain management: a comparative study of black and white Americans. J Pain. 2003;4:82–90. doi: 10.1054/jpai.2003.8. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen M, Ugarte C, Fuller I, Haas G, Portenoy RK. Access to care for chronic pain: racial and ethnic differences. J Pain. 2005;6:301–314. doi: 10.1016/j.jpain.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Racial/ethnic differences in the prevalence and impact of doctor-diagnosed arthritis--United States, 2002. MMWR Morbidity And Mortality Weekly Report. 2005;54(5):119–123. [PubMed] [Google Scholar]
- 40.Li Y, Devault CN, Van Oteghen S. Effects of extended Tai Chi intervention on balance and selected motor functions of the elderly. Am J Chin Med. 2007;35:383–391. doi: 10.1142/S0192415X07004904. [DOI] [PubMed] [Google Scholar]
- 41.Dossa A, Capitman JA. Community-based disability prevention programs for elders: predictors of program completion. J Gerontol Soc Work. 2010;53(3):235–250. doi: 10.1080/01634370903558194. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick SE, Reddy S, Lommel TS, Fischer JG, Speer EM, Stephens H, et al. Physical activity and physical function improved following a community-based intervention in older adults in Georgia senior centers. J Nutrit Elderly. 2008;27:135–154. doi: 10.1080/01639360802060223. [DOI] [PubMed] [Google Scholar]
- 43.Kovacs FM, Abraira V, Royuela A, Corcoll J, Alegre L, Cano A, et al. Minimal clinically important change for pain intensity and disability in patients with nonspecific low back pain. Spine. 2007;32:2915–2920. doi: 10.1097/BRS.0b013e31815b75ae. [DOI] [PubMed] [Google Scholar]
- 44.Allen KD, Helmick CG, Schwartz TA, DeVellis RF, Renner JB, Jordan JM. Racial differences in self-reported pain and function among individuals with radiographic hip and knee osteoarthritis: the Johnston County Osteoarthritis Project. Osteoarthritis Cartilage. 2009;17:1132–1136. doi: 10.1016/j.joca.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: non-Hispanic whites, non-Hispanic blacks, and Hispanics. J Pain. 2007;8:75–84. doi: 10.1016/j.jpain.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education [corrected] [published erratum appears in J Pain 2006 Nov;7:869-70] J Pain. 2006;7:459–468. doi: 10.1016/j.jpain.2006.01.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green CR, Ndao-Brumblay SK, Nagrant AM, Baker TA, Rothman E. Race, age, gender influences among clusters of African American and White patients with chronic pain [corrected] [published erratum appears in J Pain 2005;6:707] J Pain. 2004;5:171–182. doi: 10.1016/j.jpain.2004.02.227. [DOI] [PubMed] [Google Scholar]
- 48.Gallo LC, Penedo FJ, Espinosa de los Monteros K, Arguelles W. Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J Pers. 2009;77:1707–1746. doi: 10.1111/j.1467-6494.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- 49.Kovacs FM, Muriel A, Abriaira V, Medina JM, Castillo Sanchez MD, Olabe J. The influence of fear avoidance beliefs on disability and quality of life is sparse in Spanish low back pain patients. Spine. 2005;30:E676–E682. doi: 10.1097/01.brs.0000186468.29359.e4. [DOI] [PubMed] [Google Scholar]
- 50.Madan I, Reading I, Palmer KT, Coggon D. Cultural differences in musculoskeletal symptoms and disability. Int J Epidemiol. 2008;37:1181–1189. doi: 10.1093/ije/dyn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCracken LM, Hoskins J, Eccleston C. Concerns about medication and medication use in chronic pain. J Pain. 2006;7:726–734. doi: 10.1016/j.jpain.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Kemp CA, Ersek M, Turner JA. A descriptive study of older adults with persistent pain: use and perceived effectiveness of pain management strategies [ISRCTN11899548] BMC Geriatrics. 2005;5:12. doi: 10.1186/1471-2318-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and white patients. Pain Med. 2005;6:88–98. doi: 10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- 54.James NT, Miller CW, Brown KC, Weaver M. Pain disability among older adults with arthritis. Journal of Aging and Health. 2005;17(1):56–69. doi: 10.1177/0898264304272783. 2005/02/01/ [DOI] [PubMed] [Google Scholar]
- 55.Alschuler KN, Theisen-Goodvich ME, Haig AJ, Geisser ME. A comparison of the relationship between depression, perceived disability, and physical performance in persons with chronic pain. Eur J Pain. 2008;12:757–764. doi: 10.1016/j.ejpain.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Arnow BA, Hunkeler EM, Blasey CM, Lee J, Constantino MJ, Fireman B, et al. Comorbid depression, chronic pain, and disability in primary care. Psychosom Med. 2006;68:262–268. doi: 10.1097/01.psy.0000204851.15499.fc. [DOI] [PubMed] [Google Scholar]
- 57.Asghari A, Julaeiha S, Godarsi M. Disability and depression in patients with chronic pain: pain or pain-related beliefs? Arch Iran Med. 2008;11(3):263–269. [PubMed] [Google Scholar]
- 58.Börsbo B, Peolsson M, Gerdle B. Catastrophizing, depression, and pain: correlation with and influence on quality of life and health - a study of chronic whiplash-associated disorders. J Rehabil Med. 2008;40:562–569. doi: 10.2340/16501977-0207. [DOI] [PubMed] [Google Scholar]
- 59.Bair MJ, Wu J, Damush TM, Sutherland JM, Kroenke K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70:890–897. doi: 10.1097/PSY.0b013e318185c510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gureje O, Ademola A, Olley BO. Depression and disability: comparisons with common physical conditions in the Ibadan study of aging. J Am Geriatr Soc. 2008;56:2033–2038. doi: 10.1111/j.1532-5415.2008.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill JC, Lewis M, Sim J, Hay EM, Dziedzic K. Predictors of poor outcome in patients with neck pain treated by physical therapy. Clin J Pain. 2007;23:683–690. doi: 10.1097/AJP.0b013e3181468e67. [DOI] [PubMed] [Google Scholar]
- 62.Crisp R. Depression and occupational disability in five diagnostic groups: a review of recent research. Disabil Rehabil. 2007;29:267–279. doi: 10.1080/09638280600835267. [DOI] [PubMed] [Google Scholar]
- 63.Latza U, Kohlmann T, Deck R, Raspe H. Can health care utilization explain the association between socioeconomic status and back pain? Spine. 2004;29:1561–1566. doi: 10.1097/01.brs.0000131435.56714.15. [DOI] [PubMed] [Google Scholar]
- 64.Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis. 2009;68:1591–1595. doi: 10.1136/ard.2008.093088. [DOI] [PubMed] [Google Scholar]
- 65.Moffett JAK, Underwood MR, Gardiner ED. Socioeconomic status predicts functional disability in patients participating in a back pain trial. Disabil Rehabil. 2009;31(10):783–790. doi: 10.1080/09638280802309327. [DOI] [PubMed] [Google Scholar]
- 66.Ramírez M, Ford ME, Stewart AL, Teresi JA. Measurement issues in health disparities research. Health Serv Res. 2005;40:1640–1657. doi: 10.1111/j.1475-6773.2005.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]