Abstract
Pain frequently is an overlooked aspect of wound care, and unresolved pain can have a negative impact on wound healing. The etiology and comorbidities associated with chronic wounds can also complicate the assessment of the quantity and quality of the pain perceived by the patient. It is critical to adequately assess pain when establishing a comprehensive plan of care. This article will discuss the etiology of pain as well as provide the reader with strategies for managing the painful wound once it has been identified.
keywords: Wounds, Pain
Pain is a frequently experienced, yet oftentimes overlooked factor in wound care and wound healing. It is undeniable that pain affects wound care practice, and unresolved pain negatively impacts both wound healing and a patient's quality of life.1 Despite our increased knowledge of pain, there still remains a large gap between knowledge and implementation of pain management.2 Pain is multi-dimensional; involving both physiological and psychological components.3 The physical components include the underlying cause of the wound and pain from clinical interventions. The influence that the psychosocial factors play in the patient's perception of pain cannot be understated as well.1 Such factors include negative thoughts about pain, emotional distress, anticipatory pain and anxiety. Many clinicians are not comfortable assessing pain and are uncertain what strategies to implement to effectively address the pain issue.4 The inability to distinguish between the causative factors of wound pain makes it difficult to establish its etiology and in turn, develop an effective plan of care.
The type of pain the patient is experiencing is directly related to the type of wound. Pain associated with peripheral arterial occlusive disease (PAOD) is commonly characterized by intermittent claudication or, in the more advanced state, the presence of ischemic rest pain. Intermittent claudication is described as reproducible leg pain or cramping which occurs in the posterior calf region during bouts of activity and is relieved with rest. Pain occurs because the arterial blood flow is not great enough to meet the metabolic demands of the muscle. Ischemic rest pain occurs most frequently at night or with leg elevation and may be relieved when the extremity is placed in the dependent position.5 In contrast, venous insufficiency may be associated with an aching or deep muscle pain that may increase with prolonged posturing in the dependent position (such as standing or leg dangling). Pain occurs secondary to compromised valves and/or muscle pump failure leading to edema formation.9 The diabetic patient may feel pain secondary to neuropathy or deep structure involvement such as osteomyelitis or Charcot foot.3 The pain associated with pressure ulcers is not fully understood9 but may be associated with a number of factors including friction/shear, peri-ulcer irritation, moisture related incontinence and deep infection, to name a few.7 There have been erroneous assumptions in the past that venous ulcers, superficial and neuropathic wounds are painless.8 We must overcome these misconceptions if we are to accurately assess the true nature of pain.
The patient's expression of pain needs to be addressed by the wound care specialist and not marginalized. The key to understanding the pain process is to understand that pain is the patient's perception, not what we, the clinicians, think it should be.9 The Agency for Healthcare Research and Quality (AHRQ) guidelines stress that it should not be assumed that a patient does not have pain solely because he/she does not express it. Many times patients are unable to express their pain.10 Differences in personal, familial and cultural backgrounds also influence a patient's response to pain. Body language and non-verbal expressions must be considered and validated.2 It is imperative that the clinician know how to accurately assess, evaluate and initiate an individualized pain management regimen to meet the patient's needs.1
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.”11
A more simplistic definition, given by Margo McCaffrey, states that “pain is whatever the person experiencing it says it is, and exists whenever he or she says it exists.”12
Types of Pain
Traditionally, pain has been divided into two categories: “Nociceptive Pain” and “Neuropathic Pain.” Nociceptive pain is the normal physiological response to a painful stimulus and serves as a biologic function to warn of injury. Neuropathic pain is caused by dysfunction or damage in the nervous system.1 This is an inappropriate response wherein damaged nerves cause signals to travel in abnormal pathways.3
The Pain Pathway
Nociceptive Pain
A nociceptor is a free primary nerve ending that is found in cutaneous muscle and visceral tissues.13 They normally are silent when not stimulated. Nociceptors can be stimulated in two ways: actual injury to the tissue or changes in the tissues surrounding the area of injury. Tissue damage caused by noxious stimulation precipitates cellular changes.13 The pH changes, enzymes and mediators are activated and released and there may be ionic changes influencing membrane permeability. The inflammatory cascade is stimulated; histamine and serotonin are released increasing vasodilatation and inflammation. Some or all of these stimulate the free nociceptor nerve ending.13
After the nociceptor is stimulated, cellular changes in the nerve ending are converted to an electrical impulse in the primary afferent nerve. This impulse continues traveling and ascending to the dorsal or ventral roots of the spinal cord. The primary afferent nerves are called “first order neurons” and are mostly small, thinly myelinated A-delta fibers and small unmyelinated C-fibers. The majority of the cutaneous and visceral nociceptive primary afferent nerve fibers enter into the dorsal horn of the spinal cord. The pain impulse must pass from the primary afferent nerve to the “second order neuron” in the spinal cord for transmission to the brain. The dorsal horn of the spinal cord is considered a critical site for pain transmission.14 The impulse is passed from the primary afferent nerve to the second order neuron and ascends towards the brain in ascending tracts.13
Modulation is a physiological process that decreases the patient's perception of pain. Modulation occurs when inhibitory and facilitory input from the brain descend to the dorsal root of the spinal cord and affect the transmission of the next volley of ascending nerve impulses.31 The descending modulating impulses occur after the first volley of pain impulses reach the brain. If the modulating descending impulses are inhibitory, then the next volley of afferent impulses ascending from the injured tissue to the brain is inhibited at the spinal cord level and inhibited from reaching the second order neuron. Therefore, modulation is the body's method of decreasing pain intensity by inhibiting the ascending transmission of the pain impulse from the primary afferent neuron to the second order neuron in the spinal cord. The receptors at the dorsal horn of the spinal cord where modulation occurs are called endogenous opioid receptors. Pharmaceutical opioids bind onto these opioid receptors at the dorsal horn of the spinal cord and mimic the modulation process.13,31
Neuropathic Pain
Neuropathic pain is caused by nerve dysfunction or damage to the nervous system. Neuropathic pain is an inappropriate response, as damaged nerve fibers can lead to aberrant generation of electrical signals from the nerve endings.1,12,15 Damage to nerves can result in alteration or amplification of the pain signal. Neuropathic pain may be the result of a pathological process at any level in the nervous system, from the nociceptor, distal nerve, plexus level, dorsal root ganglion, root entry zone, and higher levels in the CNS. Nociceptive pain, in contrast, usually indicates a proper functioning nervous system and is considered physiological because it results from the activation of nociceptor.15
In neuropathic pain, the perception of pain occurs in the brain and spinal cord, with the pathological process anywhere in the nervous system. In nociceptive pain, the perception is still in the brain and spinal cord, but the origin and intensity of this pain is localized at the peripheral nociceptors, at the peripheral nerve endings.15 See Figure 1.
Figure 1.
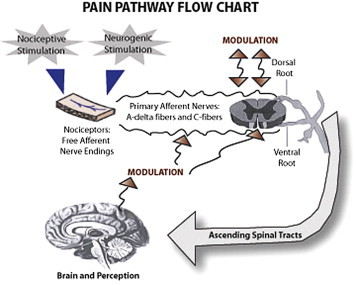
Pain Pathway Flow Chart.
Diagram of Pain Pathways.16
The Gate Control Theory of Pain
The Gate Control Theory of pain tried to integrate peripheral, central, ascending and descending, neurobiological and psychological factors into a paradigm of pain. This theory was proposed by Ronald Melzack, a Canadian psychologist, and Patrick David Wall, a British physician, in 1962,1 and again in 1965.17 According to this theory, the perception of physical pain is not a direct result of activation of nociceptors, but instead is modulated by interaction between different neurons, both pain-transmitting and non-pain-transmitting. The Gate Control Theory asserts that activation of nerves that do not transmit pain signals can interfere with signals from pain fibers and inhibit an individual's perception of pain. It theoretically explains how a stimulus that activates only non-nociceptive nerves can inhibit pain. Pain seems to be lessened when the area is rubbed because activation of non-nociceptive fibers inhibits the firing of nociceptive ones in the laminae. In transcutaneous electrical stimulation (TENS), non-nociceptive fibers are selectively stimulated with electrodes in order to produce this effect and thereby lessen pain.18
Evaluation of Pain Related to Wounds
When a patient reports pain, to what are they really referring? It is imperative for the clinician to clarify the type of pain the patient is experiencing in order to adequately manage it.
Pain is generally categorized into one of four categories:1
-
1.
Background Pain (Basal or Baseline Pain)
-
2.
Breakthrough Pain (Incident Pain)
-
3.
Procedural Pain
-
4.
Operative Pain
Background pain is related to the underlying cause of the wound, local wound factors and other related pathologies. Background pain is felt at rest, when there is no tissue manipulation or patient movement or sudden changes in the patient physical condition. Background pain is directly related to the underlying cause of the patient's wound. Another name for background pain is Basal or Baseline pain. This type of pain may be continuous or intermittent.1
Breakthrough pain is sometimes called Incident pain. Breakthrough pain is generally of rapid onset, severe intensity and brief duration. Flares related to background pain are generally mild, infrequent, slow onset and tolerated and managed easier.19 Breakthrough pain can occur during day-to-day activities such as mobilization of the patient during transfers or following dressing slippage where the wound is traumatized by the movement of the dressing over the surface of the skin. In 1990, Portenoy and Hogan, working with cancer patients, defined breakthrough pain as transient increases in pain in a patient who has stable, treated, persistent pain.4 Breakthrough pain is frequently precipitated by patient movement or activity. “End of Dose” failure is not breakthrough pain. End of dose pain occurs when regularly prescribed analgesics are inadequate in dosage or when the interval between administration of adequate dosage is too long. It is also important to note that breakthrough pain cannot occur unless the background pain is adequately controlled. Otherwise, the situation is really uncontrolled background pain rather than a spiking breakthrough pain. Background pain is not just a fluctuation in baseline pain due to inadequate pharmacological therapy. Medications used for treating breakthrough pain are usually short acting. These are sometimes called “rescue medications”. It should be clear that managing background pain is very different from the management of breakthrough pain.20
Procedural pain results from a routine intervention such as dressing removal, cleansing or dressing application.1
Operative pain is associated with any intervention that would normally be performed by a specialist and requires an anaesthetic (local or general) to manage the pain.1
Clinicians must also consider the role that Psychosocial and Environmental factors play in the causes of wound pain. Psychosocial factors such as age, environment and previous pain history can all influence a patient's experience of pain and ability to communicate their pain. Environmental factors may be one of the most important for the clinician to recognize as these are oftentimes easy to remedy and resolve for the patient. Examples of this are timing of the procedure, temperature of the room, noise level and patient positioning.1
Inflammation in Chronic Wounds
Chronic wounds and pressure ulcers are characterized by a chronic inflammatory response, which impedes healing.22 These wounds are characterized by endogenous inflammatory chemicals that lower the threshold of peripheral nociceptor stimulation. This lowering of the nociceptor threshold is called facilitated or primary hyperalgesia. When the firing threshold of the nociceptors is lowered, their responsiveness to stimulus is increased.21
Primary Hyperalgesia describes a heightened sensitivity in the area surrounding the wound, caused by a sustained area of inflammation, or a repeated stimulus to the wound. Primary Hyperalgesia refers only to the areas close to the peripheral nerve receptors.21
Hyperalgesia is often confused with a similar phenomenon called Allodynia. Allodynia describes a situation where a normal sensory stimulus, such as light touch or light pressure, provokes intense pain. The response is out of proportion to the stimulus. Allodynia is caused by central nervous system sensitization to signals transmitted by nociceptors at the site or periphery of an injury.19
Hyperalgesia may be treated by combining a non-steroidal anti-inflammatory drug (NSAID) with a mild opioid. NSAIDs decrease local inflammation at the local level, stopping the threshold lowering effect near the wound and decreasing hyperalgesia. The opioids work at the spinal cord level of modulation.22
Pain Memories and the Effects of Anxiety and Stress
In a normally functioning nervous system, an unpleasant stimulus is recognized as pain; the body's response is to protect itself and withdraw from the source. Unaddressed pain can sensitize all parts of the nervous system wherein seemingly benign sensations are perceived as painful.23 There is evidence that pain may actually become imprinted in the central nervous system as a “pain memory.” The CNS would recognize subsequent exposures to pain from patterns of environmental and sensory cues and elicit a learned response.3 According to research done by Koyama et al, expectations or anticipation can drastically impact a patient's perception of pain. When the expectation of pain was decreased, the patient's subjective experience of pain decreased and the areas of the brain responsible for pain activation also showed a decrease in activity. This research may provide insight as to how psychology (the patient's perception) plays a role in shaping the neural processes of the brain influencing the sensory experience.24 A person's expectation of pain can drastically influence their perception of pain, as the expectation can become the patient's reality. Thus, prior painful experiences, such as pain during a dressing change, can have a drastic impact on future experiences.3
Pain, undoubtedly, increases the amount of stress and anxiety that is perceived by the patient. The body perceives both psychological and physiological trauma as a threat and initiates a cascade of events to fight off the threat. The Sympathetic Nervous System (SNS) is activated in times of stress and stimulates the adrenal glands to release the stress hormones adrenaline (epinephrine) and cortisol. These hormones increase respiration, heart rate and blood pressure. Once the stressor has been removed, the parasympathetic system is activated (either cognitively or automatically) to return the body to a “normal” state.25 Cortisol is a key regulatory hormone stimulated during the stress response. Increased cortisol levels suppress the immune system by decreasing white blood cell activity (neutrophils). In a wound, this will cause decreased macrophage function and in doing so, decrease the ability to remove debris from the wound.26 Cortisol also has been linked to suppression of fibroblast proliferation and matrix degradation, affecting the duration and strength of the wound.3 Cortisol stimulates the body to produce more catecholamine, leading to vasoconstriction of small arterioles and ultimately decreasing peripheral blood supply, oxygen and nutrient transport as well as impacting the body's resistance to infection.26 The chronic presence of stress hormone ultimately leads to catabolism of the body and decreased wound healing.3
Management of Wound Pain
To formulate a pain management plan, the nature of the patient's pain must be assessed. Is this pain a change in the background pain, such as a change in the etiology, a worsening of the underlying disease process? Has the background pain been managed and is this therefore a sudden spike in pain related to patient movement? Is the pain related to procedures, such as a dressing change or wound cleansing? Does this pain suggest a new or different pathology emerging? Is this an issue with unmanaged co-morbidities?
Assessment
Correctly assessing pain is vital to establishing an effective plan of care. When assessing wound pain be specific:1,4
-
♦
The pain type (nociceptive, neuropathic or mixed)
-
♦
Duration of pain (chronic versus acute)
-
♦
Impact of the pain
-
♦
Palliative/Provocative Factors: What makes the pain worse? What makes it better?
-
♦
Quality of pain: What kind of pain is experienced (sore, aching, burning, shooting, etc)? Are there other symptoms (fever, chills, nausea or vomiting?)
-
♦
Region and radiation of pain: Where is the pain? Where does it radiate?
-
♦
Severity/Intensity of pain: Would you describe the pain as none, mild, moderate, severe, or excruciating? Rate the pain on a scale from 0 to 10, with o representing “no pain” and 10 being “the worst pain imaginable.” What is the pain intensity at its worst, best, and now?
-
♦
Temporal aspects of pain: Is the pain better or worse at any particular time of the day or night? When does it start or when does it stop? Is it intermittent or constant, or does it occur only when you're moving?
Managing Background Pain
-
1.
Treat the underlying cause: address the etiology of the wound and associated pathologies.1
-
2.
Treat local factors causing wound pain: Use local wound management protocols. Some local wound factors include: ischemia, infection, excessive dryness or excessive exudate, edema, dermatological problems and surrounding skin maceration.1
The World Health Organization developed a three-step approach to pain relief in cancer patients, and this can be modified for wound care.1,20 The inflammatory nature of chronic wounds and pressure ulcers are the basis for pharmacological pain management.
Step 1: A non-opiod analgesic (NSAID) with or without an analgesic adjuvant. Adjuvants include tricyclic antidepressants, anticonvulsants, antihistamines, benzodiazepines, steroids, and phenothiazines. Adjuvants are given for their indirect benefits in pain management.1,20
Step 2: If pain is not controlled: Continue the initial medication and add an opioid, such as codeine or tramedol, and an adjuvant.1,20
Step 3: When a patient does not respond to second-step medications, these should be discontinued and a more potent oral narcotic initiated.1,20,22
The benefits of a pharmacological approach to wound pain must be weighed against the risks before this plan is initiated. “Analgesia can facilitate ambulation, which encourages wound healing and reduces the risk of complications like deep vein thrombosis, pulmonary complications and pressure ulcers. However, the adverse effects of analgesics must be considered, effects such as respiratory depression, nausea, constipation and sedation. Analgesia may also mask further symptoms and complications.”16
Managing Breakthrough Pain
Treatment for breakthrough pain may be pharmacological and non-pharmacological. Changes in body position, movement and the management of systemic symptoms of co-morbidities such as coughing, constipation or joint pain, may help alleviate breakthrough pain.16 When breakthrough pain is predictable, such as in movement related pain, patients can be given additional pain medication preventatively. This is usually done 30 minutes before the pain provoking activity.20
Pharmacological treatment strategies for breakthrough pain include increasing the dose of the opioid, adding a stronger short acting pain medication or reducing the time interval between doses. The exact amount of the supplemental dose should be the dose what will relieve the pain without side effects. If possible, the pharmacological agent used for the treatment of breakthrough pain should be discontinued after the particular episode of breakthrough pain is resolved and the background pain is assessed as being managed. If the pharmacological agent is discontinued, it should be available to the patient if this breakthrough pain is anticipated to reoccur. When selecting supplemental doses of opioid medication, it is generally recommended to choose one that is administered by the same route as the medication used for the basal analgesic.20
Non-pharmacologic interventions for Pain
Although there is little research to support it, non-pharmacological means of pain management should not be marginalized. The power of talking to patients prior to dressing changes cannot be underestimated as well as explaining the procedures to be performed and the measures that will be taken to minimize pain. Communication prior to action will reduce the feelings of fear and anxiety.16 It has been stated that time invested prior to dressing changes is time well spent.26 Non-pharmacologic means that can assist in addressing pain including music therapy, relaxation strategies, meditation, imagery, physical activities, rest, repositioning and physical modalities such as Transcutaneous Electrical Nerve Stimulation (TENS).1,8 TENS often is hypothesized to control pain via a theory known as the “Gate Control Theory.” TENS activates large-diameter myelinated sensory fibers and blocks transmission of nociceptive input carried by small myelinated and non-myelinated pain fibers. The electrical stimulation provided by the TENS unit essentially shuts the door on the transmission of pain stimuli.27
Dressing selection
Much of the work related to pain has been conducted during dressing changes.2 In a multinational study conducted by the European Wound Management Association (EWMA), clinicians rated dressing changes as the time of greatest pain to their patients.26 In addition, dried out dressings and aggressive adhesives were most likely to cause pain during dressing removal.30 It has been demonstrated that patients report more pain with gauze dressings than with any other advanced wound care dressings.4 Wounds contain fragile tissues and the removal of an aggressive dressing can cause trauma to not only the wound, but the periwound tissue as well. One way to limit pain on dressing removal is by using dressings that promote atraumatic removal to prevent trauma from occurring.2 Such dressings include soft silicone, hydrogels, hydrofibers, and alginate dressings.30
Switching to atraumatic dressings can effectively reduce the pain associated with dressing changes. In a study of 5850 patients (reported by 656 primary care physicians), switching to a non-adherent dressing reduced wound pain in 95% of the patients with acute wounds and 88% of patients with chronic wounds. The authors of the study conclude that selecting a suitable, non-adherent dressing improves patient acceptability of dressing changes.28
It is accepted knowledge that wounds heal more quickly in a moist environment. There are multiple factors that contribute to this; moisture promotes the re-epithelization process, facilitates growth factor action and keratinocyte and fibroblast proliferation as well as enhancing collagen synthesis, angiogenesis and early wound contraction.4 One common misconception is that dressings should be changed as frequently as possible. Recent guidelines suggest that dressing changes should be performed depending on the wound characteristics, but as infrequently as possible, to decrease the chance of infection by external contaminants.3 Ovington cites that more frequent dressing changes leads to a drop in wound temperature, which causes vasoconstriction and decreased blood perfusion to the area.6 Decreased blood perfusion has been shown to drastically impair the ability of oxygen to clear bacteria from the wound leading to an increase in tissue infectability.29 A gauze dressing placed in the wound does little to impede fluid evaporation and tissue temperature measures 25°C to 27°C (77.0-80.6°F) —well below normal tissue temperature. Foams and films maintain the wound bed at 91-95 degrees.6 Additionally, gauze dressings need to be changed more frequently to prevent drying out.4
Choosing the appropriate dressing can be a daunting process. One must maintain a moisture balance, providing adequate moisture without causing maceration or dessication, both of which impede healing. If a dressing dries out in a wound, soaking may be needed to promote pain-free removal.4 which unnecessarily exposes the wound to external elements such as bacteria and decreases the wound surface temperature.3 Dessication impairs the epidermal migration necessary for wound closure.2 An alternate dressing should be chosen which can better manage the fluid needs of the wound.
In contrast, excessive fluid can lead to maceration of the tissues if the dressing cannot handle the high fluid demands of the wound. Enzyme rich fluid, if allowed to sit on the skin, can be caustic, causing pain, maceration and tissue erosion.4 Once again, it is recommended that a more appropriate dressing be chose to address the needs of the wound.
Dressings remove the visible reminder to the patient of the wound they have. They allow the patient to resume normal daily activities and provide a barrier to physical stimuli.16 The choice of the size and type of dressing can influence the patient's anxiety level during dressing changes26,3 and affect their overall quality of life.
Conclusion
Wounds are a source of great pain and anxiety for patients, leaving many feeling hopeless and depressed and greatly impairing their quality of life. There are physiological as well as psychological components to pain; both have a negative impact on healing. Clinicians have the ability to reduce these effects by understanding the etiology of pain, appropriately assessing patient reports of pain and establishing an individualized plan of care. It is imperative that clinicians accept the patient's perception of pain as valid. The benefits of pain reduction can improve healing rates and ultimately a patient's quality of life. It is the clinician's moral and ethical responsibility to be professionally competent in the most appropriate approaches to managing pain.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
References
- 1.MEP Ltd; London: 2004. “Minimizing pain at wound dressing-related procedures. A consensus document.” World Union of Wound Healing Societies' Initiative. [Google Scholar]
- 2.Price P.E., Fagervik-Morton H., Mudge E.J. “Dressing–related pain in patients with chronic wounds: An international patient perspective”. Int Wound J. 2008;5(2):159–171. doi: 10.1111/j.1742-481X.2008.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sussman C., Bates-Johnson B. 3rd Edition. Lippincott Williams and Wilkins; Baltimore: 2007. Wound Care: A Collaborative Practice Manual for Health Professionals. 46, 250, 280–287. [Google Scholar]
- 4.Woo K. “Minimising wound-related pain at dressing change: Evidence-informed practice”. Ostomy/Wound Management. 2005;51(11A(suppl)):5–6. doi: 10.1111/j.1742-481X.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtman D., Gahtan V. “Peripheral Arterial Perfusion: Is It Adequate for Wound Healing?”. Wounds. 2008;20(8):230–235. [PubMed] [Google Scholar]
- 6.Ovington L.G. “Hanging Wet to Dry Dressings Out to Dry.”. Advances in Skin and Wound Care. 2002 March/April. [Google Scholar]
- 7.Reddy M., Keast D., Fowler E., Sibbald R. “Pain in Pressure Ulcers.”. Ostomy/Wound Manage. 2003;49(4A(suppl)):30–35. [PubMed] [Google Scholar]
- 8.Woo K.Y., Sibbald R.G., Fogh K. “Assessment and management of persistent (chronic) and total wound pain.”. Int Wound J. 2008;5(2):205–215. doi: 10.1111/j.1742-481X.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranoski S., Ayello E.A. Lippincott, Williams and Wilkins; Philadelphia: 2004. Wound Care Essentials:Practice Principles. 219–222. [Google Scholar]
- 10.Bergstrom N, Bennett MA, Carlson CE, et al. Treatment of Pressure Ulcers. Clinical Practice Guideline, No. 15. Rockville, MD: U.S. Department of Health and Human Service. Public Health Service, Agency for Health Care Policy and Research. AHCPR Publication No. 95-0652. December 1994.
- 11.“Pain Terms. A list with Definitions and Notes on Usage Recommended by the IASP Subcommittee on Taxonomy”. Pain. 1979;6(3):249–252. [PubMed] [Google Scholar]
- 12.McCaffery M., Beebe A. Mosby-Year Book, Inc; St. Louis: 1989. Pain: A Clinical Manual for Nursing Practice. [Google Scholar]
- 13.Giordano J. 6th edition. CRS Press; Florida: 2002. The Neurobiology of Pain: Pain Management: A Practical Guide for Clinicians. 1089–1100. [Google Scholar]
- 14.Choiniere M. “Burn Pain: a unique challenge.”. Pain Clinical Updates. 2001;9:1–4. [Google Scholar]
- 15.Bowell M., Rosenberg S.K., Chelimsky T.C. 6th edition. CRS Press; Florida: 2002. Neuropathic Pain: Mechanisms and Management: Pain Management: A Practical Guide for Clinicians. 181–194. [Google Scholar]
- 16.Abraham S. “Pain Management in Wound Care.”. Podiatry Management. 2008 June/July:165–168. [Google Scholar]
- 17.Nicholson K., Martelli M.F., Zasler N.D. 6th edition. CRS Press; Florida: 2002. Myths and Misconception about Chronic Pain: The Problem of Mind-Body Dualism: Pain Management: A Practical Guide for Clinicians. 465–474. [Google Scholar]
- 18.Kandel E.R., Schwartz J.H., Jessell T.M. 4th edition. McGraw-Hill; New York: 2000. Principles of Neural Science. 482–486. [Google Scholar]
- 19.Portenoy R.K., Hagen N.A. “Breakthrough pain: definition, prevalence and characteristics”. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 20.WHO . WHO; Geneva: 1996. “The Significance of Breakthrough Pain in Cancer: Cancer Pain Release”. [Google Scholar]
- 21.Popescu A., Salcido R.S. “Wound Pain: A Challenge for the Patient and the Wound Care Specialist”. Adv in Skin and Wound Care. 2004 doi: 10.1097/00129334-200401000-00010. Jan/Feb. [DOI] [PubMed] [Google Scholar]
- 22.Supernaw R. 6th edition. CRS Press; Florida: 2002. Drug Management of Pain: Pain Management: A Practical Guide for Clinicians. 435–439. [Google Scholar]
- 23.Wulf H., Baron R. Medical Education Partnership Ltd; London: 2004. “The theory of pain.” EWMA position document. 8–11. [Google Scholar]
- 24.Koyama K., McHaffie J.G., Laurienti P.J., Coghill R.C. “The subjective experience of pain: Where expectations become reality.”. PNAS. 2005;102(36):12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.“Stress: How Does Stress Affect your Body?” American Psychological Association. Available at: http://www.nlm.nih.gov/medlineplus/stress.html#cat9. Accessed October 6, 2008.
- 26.Moffatt C., Franks P., Hollingworth H. Medical Education Partnership Ltd; London: 2004. “Understanding wound pain and trauma: An international perspective.” EWMA position document. 2–7. [Google Scholar]
- 27.Melzack R., Wall P.D. Pain mechanism: a new theory. Science. 1965;150:971–976. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 28.Meaume S., Téot L., Lazareth I., Martini J., Bohbot S. “The importance of pain reduction through dressing selection in routine wound management: the MAPP study.”. J Wound Care. 2004;13(10):409–413. doi: 10.12968/jowc.2004.13.10.27268. [DOI] [PubMed] [Google Scholar]
- 29.McCulloch J., Kloth L., Feedar J. 2nd Ed. F.A. Davis Company; Philadelphia: 1995. Wound Healing: Alternatives in Wound Management. 359. [Google Scholar]
- 30.Moffatt C., Franks P., Hollingworth H. Medical Education Partnership Ltd; London: 2004. “Pain at wound dressing changes; a guide to management”: EWMA position document. 12–17. [Google Scholar]
- 31.Cox R.C., Min D., Essman J. 6th edition. CRS Press; Florida: 2002. Variables in the Sensation and Perception of Pain: Pain Management: A Practical Guide for Clinicians. 817–824. [Google Scholar]


