Abstract
The hypocotyl of Arabidopsis is well suited for the analysis of cell elongation because it elongates without cell division. We have isolated a new class of recessive mutants, petit1 (pet1), which are defective in aspects of hypocotyl elongation. The short-hypocotyl phenotype of pet1 is caused by shortened cells. The cells of the elongation zone of the hypocotyl are often deformed. pet1 also shows defects in elongation of the roots, flower stalk, leaves, petals, pedicels, and siliques, and these defects cannot be repaired by the application of auxin, gibberellin, brassinolide, or an inhibitor of ethylene biosynthesis. The short-hypocotyl phenotype of pet1 is pronounced only in growth medium supplemented with sucrose, which has promotive effects on hypocotyl elongation. In pet1 this effect is much reduced, causing the sucrose-dependent short-hypocotyl phenotype of pet1. pet1 accumulates more soluble sugars than the wild type and also shows more intensive iodo-starch staining in the cotyledon and hypocotyl. These results indicate that PETIT1 is involved in a sugar-dependent elongation process that may include correct assembly of expanding cell wall architecture.
The process of cell elongation is a critical aspect of morphogenesis in plants, and is thought to be regulated by several intrinsic and extrinsic factors. Over the years, physiological and biochemical studies have revealed many of the components involved in the process (Ray, 1987). Several of the known phytohormones act as intrinsic regulators of cell elongation: GA, auxin, and BR have a stimulatory function, whereas ethylene, ABA, and cytokinin have an inhibitory function (Davies, 1995). Coordinated interplay of these hormones triggers cell wall expansion through the synthesis of new wall components, their regulated incorporation into the existing architecture, and the loosening of the structure. Using biochemical methods, hydrolases and xyloglucan glycosyltransferases have been identified as potential wall-loosening enzymes (Fry, 1993; Nishitani, 1995). Expansins have also been identified as molecules that actually confer extensibility to isolated cell walls and seem to act on a matrix polymer that is tightly bound to the surface of cellulose microfibrils (Cosgrove, 1997).
Molecular genetics provides a powerful tool for the dissection of many developmental processes in plants, including cell elongation. For example, dwarf mutants have been analyzed with respect to cell elongation, and it has been shown that many of them are defective in the biosynthesis of phytohormones. GA-deficient mutants were identified in many species, including Arabidopsis and pea, and used to clarify pathways of GA biosynthesis and function in cell elongation. The corresponding genes that encode the enzymes required for GA biosynthesis have been successfully cloned (Hedden and Kamiya, 1997). BR-deficient mutants have also been identified in Arabidopsis and pea, and the DET2, CPD, and DIM/CBB1/DWF1 genes of Arabidopsis, which code for an enzyme involved in BR biosynthesis, have been cloned (Takahashi et al., 1995; Kauschmann et al., 1996; Li et al., 1996; Szekeres et al., 1996). An auxin-deficient mutation, uzu, so far identified only in barley, affects the cell-elongation process through defects in loosening of the cell wall (Inouhe et al., 1982; Sakurai and Kuraishi, 1984).
Mutations with a dwarf phenotype affecting cell wall components have also been identified. In Arabidopsis mur1 is completely deficient in the cell wall polysaccharide Fuc. In elongating stem segments of mur1 plants, the force required to break their primary walls upon longitudinal stretching is less than one-half of that in wild-type plants, but the overall anatomy of the segments and the wall thickness are not obviously altered, indicating that mur1 has a defect in the intrinsic mechanical properties of the walls (Reiter et al., 1993). Recently, the MUR1 gene was cloned and found to encode an isoform of GDP-d-Man-4,6-dehydratase, which catalyzes the first step in the de novo synthesis of GDP-l-Fuc (Bonin et al., 1997). A few mutants with complex changes in the monosaccharide composition of their cell walls have also been found to be dwarfs (Reiter et al., 1997). Overexpression of an Arabidopsis gene, TINY, causes a dwarf phenotype. It was recently identified by activation tagging and shown to encode a protein with homology to a class of transcription factors (Wilson et al., 1996); however, the target of the TINY gene remains unknown. The Arabidopsis RHD3 gene is also required for regulated cell enlargement and has been shown to encode an evolutionarily conserved protein with GTP-binding motifs. The RHD3 product may function in vacuole biogenesis and may control cell enlargement by increasing the size of vacuoles (Wang et al., 1997). The Arabidopsis SABRE gene is involved in a pathway antagonistic to the ethylene pathway for cell enlargement and has been shown to encode a novel protein conserved in several plant species (Aeschbacher et al., 1995).
To identify new components involved in cell elongation, we isolated a series of mutants defective in hypocotyl elongation. The hypocotyl of Arabidopsis is a good system for the analysis of cell elongation because after germination it elongates without cell division (Desnos et al., 1996; Gendreau et al., 1997). Taking advantage of simple hypocotyl elongation, many loci involved in the ethylene response and photomorphogenesis have been discovered (Chory, 1993; Ecker, 1995). Here we describe a new locus, PETIT1 (PET1), which is essential for cell elongation in various organs such as the hypocotyl, root, flower stalk, leaf, petal, pedicel, and silique. The growth defect of the mutant was only obvious on medium containing Suc, which had promotive effects on hypocotyl elongation in Arabidopsis; these effects were not seen in pet1. In addition, we found that the pet1 defect was often associated with a deformation of hypocotyl structure. The growth analysis of pet1 mutants in the present study suggests an unexpected relationship between cell wall synthesis and the control by Suc of the processes of cell elongation.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis ecotype Landsberg erecta was used as the wild type. Seeds mutagenized with fast neutrons were purchased from Lehle Seeds (Round Rock, TX). Mutants that were back-crossed twice were used for phenotypic characterization.
Seeds were surface sterilized with 1.5% (v/v) sodium hypochlorite and 0.02% (v/v) Triton X-100 for 5 min with vigorous shaking, and then washed several times with sterile water. Seeds chilled at 4°C for 2 to 4 d in water were usually plated onto one-half-strength Murashige-Skoog medium supplemented with Gamborg B5 vitamins and 1% Suc (pH 5.7–5.8). The plates were illuminated with continuous white light (43 W m−2) for 24 h at 22°C to induce germination. In the present study the age of seedlings is described as the time after transfer from 4°C to 22°C. Seeds were sometimes sown onto rock wool surrounded with a 1:1 (v/v) mixture of vermiculite and Metro-Mix 350 (Scotts-Sierra Horticultural Co., Marysville, OH) in a pot and grown at 22°C under continuous light. Plants were subirrigated with water or 1000-fold-diluted Hyponex (Murakami-Bussan, Tokyo, Japan) every 2 d.
Light Sources
White light (43 W m−2) was obtained from five 40-W white fluorescent light bulbs (FL40SS W/37, Sanyo, Tokyo, Japan). Dim white light (0.42 W m−2) was obtained from the same white fluorescent light bulbs filtered through a black acrylic sheet (no. 909, Takiron, Osaka, Japan). Broad-band green light (3.8 W m−2) was obtained from five 20-W white fluorescent light bulbs (FL20SS EX-N/18, Matsushita, Osaka, Japan) filtered through a green plastic film (maximum wavelength = 512 nm, maximum transmittance = 45.7%, one-half bandwidth = 69 nm). Fluence rates were measured with an optometer (model 370, Graseby Optronics, Orlando, FL).
Measurement of Length
The length of plant organs was measured from an image of 15 to 30 seedlings taken with a CCD (charge-coupled device) camera and captured to a desktop computer as described previously (Kurata and Yamamoto, 1997).
Microscopy
For scanning electron microscopy, seedlings were fixed overnight in FAA (5% [v/v] acetic acid, 45% [v/v] ethanol, and 5% [v/v] formaldehyde), and then dehydrated in a graded ethanol series at room temperature. Isoamyl acetate was then gradually substituted for the ethanol and the seedlings were critical-point dried in liquid CO2. After mounting of individual samples on stubs for scanning electron microscopy, they were sputter-coated with gold using an ion sputter coater (model JFC-1100, Jeol), and analyzed using a scanning electron microscope (model JSM-T20, Jeol). For light microscopy, seedlings were fixed overnight in FAA and dehydrated as described above. Completely dehydrated samples were embedded in Technovit 7100 (Kulzer, Wehrheim, Germany) according to the method of Tsukaya et al. (1993). Sections 5 μm thick were cut with Histoknives (Kulzer) on a microtome (model RM2135, Leica), affixed to glass slides, and stained with 0.1% (w/v) toluidine blue at room temperature for 1 min. Specimens were examined with a microscope (Axioplan, Zeiss) and photographed under bright-field illumination. For measurements of number or length of cells, seedlings were examined with the light microscope after immersion in India ink.
Chemical Assays
Anthocyanin was extracted from 20 seedlings, as described by Peters et al. (1989), and the amount was estimated from the A535 value. Total chlorophyll was estimated as described previously (Kurata and Yamamoto, 1997).
Soluble sugars were determined enzymatically according to the method of Heim et al. (1993). They were extracted from whole seedlings with 80% (v/v) ethanol at 80°C for 2 h, and after evaporation were dissolved in sterile water. Suc, Glc, and Fru were determined enzymatically using sugar-determination kits (Boehringer Mannheim). For iodo-starch staining, seedlings were immersed in 100% ethanol to extract chlorophyll. Bleached samples were stained in 0.2 n HCl containing 5.7 mm I2 and 43.3 mm KI (Casper et al., 1985), washed in 100% ethanol, and soaked in water.
Genetic Mapping
The PET1 locus was mapped using a combination of CAPS and RFLP molecular markers (Konieczny and Ausubel, 1993) and morphological markers. pet1-1 was outcrossed with the Columbia (Col-0) ecotype, and the F2 generation was analyzed. In this population, the erecta mutation on chromosome 2 was found to be linked to pet1. CAPS analysis with mi238 was carried out using genomic DNA prepared from 46 F2 pet1 plants using the phenol/SDS method (Liu et al., 1995). Genomic DNA from the F3 lines was used for the RFLP analysis with mi139 and mi148. The RFLP marker mi238 was sequenced and converted to a CAPS marker (T. Kurata and K.T. Yamamoto, unpublished data). Linkage was calculated along with the Kosambi mapping function described by Koornneef and Stam (1992).
RESULTS
Isolation and Genetic Characterization of Short-Hypocotyl Mutants
We initially screened light-hypersensitive mutants, which have a shorter hypocotyl than the wild type, under weak, continuous, far-red light in sugar-containing solidified medium. From 20,000 plants from the M2 population mutagenized with fast neutrons and 4,900 lines mutagenized by T-DNA insertion (Feldmann, 1991), we picked out 18 and 4 mutant lines, respectively. We excluded the typical deetiolation mutants, which displayed photomorphogenetic phenotypes in the dark: short hypocotyl, expanded cotyledon, initiation of foliage leaves, and accumulation of anthocyanin (Chory, 1993). The mutants we isolated exhibited the short-hypocotyl phenotype both in the dark and in the light, but did not show the other photomorphogenetic responses in the dark. We assigned four complementation groups by carrying out complementation tests of these mutants (data not shown). We describe one of them, which we designated pet1.
pet1 consisted of two allelic lines, both of which were isolated from fast-neutron-mutagenized M2 lines. When they were back-crossed to the wild type, the F1 progeny had a wild-type phenotype, indicating that the mutation was recessive to wild type. Segregation analysis of the F2 progeny indicated that these mutants were monogenic in terms of the short-hypocotyl phenotype (data not shown). The F1 progeny of the two mutants had the short-hypocotyl phenotype, indicating that they were allelic. We did not observe any allele-specific variations of phenotype between the two allelic mutants other than those described below.
pet1 was mapped to the region of chromosome 2 between mi139 and mi238. It was located 2.2 ± 1.5 centimorgans south of mi139 and 7.5 ± 2.8 centimorgans north of mi238, and tightly linked to mi148 (no crossover was found in 92 chromosomes). In this region two mutations, compacta2 (cp2) (Koornneef et al., 1983) and superroot (sur) (Boerjan et al., 1995), which cause a short-hypocotyl phenotype in the dark, have been mapped previously. Complementation tests between pet1-1 and cp2 or sur demonstrated that pet1 was a new mutation (data not shown).
Phenotype at the Seedling Stage
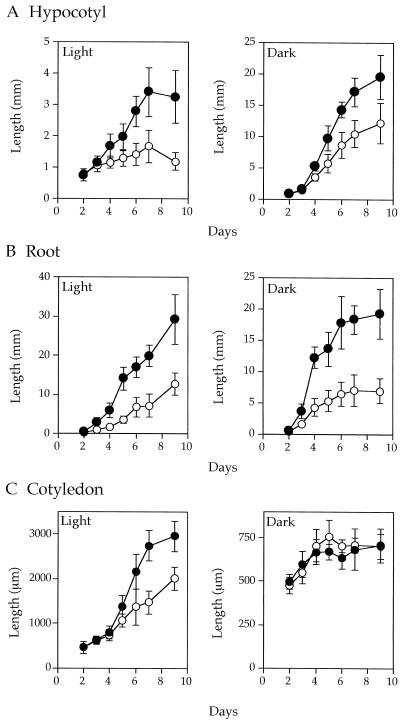
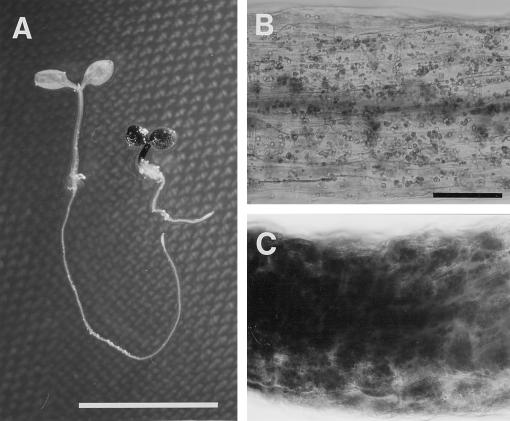
At the postgermination stage (2 d old), dark- and light-grown wild-type seedlings had similar morphologies, including hook formation, etiolated cotyledon, and unelongated hypocotyl and root, whereas pet1-1 had no hook and had paler cotyledons than the wild type. Hypocotyls of pet1-1 were as long as those of the wild type (Fig. 1A). Fully grown hypocotyls of dark- and light-grown pet1-1 seedlings (7 d old) were shorter than those of the wild type by factors of 1.5 and 2, respectively (Figs. 1B and 2A). Growth kinetics of the pet1-1 hypocotyl (Fig. 2A) showed that the short-hypocotyl phenotype did not result from delayed germination.
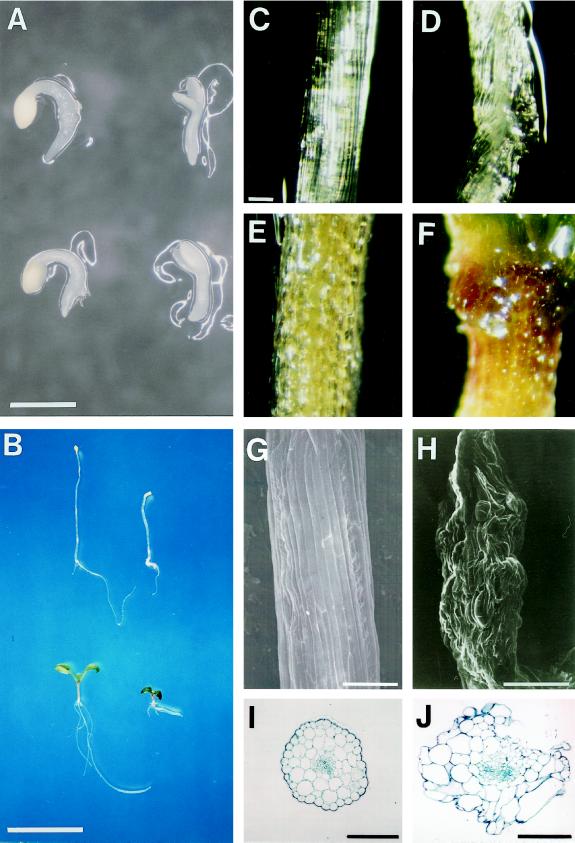
Figure 1.
Morphologies of dark- and light-grown wild-type and pet1 seedlings. A and B, Two- (A) and seven-day-old (B) wild-type (left) and pet1-1 seedlings (right) were grown at 22°C for 1 and 6 d in the dark (top) or light (bottom) after light exposure for 1 d to promote germination. C to F, Surface view of the upper part of the hypocotyl of 7-d-old wild-type (C and E) and pet1-1 (D and F) seedlings grown in the dark (C and D) or the light (E and F). G to J, Scanning electron micrographs (G and H) and cross-sections (I and J) of the upper part of the hypocotyl of 13-d-old dark-grown wild-type (G and I) and pet1-1 (H) and pet1-2 (J) seedlings. Scale bars: A, 1 mm; B, 1 cm; C to G, 100 μm; H, 400 μm; I, 100 μm; and J, 200 μm.
Figure 2.
Elongation kinetics of hypocotyl (A), root (B), and cotyledon (C), of dark- and light-grown wild-type (•) and pet1-1 seedlings (○). A different set of about 20 seedlings was used for each measurement. Vertical bars indicate sd.
pet1-1 also showed defects in the elongation of roots and cotyledons (Fig. 1B). Root growth of light-grown pet1-1 seedlings was slower than that of the wild type. Root length of dark-grown seedlings reached a maximum about 6 d after germination, and the maximum length of pet1 roots was about 3 times shorter than that of the wild type (Fig. 2B). The elongation of root hairs was not affected in pet1 (data not shown), indicating that PET1 is not required for tip growth. Cotyledons of the wild type elongated rapidly after 4 d of growth in the light, but pet1-1 cotyledons elongated more slowly during this period (Fig. 2C). In the dark, cotyledons of both genotypes elongated at a similar rate, which was much reduced compared with that in the light, confirming that pet1 is not a deetiolation mutant that shows expansion of cotyledons even in the dark (Chory, 1993).
To investigate the defect in hypocotyl elongation at a cellular level, we conducted a microscopic analysis of hypocotyl cells of light-grown seedlings (Table I). On d 2 after germination, epidermal cells of pet1-1 hypocotyls were of regular shape and their length and width were the same as those of the wild type. In both wild type and pet1-1, epidermal cells then started to expand, but by d 7, pet1-1 cells were significantly shorter than those of the wild type. We did not observe significant differences in either width or number of hypocotyl epidermal cells within a cell file between wild type and pet1. These results indicate that the short-hypocotyl phenotype of pet1 derives from defects in the cell-elongation process.
Table I.
Length, width, and number of hypocotyl epidermal cells of light-grown wild-type and pet1-1 seedlings at d 2 and 7 after germination
| d 2
|
d 7
|
|||
|---|---|---|---|---|
| Wild type | pet1-1 | Wild type | pet1-1 | |
| Cell length (μm)a | 48.1 ± 13.2 | 40.5 ± 5.4 | 149 ± 33b | 87.4 ± 31.3b |
| Cell width (μm)a | 15.5 ± 2.2 | 15.8 ± 1.9 | 33.3 ± 3.0 | 35.1 ± 3.4 |
| Cell no.c | 16.6 ± 1.7 | 16.7 ± 1.3 | 15.5 ± 0.9 | 16.2 ± 0.4 |
Six to thirty cells of at least five seedlings were measured.
Significant difference by Student's t test (P < 0.05).
Two to three cell files were measured for seven seedlings.
Some light- and dark-grown pet1 seedlings exhibited surface irregularities in the upper part of their hypocotyls (Fig. 1, C–F). In seedlings grown for 10 d in the dark, deformation of the hypocotyl surface occurred in about 6% and 12% of pet1-1 and pet1-2, respectively. Longer culture in the dark increased both the frequency and the extent of the irregularities: as many as 38% of 13-d-old dark-grown pet1-2 seedlings showed the abnormal hypocotyl surface, and the deformation became as dramatic as that shown in Figure 1H. Some of these seedlings also had foliage leaves that were twisted or swollen. Examination of the distorted regions of pet1-2 by light microscopy revealed that many epidermal, cortical, and endodermal cells appeared swollen, but some epidermal cells were compressed (Fig. 1J). These results are consistent with the surface view of the deformed hypocotyl.
It is noteworthy that pet1 accumulated anthocyanin in the light (Fig. 1F). Extraction and quantitation of anthocyanin and chlorophyll showed that pet1 contained about 3- to 4-fold more anthocyanin than the wild type, but had about a 2-fold lower chlorophyll content in the light.
Phenotype at the Adult Stage
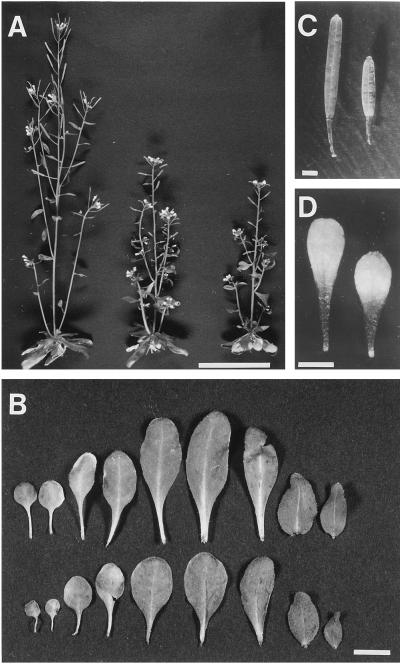
Figure 3A shows adult pet1 plants grown in soil, which clearly exhibit semi-dwarf characteristics. A time-course study of elongation of the first flower stalk showed that pet1 started to bolt at the same time as the wild type on d 25, but its stem elongated more slowly, resulting in an approximately 1.5-fold reduction in height compared with the wild type at maturity. Defects in the elongation process were also obvious in rosette leaves (Fig. 3B); the radius of pet1 rosettes was less than that of the wild type. A reduction in the length of petioles and leaf blades was obvious in pet1-1, but the width of the leaf blade was essentially the same as that of the wild type. A more significant reduction in the length of leaf blades appeared in leaves that developed later (Fig. 3B). A decrease in length was also observed in pedicels, siliques, and petals (Fig. 3, C and D). Accumulation of anthocyanin was also observed in early developing flower buds of pet1 plants (data not shown).
Figure 3.
Adult-plant phenotype of wild-type and pet1 grown in soil at 22°C under continuous white light. A, Overall morphologies of 40-d-old wild-type, pet1-1, and pet1-2 plants from left to right. Scale bar = 5 cm. B, Leaves of the wild type (top row) and pet1-1 (bottom row). From left to right are shown seven rosette leaves and two cauline leaves. Scale bar = 1 cm. C and D, Silique and petal of wild-type (left) and pet1-1 (right) plants, respectively. Scale bars = 1 mm.
GA, Auxin, BR, and AVG Did Not Repair the Short-Hypocotyl Phenotype
We conducted feeding experiments to determine whether the pet1 phenotype was caused by a deficiency in phytohormones. Additions of GA3 (10−10–10−5 m), IAA (10−10–10−5 m), or BR (10−10–10−6 m) to the culture medium did not repair the short-hypocotyl phenotype. Alternatively, because ethylene inhibits cell elongation, pet1 could be an overproducer of ethylene; therefore, we examined the effects of an inhibitor of ethylene biosynthesis, AVG. The short-hypocotyl phenotype of pet1 was not reversed by the addition of AVG (10−8–10−5 m). These results indicate that pet1 is neither a hormone-deficient mutant nor an ethylene-overproducing mutant.
Influence of Suc on Hypocotyl Elongation
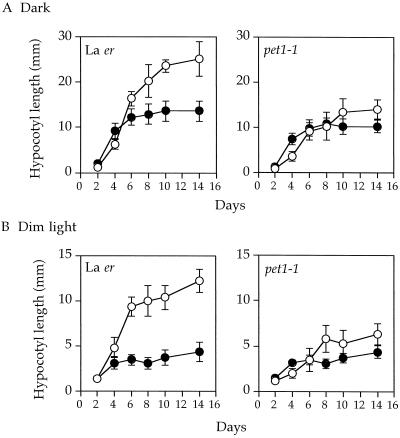
Arabidopsis was usually grown on a medium containing 1% Suc, under which conditions pet1 hypocotyls were shorter than those of the wild type. However, on a Suc-free medium, the short-hypocotyl phenotype almost disappeared, although pet1 hypocotyls were still about 15% shorter than those of the wild type (Fig. 4A). To further characterize the effect of Suc on hypocotyl elongation, the kinetics of hypocotyl elongation were measured in the presence or absence of Suc. Because under normal white-light conditions seedlings are short as a result of the inhibitory effects of light on hypocotyl elongation, we checked hypocotyl growth under dim white light so that changes in elongation could be detected more easily (Fig. 4B). Suc was found to affect the maximum length of hypocotyl that was reached 8 to 10 d after germination. The addition of 1% Suc to the growth medium increased the maximum length of wild-type hypocotyls both in the dark and in dim light, and the Suc-induced increase was larger in light-grown than in dark-grown seedlings. In the pet1 mutant the Suc-induced increase in length was much smaller than in the wild type, resulting in the obvious short-hypocotyl phenotype of pet1 in the presence of Suc. This suggests that the pet1 mutant could not use the exogenously supplied Suc for elongation as efficiently as the wild type. We then investigated the effect of Suc concentration on the maximum length of wild-type hypocotyls and found that the optimal concentration was 1%. The promotive effects decreased gradually at higher concentrations (data not shown).
Figure 4.
Effects of 1% Suc in the growth medium on hypocotyl elongation of wild-type and pet1-1 seedlings grown in the dark (A) or under continuous dim light (B). Seedlings were grown in the absence (•) or presence (○) of 1% Suc. A different set of about 20 seedlings was used for each measurement. Vertical bars indicate sd.
Overaccumulation of Soluble Sugars and Starch in pet1 Mutants
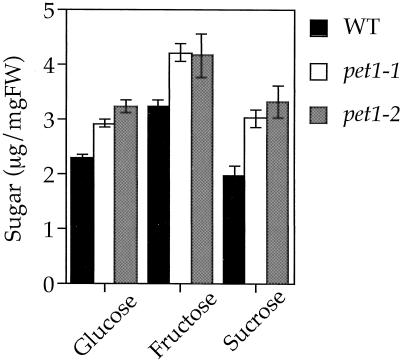
It was expected that if pet1 could not use exogenously added Suc for cell elongation, accumulation of sugar might be greater in pet1 than in the wild type. We tested this possibility directly by quantitation of soluble sugars. Figure 5 shows that pet1 mutants grown in a medium supplemented with Suc contained more soluble sugars (Suc, Glc, and Fru) than the wild type. We also stained the seedlings with iodine to detect starch (Fig. 6). Weak staining was observed in the cotyledons, the upper parts of the hypocotyl, and in the root tips of the wild type. In hypocotyls of the wild type, starch granules were observed throughout the cytoplasm. Staining of hypocotyls and cotyledons was more intense in pet1 than in the wild type, but was similar for root tips. These results are consistent with the hypothesis that growth defects of pet1 mutants are related to the deficiency in usage of sugars for cell elongation.
Figure 5.
Quantitation of water-soluble sugars in 6-d-old wild-type and pet1 seedlings grown in the dark at 22°C in the presence of 1% Suc. Vertical bars indicate sd. FW, Fresh weight; WT, wild type.
Figure 6.
Iodine staining of starch of 6-d-old wild-type and pet1-1 seedlings grown under continuous dim light in the presence of 1% Suc. A, Wild type (left) and pet1-1 (right). B and C, Magnified hypocotyl cells of wild-type and pet1-1 seedlings, respectively. Note the intensive staining of starch granules of pet1-1. Scale bars: A, 1 cm; B, 100 μm.
Effect of Green-Light Irradiation in a Sugar-Free Medium
The observation that exogenously supplied Suc induced the pet1 short-hypocotyl phenotype raised the question of whether sugars produced endogenously by photosynthesis could also cause a similar phenotype. Because photosynthetically active blue and red light inhibit hypocotyl growth (Kendrick and Kronenberg, 1994), we grew seedlings under continuous green light, which is the least effective wavelength for inhibition of growth (Goto et al., 1993). Exposure to green light induced a small but significant difference in hypocotyl length between wild type and pet1-1, even in the absence of Suc (Table II). We also tested the effects of the photosynthesis inhibitor DCMU on this phenomenon. Upon addition of 1 μm DCMU, a concentration that has been found to completely inhibit photosynthesis in Arabidopsis (Kurata and Yamamoto, 1997), the difference in hypocotyl length between the wild type and pet1 under green light disappeared as a result of a decrease in the length of the wild type (Table II). These results suggest that the short-hypocotyl phenotype of pet1 is a consequence of its inability to use sugars for hypocotyl growth, whether they are supplied in the medium or produced endogenously by photosynthesis.
Table II.
Effects of continuous green light on hypocotyl length in the absence of Suc
| Genotype | −DCMU
|
+DCMU
|
||||
|---|---|---|---|---|---|---|
| Dark | Dim white | Green | Dark | Dim white | Green | |
| mm | ||||||
| Wild type | 13.0 ± 1.0 | 3.2 ± 0.5 | 4.7 ± 0.8a | 12.9 ± 1.0 | 3.1 ± 0.6 | 3.1 ± 0.4 |
| pet1-1 | 10.5 ± 1.2 | 3.3 ± 0.5 | 3.2 ± 0.7a | 10.3 ± 1.9 | 3.1 ± 0.5 | 3.0 ± 0.4 |
Seedlings were initially grown under continuous white light (43 W m−2) for 24 h and were then grown in the dark or in dim white light (0.42 W m−2) or green light (3.8 W m−2) for 5 d at 22°C in the presence (+DCMU) or absence (−DCMU) of 1 mm DCMU.
Significant difference between wild type and pet1-1 by Student's t test (P < 0.05).
DISCUSSION
Our results demonstrate that PET1 is essential for cell elongation throughout Arabidopsis development in a variety of organs, including the hypocotyl, root, leaf, inflorescence stem, petal, pedicel, and silique (Figs. 1B and 3). Microscopy revealed that the reduced elongation in the hypocotyl was associated with a reduction in cell length, rather than in cell number. Cell elongation in plants is known to be regulated by the action of phytohormones (Evans, 1984); however, the possibility that the pet1 phenotype is a result of a deficiency in GA, auxin, or BR synthesis was likely to be ruled out by feeding experiments. Furthermore, we tested the possibility that PET1 acts in opposition to the ethylene pathway and promotes cell elongation rather than radial cell expansion, as proposed for the SABRE gene product (Aeschbacher et al., 1995). This possibility was also ruled out by the finding that an inhibitor of ethylene biosynthesis, AVG, did not reverse the pet1 phenotype. These results suggest that PET1 acts independently of the phytohormones tested.
The short-hypocotyl phenotype of pet1 mutants is obvious only when Suc is supplied in the culture medium. In the absence of Suc, no significant differences in hypocotyl length were observed between the wild type and pet1 in the light (Fig. 4B), and in the dark there was only a 15% reduction in pet1 (Fig. 4A). The short-hypocotyl phenotype of pet1 is associated with an inability to increase hypocotyl growth upon the addition of Suc; this is in contrast to the wild type, in which significant elongation occurs. Therefore, the pet1 phenotype is conditional, depending on the presence of Suc. In the wild type, photosynthesis driven by green light was able to significantly promote hypocotyl elongation in the absence of Suc, but this treatment did not increase hypocotyl elongation of pet1 (Table II). This suggests that in the wild type the photosynthetic product(s) can at least partially replace the effect of Suc in the culture medium. Adult pet1 plants grown auxotrophically are shorter than wild-type plants (Fig. 3A), which is consistent with this conclusion. The conditional phenotype of pet1 suggests that pet1 defects are related to the use of sugars in the process of cell elongation.
Several conditional growth mutants have been reported. Conditional root-expansion mutants are conditional on the growth rate of roots. They exhibit reduced growth and abnormal expansion of their roots only when cultured in optimum growth conditions in terms of temperature, light, and Suc concentration (4%) of nutrient medium (Benfey et al., 1993; Hauser et al., 1995). pet1 is distinct from the conditional root-expansion class of mutants because growth retardation of pet1 is obvious in the light, in which the growth rate is significantly reduced, if 1% Suc is added to the medium (Fig. 4).
In the presence of Suc a deformation of hypocotyl structure is sometimes seen in pet1 seedlings, raising the possibility that the cell wall might be a target site of PET1. Similar structural irregularities have been reported in procuste1 (prc1), another conditional growth mutant of Arabidopsis that has a hypocotyl-elongation defect only in the dark (Desnos et al., 1996). The aerial phenotype of prc1 grown in the light is indistinguishable from the wild type at both the seedling and adult stages. The elongation defect observed in the dark is always associated with a deformation of the hypocotyl surface that results from an uncontrolled swelling and compression of epidermal, cortical, or endodermal cells. The irregularities can be phenocopied by the treatment of wild-type seedlings with an inhibitor of cellulose biosynthesis. PRC1 is suggested to play a role in the correct assembly of the expanding cell wall. A mutation in the MUR1 locus that encodes an enzyme in the first step of de novo synthesis of GDP-l-Fuc (Bonin et al., 1997) causes Fuc deficiency in cell walls; consequently, a deformed, short-hypocotyl phenotype occurs (Desnos et al., 1996). In pet1 deformation of the hypocotyl is not always observed. However, the occasional occurrence of an irregular hypocotyl surface suggests that smaller defects occur in the cell wall architecture of pet1 seedlings even if they are not detectable microscopically. In fact, in the dark, defects in elongation are more severe in prc1 than in pet1. The irregular hypocotyl structure of pet1 suggests that, like PRC1, the cell wall is a target site of PET1. Cell wall architecture could be affected by Suc availability in the cell, and PET1 might be needed for the correct organization of the cell wall in the presence of Suc. An apparent defect of pet1 in the use of Suc may result from the deformation of hypocotyl structure in the presence of Suc.
Overaccumulation of sugars (Fig. 5) and starch (Fig. 6) was observed in pet1 seedlings. pet1 also contained more anthocyanin and less chlorophyll than the wild type. This is probably because, in Arabidopsis, accumulation of anthocyanin is induced (Tsukaya et al., 1991; Koch, 1996) and chlorophyll biosynthesis is repressed (Koch, 1996; Jang et al., 1997) by sugars. The dwarf mutants of Arabidopsis, det2-1 and ga1-3, which are deficient in BR (Li et al., 1996) and GA (Sun and Kamiya, 1994), respectively, also showed overaccumulation of starch in the medium supplemented with Suc (data not shown). In contrast to pet1, however, they always showed the short-hypocotyl phenotype independent of the presence of Suc. Therefore, accumulation of sugars and starch in pet1 does not seem to be a cause but a consequence of its dwarfness. It also suggests that Suc uptake is not likely to be affected by the pet1 mutation.
In conclusion, PET1 seems to be required for cell elongation and correct cell wall structure of the hypocotyl in the presence of Suc. Map-based cloning of the PET1 locus in progress in our laboratory will enable us to gain further knowledge of the molecular basis underlying the complex network of growth control in plants.
ACKNOWLEDGMENTS
The authors thank S. Hosono for his help in the initial screening of the mutants, and Drs. J. Chory (Salk Institute, San Diego, CA) and W. Boerjan (Gent University, Belgium) for det2-1 and sur seeds, respectively. We also thank the Arabidopsis Biological Resource Center (Ohio State University, Columbus) for the cp2 and ga1-3 seeds and for the RFLP clones mi139, mi148, and mi238.
Abbreviations:
- AVG
aminoethoxyvinylglycine
- BR
brassinolide
- CAPS
cleaved-amplified polymorphic sequence
- RFLP
restriction fragment-length polymorphism
Footnotes
This work was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan (no. 05276102 to K.T.Y.).
LITERATURE CITED
- Aeschbacher RA, Hauser MT, Feldmann KA, Benfey PN. The SABRE gene is required for normal cell expansion in Arabidopsis. Genes Dev. 1995;9:330–340. doi: 10.1101/gad.9.3.330. [DOI] [PubMed] [Google Scholar]
- Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser M-T, Aeschbacher RA. Root development in Arabidopsis: four mutants with dramatically altered root morphogenesis. Development. 1993;119:57–70. doi: 10.1242/dev.119.Supplement.57. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W,Bellini C, Caboche M, Onckelen HV, Van Montagu M, Inze D (1995) superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed]
- Bonin CP, Potter I, Vanzin GF, Reiter W-D. The MUR1 gene of Arabidopsis thaliana encodes an isoform of GDP-d-mannose-4,6-dehydratase, catalyzing the first step in the de novo synthesis of GDP-l-fucose. Proc Natl Acad Sci USA. 1997;94:2085–2090. doi: 10.1073/pnas.94.5.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper T, Huber SC, Somerville C. Alteration in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985;79:11–17. doi: 10.1104/pp.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J. Out of darkness: mutants reveal pathways controlling light-regulated development in plants. Trends Genet. 1993;9:167–172. doi: 10.1016/0168-9525(93)90163-c. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ (ed) (1995) Plant Hormones: Physiology, Biochemistry and Molecular Biology, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Desnos T, Orbovic V, Bellini C, Kronenberger J, Caboche M, Traas J, Höfte H. Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in the dark- and light-grown Arabidopsis seedlings. Development. 1996;122:683–693. doi: 10.1242/dev.122.2.683. [DOI] [PubMed] [Google Scholar]
- Ecker JR. The ethylene signal transduction pathway in plants. Science. 1995;268:667–675. doi: 10.1126/science.7732375. [DOI] [PubMed] [Google Scholar]
- Evans ML. Functions of hormones at the cellular level of organization. In: Scott TK, editor. Hormonal Regulation of Development II. Encyclopedia of Plant Physiology, New Series, Vol 10. Berlin: Springer-Verlag; 1984. pp. 23–79. [Google Scholar]
- Feldmann KA. T-DNA insertional mutagenesis in Arabidopsis: mutational spectrum. Plant J. 1991;1:71–82. [Google Scholar]
- Fry SC. Loosening the ties. Curr Biol. 1993;3:355–357. doi: 10.1016/0960-9822(93)90199-x. [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto N, Yamamoto KT, Watanabe M. Action spectra for inhibition of hypocotyl growth of wild-type plants and of the hy2 long-hypocotyl mutant of Arabidopsis thaliana L. Photochem Photobiol. 1993;57:867–871. [Google Scholar]
- Hauser MT, Morikami A, Benfey PN. Conditional root expansion mutants of Arabidopsis. Development. 1995;121:1237–1252. doi: 10.1242/dev.121.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden P, Kamiya Y. Gibberellin biosynthesis: enzymes, genes and their regulation. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:431–460. doi: 10.1146/annurev.arplant.48.1.431. [DOI] [PubMed] [Google Scholar]
- Heim U, Weber H, Baumlein H, Wobus U. A sucrose-synthase gene of Vicia faba L.: expression pattern in developing seeds in relation to starch synthesis and metabolic regulation. Planta. 1993;191:394–401. doi: 10.1007/BF00195698. [DOI] [PubMed] [Google Scholar]
- Inouhe M, Sakurai N, Kuraishi S. Growth regulation of dark-grown dwarf barley coleoptile by the endogenous IAA content. Plant Cell Physiol. 1982;23:689–698. [Google Scholar]
- Jang J-C, León P, Zhou L, Sheen J (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- Kendrick RE, Kronenberg GHM (eds) (1994) Photomorphogenesis in Plants, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Stam P. Genetic analysis. In: Koncz C, Chua N-H, Schell J, editors. Methods in Arabidopsis Research. Singapore: World Scientific Publishing; 1992. pp. 83–99. [Google Scholar]
- Koornneef M, van Eden J, Hanhart CJ, Stam P, Braaksma FJ, Feebstra WJ. Linkage map of Arabidopsis thaliana. J Hered. 1983;74:265–272. [Google Scholar]
- Kurata T, Yamamoto KT. Light-stimulated root elongation in Arabidopsis thaliana. J Plant Physiol. 1997;151:346–351. [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development in Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Liu Y-G, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995;8:457–463. doi: 10.1046/j.1365-313x.1995.08030457.x. [DOI] [PubMed] [Google Scholar]
- Nishitani K. Endo-xyloglucan transferase, a new class of transferase involved in cell wall construction. J Plant Res. 1995;108:137–148. [Google Scholar]
- Peters JL, Tuinen AV, Adamse P, Kendrick RE, Koornneef M. High pigment mutants of tomato exhibit high sensitivity for phytochrome action. J Plant Physiol. 1989;134:661–666. [Google Scholar]
- Ray PM (1987) Principles of plant cell expansion. In DJ Cosgrove, DP Knievel, eds, Physiology of Cell Expansion during Plant Growth. American Society of Plant Physiologists, Rockville, MD, pp 1–17
- Reiter W-D, Chapple C, Somerville CR. Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J. 1997;12:335–345. doi: 10.1046/j.1365-313x.1997.12020335.x. [DOI] [PubMed] [Google Scholar]
- Reiter W-D, Chapple CCS, Somerville CR. Altered growth and cell walls in a fucose-deficient mutant of Arabidopsis. Science. 1993;261:1032–1035. doi: 10.1126/science.261.5124.1032. [DOI] [PubMed] [Google Scholar]
- Sakurai N, Kuraishi S. Sugar compositions, intrinsic viscosities and molecular weights of hemicellulosic polysaccharides of the coleoptile cell walls in a semi-brachitic and a normal type barley. Plant Cell Physiol. 1984;25:955–963. [Google Scholar]
- Sun T-P, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua N-H. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Naito S, Redei GP, Komeda Y. A new class of mutation in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development. 1993;118:751–764. [Google Scholar]
- Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from Petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lockwood SK, Hoeltzel MF, Schiefelbein JW. The ROOT HAIR DEFECTIVE3 gene encodes an evolutionarily conserved protein with GTP-binding motifs and is required for regulated cell enlargement in Arabidopsis. Genes Dev. 1997;11:799–811. doi: 10.1101/gad.11.6.799. [DOI] [PubMed] [Google Scholar]
- Wilson K, Long D, Swinburne J, Coupland G. Plant Cell. 1996;8:659–671. doi: 10.1105/tpc.8.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]