Abstract
Background
Small clinical studies suggest adjunctive use of acoustic pressure wound therapy (APWT) may enhance wound healing, even in challenging patients. This noncontact low-frequency, nonthermal ultrasound therapy for assisting with the debridement of necrotic tissue from challenging wounds is generally better tolerated by patients for whom treatment-related wound pain, anticoagulation, or medical instability precludes sharp, surgical, or mechanical debridement.
Objective
To evaluate changes in amount of devitalized tissue, amount and type of wound drainage, and wound surface area after administration of APWT.
Design
Retrospective chart review of 48 consecutive patients treated with adjunctive APWT at a single center between January 2006 and October 2007.
Methods
Paired comparisons of baseline versus posttreatment values for wound area, tissue characteristics, drainage, and pain were analyzed. Time, frequency, and duration of APWT and treatment-related adverse events were collected.
Results
APWT was administered a mean of 2.1 times per week for a mean of 4.1 minutes per session. Mean duration of therapy was 5.5 weeks. Median wound area was reduced by 92% from baseline to end of APWT (6.2 cm2 to 0.2 cm2,P < .0001). The proportion of wounds with >75% granulation tissue increased from 37% to 89% (P < .0001). The proportion of wounds without fibrin slough or eschar increased from 31% to 75% (P < .0001) and from 72% to 94% (P = .02), respectively.
Limitations
Retrospective design, lack of control group, small sample population.
Conclusion
As an adjunct to conventional wound management, APWT appears to improve parameters associated with wound healing, including increased tissue granulation, decreased necrotic tissue, and decreased wound area.
Keywords: Chronic wounds, Noncontact low-frequency, Ultrasound, Adjunctive therapy, Acoustic pressure wound therapy
Introduction
Wounds in which the ordered cellular and molecular processes that lead to healing in acute wounds are disrupted present a persistent challenge for the physical therapist practicing wound management. Healing of these challenging wounds requires proper preparation of the wound bed and treatment of underlying medical conditions that contribute to delayed healing.
In wound bed preparation, the presence of necrotic debris is a major inhibitor of the healing process. In addition to providing a fertile environment for overgrowth of bacteria, the debris itself slows both the granulation process and the progression of wound contraction.1 Debridement to remove necrotic or infected tissue from the wound bed reduces the bacterial burden and its metabolic byproducts that inhibit the tissue repair process.1,2 Beyond simply removing necrotic tissue, debridement serves to stimulate host repair cells to engage in the cellular processes required for the wound to heal.3 Ultimately, debridement creates an environment in which the combination of normal host repair mechanisms with other wound care modalities can achieve wound healing.
There is no shortage of techniques for debridement of chronic wounds. Autolytic and enzymatic debridement aim to break down necrotic tissue by employing either moisture-retentive dressings to retain endogenous enzymes for breaking down slough and eschar (autolytic debridement) or topically applied chemicals capable of emulsifying necrotic tissue (enzymatic debridement). For adherent slough and eschar, sharp or surgical debridement or mechanical debridement (eg, scrubbing, wet-to-dry dressings, pulsatile lavage) is often indicated to remove nonviable tissue. However, many patients cannot tolerate the pain associated with these debridement techniques, and these techniques may be contraindicated in cases of anticoagulation or medical instability. Low-frequency, nonthermal ultrasound therapy offers an alternative to the use of sharp or mechanical force to loosen and dislodge adherent necrotic tissue.
Unlike the high-frequency, thermal ultrasound traditionally used for musculoskeletal therapy and fetal monitoring applications, which operates in the 1- to 3-MHz range, low-frequency ultrasound therapy operates at 20 to 40 kHz. Moreover, low-frequency ultrasound does not require a conduction medium of gel or water immersion to transfer acoustic energy to the treatment site. The nonthermal ultrasound waves delivered via low-frequency ultrasound reach the wound bed via either a solution (in contact low-frequency ultrasound) or a fine saline mist (in noncontact low-frequency ultrasound). Contact low-frequency ultrasound systems use acoustic vibration and irrigation solution to cut away necrotic tissue and cleanse wounds. In contrast, noncontact low-frequency ultrasound delivers acoustic energy to wound tissues via an atomized, sterile saline mist without the device touching the wound or fluid aggressively flushing through the wound. Called acoustic pressure wound therapy (APWT), this noncontact low-frequency ultrasound therapy promotes wound healing through wound cleansing and maintenance debridement by removal of yellow slough, fibrin, tissue exudates, and bacteria (per U.S. Food and Drug Administration marketing clearance). In addition, Kavros and Schenck observed a bactericidal effect of APWT in a preliminary, in vitro investigation. When APWT was applied to Staphylococcus aureus, Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant enterococci cells, scanning and transmission electron micrographs showed cell wall destruction of those cells exposed to APWT but not those exposed to a saline-drip control.4
Patients who cannot tolerate sharp, surgical, or mechanical debridement therapies because of pain, anticoagulation, or medical instability are typically able to tolerate APWT. It is speculated that the lack of mechanical force applied to the wound bed with APWT results in an absence of treatment-related pain for the patient. Furthermore, APWT may even provide some palliative benefit. In a small, retrospective study of 15 patients with painful chronic wounds, a statistically significant reduction in mean wound pain was observed within 2 to 4 weeks of starting APWT.5 In addition, reduced need for narcotic pain medications was observed anecdotally in this study.
The value of APWT in promoting healing is supported by published literature demonstrating clinically relevant improvements in healing rate or time to healing. APWT was shown to improve healing time in a randomized, sham-controlled trial of 55 patients with chronic diabetic foot ulcers6 and a randomized trial of 70 patients with wounds complicated by chronic critical limb ischemia.7 In addition, single-arm studies have reported improvements in healing rate with APWT for chronic lower extremity wounds, compared with either a historical control8 or a baseline standard-of-care period.4
A debridement therapy that can painlessly assist in the removal of adherent necrotic tissue and stimulate the formation of healthy granulation tissue is appealing to both physical therapists practicing wound management and their patients. Given that the ultimate goal is to expedite wound healing, this study was undertaken to assess the impact of adjunctive APWT on the healing progression of challenging wounds.
Methods
Stalled wounds, particularly those with adherent necrotic tissue, are a persistent challenge in outpatient wound care practice. The researchers began using APWT to assist with the loosening and removal of necrotic tissue and to stimulate granulation in these challenging wounds. The use of APWT quickly evolved to include treatment of acute wounds in patients presenting with comorbidities or bacterial colonization that would likely lead to delayed wound closure. These comorbidities included, but were not exclusive to, diabetes mellitus, arterial insufficiency, and long-term steroid dependency. A single-site, retrospective chart review was conducted of consecutive patients who presented to the center for wound care between January 2006 and October 2007 and were treated with APWT as an adjunct to physical therapy wound management. The objective was to evaluate wound healing, specifically the change in amount of devitalized tissue, change in amount and type of wound drainage, and change in wound surface area.
Study Population
Patients treated with APWT during the study period and who met the entrance criteria were considered for this retrospective analysis. Eligible patients were those aged 18 years and older who presented with a wound of any etiology and received APWT to the wound an average of at least 2 times per week during the study period. Patients were excluded from this analysis if their wound was not appropriate for APWT, therapy was provided less than an average of 2 times per week, or they were diagnosed with a terminal illness with life expectancy of less than 6 months.
The study protocol was approved by the Clarian Health Institutional Review Board, which determined that an additional consent form for this analysis was not required because all patients in this analysis had signed a general consent form prior to receiving treatment. Furthermore, this study is a retrospective chart review, which involves only minimal risk of loss of confidentiality. The patients had already received treatment and were not contacted for active involvement in the study. Chart review was the only means of data collection.
Study Treatments
All therapeutic interventions used during the study period were recorded, including APWT. Physical therapy wound management consisted of wound bed preparation with the goal of providing the wound with the most optimal healing potential. Wound bed preparation in this context refers to the following: selective and nonselective forms of debridement, dressing selection focusing on achieving an optimal moisture and bacterial balance, multilayer compression, patient education, and therapeutic modalities. Therapeutic modalities included high-voltage pulsed current, negative pressure wound therapy, and pulsed lavage with suction. APWT was provided according to the recommended treatment algorithm provided by the manufacturer, which suggests a treatment time per session dependent on the total surface area of the wound. In general, treatment time increases as the total wound surface area increases. The treatment algorithm includes wound surface area from <10 cm2 to 180 cm2, with treatment times ranging from 3 to 20 minutes.
The APWT device (MIST Therapy System, Celleration, Inc., Eden Prairie, Minnesota) generates low-frequency (40 kHz), nonthermal ultrasound waves that are transferred to the wound bed by a gentle mist of sterile saline without direct contact of the device with the wound tissues. The device is a compact, portable unit consisting of a generator, transducer, and disposable applicator that uses prepackaged sterile saline. The disposable applicator contains an on-off valve that controls the flow of sterile saline to the ultrasound transducer surface. APWT is contraindicated in cases in which an electronic implant or prosthesis is near the treatment site (eg, near or over the heart or thoracic area in a patient with a cardiac pacemaker) or in cases in which the treatment site is on the lower back during pregnancy, over a pregnant uterus, or over an area of malignancy. Precautions should also be taken in cases in which traditional thermal ultrasound is contraindicated (eg, presence of deep vein thrombosis or over the brain, genitalia, or growth plates).
Data Collection
Baseline clinical parameters collected were medical history (including comorbidities), appropriate laboratory and imaging studies as indicated (including ankle-brachial index), history and etiology of the treated wound, wound measurements and characteristics, and pain ratings reported by patients using the visual analog scale (VAS). All treatment modalities used during the study period were recorded. For APWT, data collection included time, frequency, and duration of treatment; total number of treatments; and treatment-related adverse events. Adverse events were reported and collected at each patient visit. At the completion of APWT, wound size, wound characteristics, and VAS pain scores were analyzed.
Study Assessments
The primary effectiveness endpoint was the percentage change in wound area from baseline to the end of APWT. Wound area was calculated as the greatest length times the greatest width that is perpendicular to the length with a head-to-toe anatomic orientation. Percentage change in wound area from the beginning to the end of APWT was calculated as: [(beginning area − ending area) / (beginning area)] × 100. The primary safety endpoint was the proportion of patients experiencing at least 1 device- or treatment-related adverse event during the study period. Secondary endpoints included changes in amount and type of wound drainage, percentage of devitalized tissue, and pain ratings from beginning to end of APWT.
Statistical Analysis
Statistical analyses were performed with SAS Version 9.1.3 (SAS Institute, Cary, North Carolina). This retrospective analysis was performed with a study population of consecutive patients treated with adjunctive APWT between January 2006 and October 2007. Formal power calculations to determine sample size were not performed. Descriptive statistics were performed to characterize the patients and their wounds at the start and end of APWT. Paired comparisons between parameters at baseline and end of treatment were made using the Wilcoxon signed rank test for continuous variables and the McNemar test for categorical variables.
Results
Patient and Wound Characteristics
Between January 2006 and October 2007, 48 patients (50 wounds) meeting the study eligibility criteria were treated with APWT in addition to conventional physical therapy wound management. Baseline patient characteristics are shown in Table 1. Mean age was 54 years. Men and whites accounted for more than 60% of the study population. The majority of patients presented with comorbid medical conditions, the most common of which were conditions related to the cardiovascular (57%), integumentary (40%), and musculoskeletal (38%) systems. One-quarter of patients had diabetes mellitus.
Table 1.
Patient Characteristics at Baseline
| Demographics | Patients (N = 48) % (n) |
|---|---|
| Male | 67 |
| Mean age in years (range) | 54 (19-88) |
| Race | |
| Black or African American | 25 (12) |
| White | 63 (30) |
| Hispanic or Latino | 10 (5) |
| Native American | 2 (1) |
| Smoking (current) | 27 (13) |
| Cardiovascular or vascular disorder | 57 (27)a |
| Hematologic disorder | 17 (8)a |
| Neurological or psychological disorder | 21 (10)a |
| Diabetes | 25 (12) |
| Gastrointestinal disorder | 23 (11)a |
| Musculoskeletal disorder | 38 (18) |
| Integumentary disorder | 40 (19)a |
N = 47 patients with responses.
Wound characteristics at baseline are shown in Table 2. More than 70% of the wounds were located on the lower extremities, including the leg, ankle, and foot. This was a diverse wound population in terms of etiology. The most common etiology, venous insufficiency, accounted for 26% of the wounds; the other wounds were attributed to 18 different etiologies. On average, wounds in this study had been present for nearly 23 weeks, although the range of chronicity was broad, ranging from 0 to 220 weeks, resulting in a median chronicity of 7 weeks. Infection was confirmed by a swab culture in 5 wounds at baseline and 1 wound at the end of treatment. Methicillin-resistant Staphylococcus aureus was isolated in 3 of the 5 infected wounds at baseline and in none at the end of treatment. Swab cultures were performed only in wounds that presented with 2 or more of the cardinal signs of infection including rubor, tumor, dolor, calor, and purulent drainage. Of the wounds that were cultured, infection was confirmed in 5 wounds at baseline and 1 wound at the end of treatment. MRSA was isolated in 3 of 5 infected wounds at baseline and in 0 at the end of treatment. The 5 wounds that presented with confirmed infections were treated systemically with antibiotics as indicated and locally with the use of antimicrobial topicals and dressings.
Table 2.
Wound Characteristics at Baseline
| Characteristic | Data N = 50 |
|---|---|
| Mean / median chronicity in weeks (SD; range) | 22.8 / 7.0(42.4; 0-220.0) |
| Mean / median wound area, cm2 (SD; range) | 27.8 / 6.2(65.0;0-400) |
| Location, % (n) | |
| Sacrum | 4 (2) |
| Leg | 46 (23) |
| Ankle | 10 (5) |
| Foota | 16 (8) |
| Otherb | 24 (12) |
| Etiology,c % (n) | |
| Pressure | 4 (2) |
| Venous insufficiency | 26 (13) |
| Arterial insufficiency | 8 (4) |
| Surgery | 10 (5) |
| Trauma | 8 (4) |
| Neuropathy | 8 (4) |
| Otherd | 36 (18) |
Includes 4 diabetic foot ulcers.
Other locations (no.): abdomen (1), arm (2), finger (1), hand (1), natal cleft (1), toe (3), coccyx (1), ischium (1), elbow (1).
Etiologies for the 4 diabetic foot ulcers are surgery (1) and neuropathy (3).
Other etiologies (no.): abscess/cellulitis (1), allergic vasculitis (1), burn (3), cellulitis (1), gunshot wound (2), IV infiltrate (1), methicillin-resistant Staphylococcus aureus (2), originally surgical but now pressure (1), paint injection (1), sickle cell (1), suspected spider bite (1), venous or arterial (2), undetermined (1).
Treatment Characteristics
On average, patients received APWT treatments 2.1 times per week (range, 0.8-3.7 times), for a mean treatment time of 4.1 minutes per session (range, 1.5-12.0 minutes). The mean duration of APWT per patient over the course of treatment was 5.5 weeks, with a range of 0.4 to 25 weeks. No treatment-related adverse events were reported.
Wound Healing
Among the 24% (n = 12) of wounds that closed completely during the study period, the mean time to closure was 4.3 weeks (range, 1.6-8.1 weeks). As illustrated in Figure 1, median wound area was reduced by 92% from 6.2 cm2 (range, 0-400 cm2) at baseline to 0.2 cm2 (range, 0-63 cm2) after APWT treatment (P < .0001). Wound volume could not be analyzed due to a predominance of wounds with non-measureable depth. Ultimately, paired data and measurable baseline wound volume were available for only 12 wounds.
Figure 1.
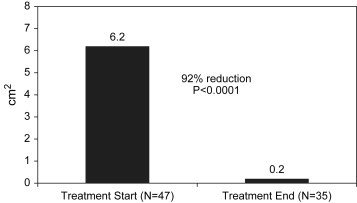
Change In Median Wound Surface Area From Start to End of APWT.
The mean wound area at baseline (27.8 cm2) reflects a data set skewed by a few very large wounds, the 5 largest of which measured 400, 150, 127, 106 and 75 cm2. In this wound population, the median wound area of 6.2 cm2 better reflects the average wound area because the mean is skewed by the larger wounds. Similarly, at the end of APWT, a few very large wounds again skew the mean (5.5 cm2) to a value substantially higher than the median (0.2 cm2).
In addition to the reduction in wound area, changes in tissue quality and drainage of the wounds reflect clinically relevant improvement after APWT administration (Table 3). First, the proportion of wounds with >75% granulation tissue increased significantly, from 37% at baseline to 89% at the end of treatment (P < .0001). Second, the proportion of periwound skin rated as normal increased significantly, from 28% at baseline to 64% at the end of treatment (P = .0002). Third, the amount of wound drainage was reduced significantly, although the type of drainage did not change appreciably during the study period. At baseline, wound drainage was rated as either moderate or scant in 89% of wounds, whereas at the end of APWT treatment, the ratings had shifted such that 83% of wound drainage was rated as either scant or none (P = .03). Also shown in Table 3, undermining, tunneling, odor, and maceration were uncommon in this study population and did not change significantly after APWT.
Table 3.
Wound Tissue and Drainage Characteristics From Start to End of APWT (N = 50)
| Wound Characteristic | Baseline % (n) | End of Treatment % (n) | P Valuea |
|---|---|---|---|
| Amount of healthy granulation tissue | <.0001b | ||
| Complete closure (%) | 0 | 24 (12) | |
| 76-99 | 37 (18) | 65 (32) | |
| 51-75 | 2 (1) | 0 | |
| 26-50 | 12 (6) | 0 | |
| 1-25 | 16 (8) | 4 (2) | |
| None | 33 (16) | 6 (3) | |
| Peri-wound skin | .0002c | ||
| Normal | 28 (14) | 64 (32) | |
| Irritation | 12 (6) | 8 (4) | |
| Erythematous | 30 (15) | 2 (1) | |
| Edematous | 4 (2) | 2 (1) | |
| Callus | 18 (9) | 10 (5) | |
| Other | 36 (18d) | 16 (8e) | |
| Undermining | NSf | ||
| None | 96 (48) | 96 (48) | |
| Present | 4 (2) | 4 (2) | |
| Tunneling | .16 | ||
| None | 94 (47) | 98 (49) | |
| Present | 6 (3) | 2 (1) | |
| Odor | .10 | ||
| None | 88 (44) | 96 (48) | |
| Present | 12 (6) | 4 (2) | |
| Maceration | .17 | ||
| None | 76 (38) | 88 (42) | |
| Minimal | 6 (3) | 13 (6) | |
| Moderate | 16 (8) | 0 | |
| Maximum | 2 (1) | 0 | |
| Amount of drainage | .03 | ||
| None | 7 (3) | 27 (11) | |
| Scant | 48 (22) | 56 (23) | |
| Moderate | 41 (19) | 17 (7) | |
| Maximum | 4 (2) | 0 | |
| Type of drainage | .48 | ||
| Sanguinous | 5 (2) | 3 (1) | |
| Serous | 56 (24) | 60 (18) | |
| Serosanguineous | 33 (14) | 37 (11) | |
| Purulent | 7 (3) | 0 | |
| Amount of eschar | .019 | ||
| None | 72 (34) | 94 (45) | |
| <50% | 13 (6) | 2 (1) | |
| ≥50% | 15 (7) | 4 (2) | |
| Amount of fibrin slough | <.0001 | ||
| None | 31 (15) | 75 (36) | |
| <50% | 16 (8) | 21 (10) | |
| ≥50% | 53 (26) | 4 (2) |
McNemar test.
Comparison of >75% granulation tissue at start versus end of treatment.
Comparison of normal peri-wound skin at start versus end of treatment.
Other (no.): atrophy blanche (3), desiccated (1), dry (2), fungal (1), hemosiderin (1), hyperkeratotic (1), indurated (5), inflammation (2), macerated (1), punched out (1).
Other (no.): dry (2), induration (1), atrophy blanche (3), fungal(1), hemosiderin (1).
NS, not statistically significant. Identical distributions at baseline and follow-up preclude calculation of a P value for the McNemar test.
As shown in Table 3, the debridement effect of APWT appears to be reflected in reduced eschar and fibrin slough in the wound bed after APWT treatment. The proportion of wounds without fibrin slough increased from 31% to 75% (P < .0001), and the proportion without eschar increased from 72% to 94% (P = .02).
Pain Ratings
Patient-reported pain ratings made with the VAS (0 = no pain, 10 = equals intense pain) decreased from a mean of 3.6 at baseline to 0.8 at the end of APWT treatment. Of the 42 patients for whom pain scores were available at both baseline and the end of APWT, the mean reduction in pain was 2.6 points on the VAS (P < .0001).
Discussion
In this retrospective analysis, greater than 90% wound area reduction was achieved with the addition of a mean of 5.5 weeks of APWT to typical physical therapy wound management in challenging wounds. Among wounds that healed completely during the study period, the mean time to healing was 4.3 weeks. Although little evidence exists comparing one method of debridement with another,9 the closure or near closure of challenging wounds in 4 to 6 weeks in this study represents a clinically meaningful response, particularly in light of the painless nature of this therapeutic intervention used to assist with debridement. The reduction in wound size observed in this study was accompanied by improvements in normal periwound skin, granulation tissue formation, amount of necrotic tissue, and wound drainage over the APWT treatment period.
In this population of wounds, in which the majority had either eschar or fibrin slough in the wound bed, debridement was a key component of the wound care regimen. APWT was tolerated well by patients in terms of treatment-related pain. Moreover, significant pain reduction was observed, based on VAS ratings of patient-reported pain. A direct correlation between the reduction of pain and APWT cannot be established in this study because physical therapy wound management alone may have had a direct impact on pain without the use of APWT. Further studies are needed to conclusively determine whether decreased wound pain can be attributed to APWT. Regardless, APWT appears to offer a painless alternative to conventional sharp and mechanical debridement therapies for removing adherent necrotic tissue. In addition to significant reductions in eschar and fibrin slough, there was a significant increase in healthy granulation tissue by the end of APWT treatment.
In this sample of 42 patients, a 2.6-point reduction in mean VAS pain score after a mean 5.5 weeks of APWT was found. One small study has documented decreased wound pain after initiation of APWT in painful wounds. In their retrospective analysis of 15 consecutive patients with lower extremity wounds who received APWT, Gehling and Samies observed a statistically significant 80% reduction (6.4 points) in patient-reported VAS pain scores after 2 to 4 weeks of APWT.5 Whether APWT offers a palliative benefit or is simply a painless alternative to traditional sharp and mechanical debridement techniques known to be painful is not clear. APWT delivers acoustic pressure waves to the wound via a gentle mist of sterile saline, as opposed to a scalpel, scrubbing, or high-pressure lavage. These preliminary reports of pain relief associated with APWT warrant prospective analysis of this potential palliative benefit in painful wounds.
Two prospective, randomized studies have evaluated APWT as an adjunct to conventional wound care in chronic wounds. In a randomized, controlled study of 70 patients with chronic critical limb ischemia, Kavros et al found that a significantly greater proportion of patients treated with APWT in addition to conventional wound care achieved greater than 50% wound healing at 12 weeks than those treated with conventional wound care alone (63% vs 29%, respectively, P < .001).7 Similarly, in a randomized, double-blind study of 55 patients with recalcitrant diabetic foot ulcers (n = 27 APWT, n = 28 sham ultrasound), Ennis et al observed that a significantly higher proportion of wounds treated with APWT had healed (complete epithelialization without drainage) after 12 weeks of care than had wounds treated with the sham ultrasound therapy (40.7% vs 14.3%, P = .04).6 Ennis et al also conducted a small, single-arm study of chronic lower extremity wounds in which APWT resulted in a mean healing time of 7 weeks, compared with 10 weeks in a historical control cohort treated at the same center.8 A feasibility study of APWT in recalcitrant lower-leg and foot ulcerations, conducted by Kavros et al, observed mean percentage wound volume reduction of 94.9% ± 9.8% in the group treated with APWT in addition to conventional care, compared with 37.3% ± 18.6% in the baseline standard-of-care group (P < .0001).4
The current analysis is retrospective in design and small in sample size. In addition, the single-arm design precludes assessment of the comparative efficacy of APWT with other wound-healing modalities. In this study, wounds were treated with other forms of debridement and therapeutic modalities in addition to APWT. Because of the lack of a control wound population, the possibility that these wounds would have progressed in a similar manner without APWT cannot be excluded. However, this research is the first to contribute data on the use of APWT as a painless debridement therapy in wounds frequently complicated by necrosis. As clinicians endeavor to make evidence-based therapy decisions, it will be important to make use of existing data sets to evaluate therapy options. Admittedly, large, prospective studies with randomized and blinded design are the gold standard of scientific evidence. However, retrospective analyses of this nature can begin to identify clinically meaningful therapeutic benefits in a field of clinical medicine not replete with the resources necessary to conduct large, rigorously controlled studies. Furthermore, research in a real-world, clinical practice setting where patients are not carefully selected and treatment conditions not rigorously controlled provides important insight into the effectiveness of a given therapy when delivered in a typical care setting.
Clearly, physical therapists have a number of debridement therapies at their disposal to remove necrotic tissue from challenging wounds in an effort to stimulate the natural wound-healing process. Based on the findings of this study and others, it appears that APWT is one therapy that may contribute to wound healing via debridement without the concerns of pain, anticoagulation, or existing comorbidities often associated with sharp and mechanical debridement.
Footnotes
Conflict of interest: Statistical and manuscript support were funded by Celleration, Inc. The authors report no financial interest in Celleration, Inc., and received no funding for the study or their work on the manuscript.
References
- 1.Ennis W.J., Meneses P. Factors impeding wound healing. In: Kloth L.C., McCulloch J.M., editors. Wound Healing: Alternatives in Management. F.A. Davis Company; Philadelphia, PA: 2002. pp. 68–96. [Google Scholar]
- 2.Bryant W.M. Wound healing. Clin Symp. 1977;29(3):1–36. [PubMed] [Google Scholar]
- 3.Enoch S., Harding K. Wound bed preparation: the science behind the removal of barriers to healing. Wounds. 2003;15(7):213–229. [Google Scholar]
- 4.Kavros S.J., Schenck E.C. Use of noncontact low-frequency ultrasound in the treatment of chronic foot and leg ulcerations: a 51-patient analysis. J Am Podiatr Med Assoc. 2007;97(2):95–101. doi: 10.7547/0970095. [DOI] [PubMed] [Google Scholar]
- 5.Gehling M.L., Samies J.H. The effect of noncontact, low-intensity, low-frequency therapeutic ultrasound on lower-extremity chronic wound pain: a retrospective chart review. Ostomy Wound Manage. 2007;53(3):44–50. [PubMed] [Google Scholar]
- 6.Ennis W.J., Foremann P., Mozen N. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manage. 2005;51(8):24–39. [PubMed] [Google Scholar]
- 7.Kavros S.J., Miller J.L., Hanna S.W. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: the Mayo Clinic experience, 2004-2006. Adv Skin Wound Care. 2007;20(4):221–226. doi: 10.1097/01.ASW.0000266660.88900.38. [DOI] [PubMed] [Google Scholar]
- 8.Ennis W.J., Valdes W., Gainer M., Meneses P. Evaluation of clinical effectiveness of mist ultrasound therapy for the healing of chronic wounds. Adv Skin Wound Care. 2006;19(8):437–446. doi: 10.1097/00129334-200610000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bradley M., Cullum N., Sheldon T. The debridement of chronic wounds: a systematic review. Health Technol Assess. 1999;3(17 Pt 1) iii–iv, 1–78. [PubMed] [Google Scholar]


