Neutrophil-derived TRAIL induces apoptosis of alveolar macrophages, limiting the spread of S. pneumoniae infection.
Abstract
Apoptotic death of alveolar macrophages observed during lung infection with Streptococcus pneumoniae is thought to limit overwhelming lung inflammation in response to bacterial challenge. However, the underlying apoptotic death mechanism has not been defined. Here, we examined the role of the TNF superfamily member TNF-related apoptosis-inducing ligand (TRAIL) in S. pneumoniae–induced macrophage apoptosis, and investigated the potential benefit of TRAIL-based therapy during pneumococcal pneumonia in mice. Compared with WT mice, Trail−/− mice demonstrated significantly decreased lung bacterial clearance and survival in response to S. pneumoniae, which was accompanied by significantly reduced apoptosis and caspase 3 cleavage but rather increased necrosis in alveolar macrophages. In WT mice, neutrophils were identified as a major source of intraalveolar released TRAIL, and their depletion led to a shift from apoptosis toward necrosis as the dominant mechanism of alveolar macrophage cell death in pneumococcal pneumonia. Therapeutic application of TRAIL or agonistic anti-DR5 mAb (MD5-1) dramatically improved survival of S. pneumoniae–infected WT mice. Most importantly, neutropenic mice lacking neutrophil-derived TRAIL were protected from lethal pneumonia by MD5-1 therapy. We have identified a previously unrecognized mechanism by which neutrophil-derived TRAIL induces apoptosis of DR5-expressing macrophages, thus promoting early bacterial killing in pneumococcal pneumonia. TRAIL-based therapy in neutropenic hosts may represent a novel antibacterial treatment option.
Streptococcus pneumoniae is the most prevalent pathogen, and is responsible for causing community-acquired pneumonia in humans. Despite the fact that all clinically relevant serotypes of S. pneumoniae are susceptible against the most common antibiotics, S. pneumoniae remains a significant cause of morbidity and lethality worldwide (Welte et al., 2012). Therefore, the development of novel antibiotic-independent therapeutic strategies is urgently needed to decrease the disease burden associated with pneumococcal infections of the lung.
Because of their pivotal role in bacterial phagocytosis and orchestration of innate immune responses to bacterial infections, alveolar macrophages represent the first line of lung protective immunity against inhaled S. pneumoniae (Calbo and Garau, 2010). Recruited neutrophils support macrophages in lung bacterial clearance during established pneumonia (Knapp et al., 2003; Herbold et al., 2010; Calbo and Garau, 2010), and resident alveolar and lung macrophages, along with inflammatory recruited exudate macrophages, critically contribute to resolution of lung inflammation (Knapp et al., 2003; Winter et al., 2007).
An important feature of S. pneumoniae–induced lung infection is the rapid induction of apoptosis in alveolar macrophages within 24 h, resulting in a transient depletion of alveolar macrophages from distal airways (Paton, 1996; Rubins et al., 1996; Dockrell et al., 2003; Knapp et al., 2003; Maus et al., 2004, 2007; Winter et al., 2007; Taut et al., 2008; Hahn et al., 2011b). Inhibition of S. pneumoniae–induced macrophage apoptosis decreases lung pneumococcal clearance, thereby promoting invasive pneumococcal disease progression in mice (Dockrell et al., 2003; Marriott et al., 2005). Conversely, activation of apoptotic cascades in macrophages and neutrophils limits pathogen-driven inflammatory cascades during pneumococcal disease (Marriott et al., 2004, 2006). Moreover, phagocytosis of apoptotic macrophages by lung macrophages down-regulates the overall inflammatory response and decreases invasive disease progression of pneumococcal pneumonia (Fadok et al., 1998; Marriott et al., 2006). Together, these data suggest that macrophage apoptosis is protective in terms of limiting excessive proinflammatory responses during pneumococcal lung infections.
The TNF superfamily member TNF-related apoptosis-inducing ligand (TRAIL) exhibits a complex ligand/receptor cross-talk (Schneider et al., 2003). In humans, four membrane-bound TRAIL receptors have been identified, of which TRAIL-R1 (DR4) and TRAIL-R2 (DR5) are apoptosis-inducing receptors, and TRAIL-R3 (DcR1) and TRAIL-R4 (DcR2) act as “decoy” receptors because of absent or nonfunctional death domains (Ashkenazi and Dixit, 1999). In mice, three decoy receptors, but only one death-mediating receptor for TRAIL, death receptor 5 (DR5), have been identified (Wu et al., 1999; Schneider et al., 2003). Previously, a role for caspases and TNF superfamily member Fas ligand has been established in lung infection models (Ali et al., 2003; Matute-Bello et al., 2005). More recently, there has been emerging evidence for a role of TRAIL to induce apoptosis in leukocyte subsets (Katsikis et al., 1997; Renshaw et al., 2003; Zheng et al., 2004; Lum et al., 2005; McGrath et al., 2011; Zhu et al., 2011), alveolar epithelial cells, and other host cell-types in models of LPS-induced acute lung injury, peritonitis (McGrath et al., 2011), as well as viral and bacterial infections (Zheng et al., 2004; Ishikawa et al., 2005; Hoffmann et al., 2007; Brincks et al., 2008, 2011; Stary et al., 2009; Cziupka et al., 2010; Zhu et al., 2011). These data collectively demonstrate that TRAIL plays a role in inducing apoptosis in different cell types in pulmonary inflammation and infection models.
Despite the increased acknowledgment that TRAIL is a key player in several immune reactions within the lung, there are currently no data available regarding the role of TRAIL in macrophage apoptosis and disease progression in bacterial pneumonia induced by the major prototype lung-tropic pathogen, S. pneumoniae. Our data reveal a novel neutrophil-macrophage cross talk mechanism by which alveolar accumulating neutrophils responding to the infection secrete TRAIL that induces alveolar macrophage apoptosis and regulates bacterial killing subsequent to pneumococcal challenge. Importantly, we also show for the first time that treatment of neutropenic mice with agonistic anti-DR5 antibody compensates for the lack of neutrophil-derived TRAIL, and significantly improves survival of pneumococcal pneumonia. This finding may be of great interest for future antibiotic-independent immunomodulatory strategies in immunocompromised patients at risk of acquiring bacterial infections. The implications of these findings will be discussed.
RESULTS
Trail−/− mice exhibit increased mortality and decreased bacterial clearance upon challenge with S. pneumoniae
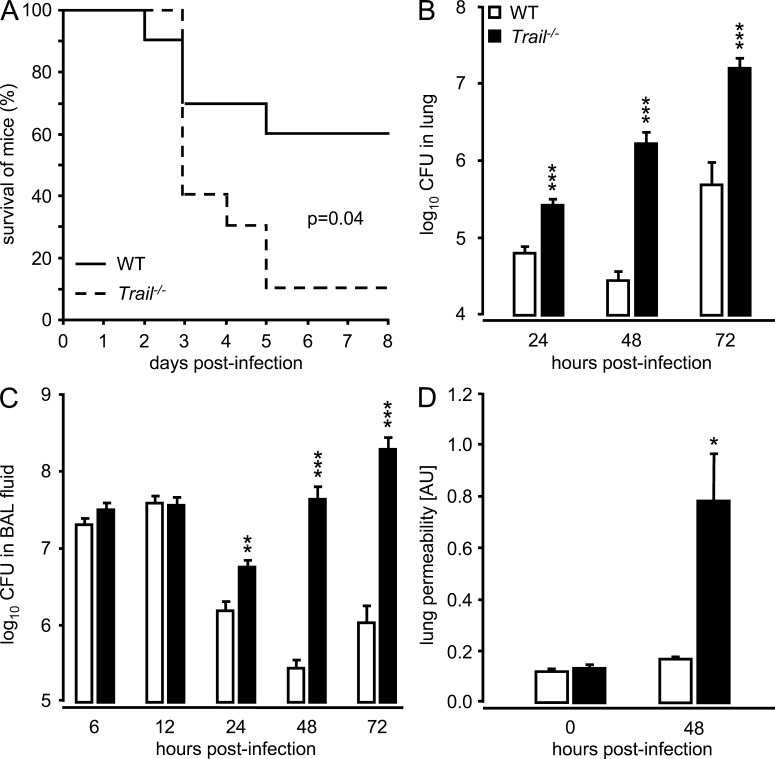
We initially examined the survival of WT and Trail−/− mice challenged with two different strains of S. pneumoniae, either causing focal pneumonia (serotype 19) or invasive pneumococcal disease (serotype 2) in mice. Trail−/− mice demonstrated a significantly increased mortality, relative to WT mice, after infection with serotype 19 S. pneumoniae (Fig. 1 A). Similarly, there was significantly increased mortality of Trail−/− mice after infection with highly virulent serotype 2 S. pneumoniae compared with WT mice (unpublished data). In line with the observed increased mortality, Trail−/− mice exhibited major defects in purging bacterial loads of S. pneumoniae in lung distal airspaces (Fig. 1, B and C). Specifically, we observed a dramatic outgrowth of pneumococci in the lungs of Trail−/− mice at 24 h until 72 h after infection, whereas WT mice were able to control bacterial spread in lung distal airspaces. Consistent with the increased mortality and decreased control of infection, Trail−/− mice displayed significantly increased lung leakage on day 2 after pneumococcal infection compared with WT mice (Fig. 1 D). Together, these data show that TRAIL is indispensible for survival of pneumococcal lung infection in mice.
Figure 1.
Effect of TRAIL on survival and bacterial clearance in mice infected with S. pneumoniae. WT and Trail−/− mice were infected with S. pneumoniae (107 CFU/mouse). Survival at the indicated time points after infection (A; n = 10 mice per group) and bacterial loads of S. pneumoniae (B and C) in lung tissue, and BAL fluids were measured at the indicated time points. Values in B and C are shown as mean ± SEM of n = 5–8 mice (6 and 12 h after infection) or n = 10–13 mice (24–72 h after infection) per treatment group. Experiments in A–C were performed two times. (D) Lung permeability (arbitrary units, AU) was measured at 48 h in WT and Trail−/− mice infected with S. pneumoniae. Data in D are given as mean ± SEM of n = 3 mice per group, and the experiment was repeated two times with similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001, relative to WT mice.
S. pneumoniae triggers increased TRAIL mRNA and protein expression in the lungs of mice
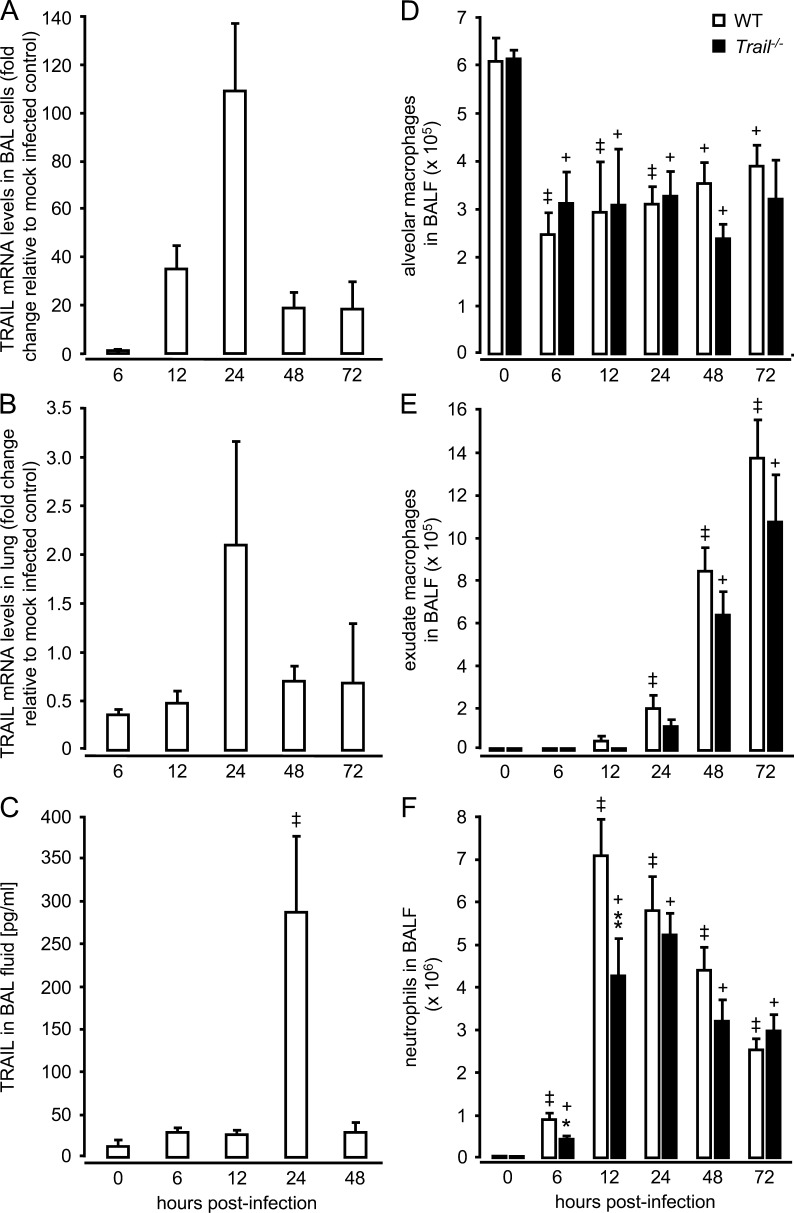
To determine how the kinetics of TRAIL expression relate to the extent of the bacterial infection, we next analyzed TRAIL mRNA and protein expression in the lungs of S. pneumoniae–infected WT mice. TRAIL gene expression was strongly induced at 24 h after infection, both in unfractionated bronchoalveolar lavage (BAL) cells and in lung tissue of WT mice challenged with S. pneumoniae (Fig. 2, A and B). Moreover, TRAIL protein levels in BAL fluids increased ∼4-fold over background (0 h) levels at 24 h after infection (Fig. 2 C). These data illustrate that TRAIL is induced in the lungs of WT mice challenged with S. pneumoniae, and peak TRAIL expression coincides with the control of the infection in WT mice.
Figure 2.
Pulmonary TRAIL mRNA and protein expression and alveolar leukocyte recruitment in response to infection with S. pneumoniae. WT mice were either mock-infected or were infected with S. pneumoniae (107 CFU/mouse). At the indicated time points, mice were subjected to BAL and lungs were removed. Unfractionated BAL cells (A) and lung tissue from washed lungs (B) were subjected to real-time RT-PCR analysis of TRAIL mRNA expression or ELISA analysis of soluble TRAIL protein expression in BAL fluids (C). Values are shown as mean ± SEM of n = 3 mice for TRAIL mRNA analysis (A and B), and n = 6 mice for analysis of soluble TRAIL in BAL fluids (C). The experiment was repeated two times with similar results. ++, P < 0.01 relative to baseline (0 h) values. (D-E) Resident alveolar macrophages (D), inflammatory-recruited exudate macrophages (E), and neutrophils (F) determined in BAL fluids of S. pneumoniae–infected WT and Trail−/− mice. Values are shown as mean ± SEM of n = 5–8 mice (6 and 12 h after infection) or n = 10–13 mice (24–72 h after infection) per time point and treatment group. Results are representative of two independently performed experiments. *, P < 0.05; **, P < 0.01 relative to WT mice. +, P < 0.05; ++, P < 0.01, relative to baseline (0 h) values.
The observed differences in mortality and bacterial loads between WT and Trail−/− mice could be explained by differences in immune cell recruitment into the infected lungs. Thus, we next examined alveolar leukocyte recruitment in WT and Trail−/− mice infected with S. pneumoniae. Both WT and Trail−/− mice responded with a significant depletion of resident alveolar macrophages in their bronchoalveolar space after infection with S. pneumoniae, with no overt differences noted between groups (Fig. 2 D). We also observed similar numbers of alveolar-recruited exudate macrophages in BAL fluids of WT and Trail−/− mice (Fig. 2 E). Notably, TRAIL deficiency did not affect alveolar neutrophil recruitment (Fig. 2 F), i.e., when major differences in bacterial loads between WT and Trail−/− mice were observed (Fig. 1, B and C). Interestingly, the peak of alveolar neutrophil accumulation observed in WT mice at 12–24 h after infection coincided with peak TRAIL mRNA and protein levels in total BAL cells, which consisted of >95% neutrophils. Thus, these data suggest that primarily alveolar-recruited neutrophils contributed to the observed rise in TRAIL in the lungs of WT mice infected with S. pneumoniae (Fig. 2, A–C).
TRAIL deficiency results in necrotic death of lung macrophages in response to pneumococcal infection
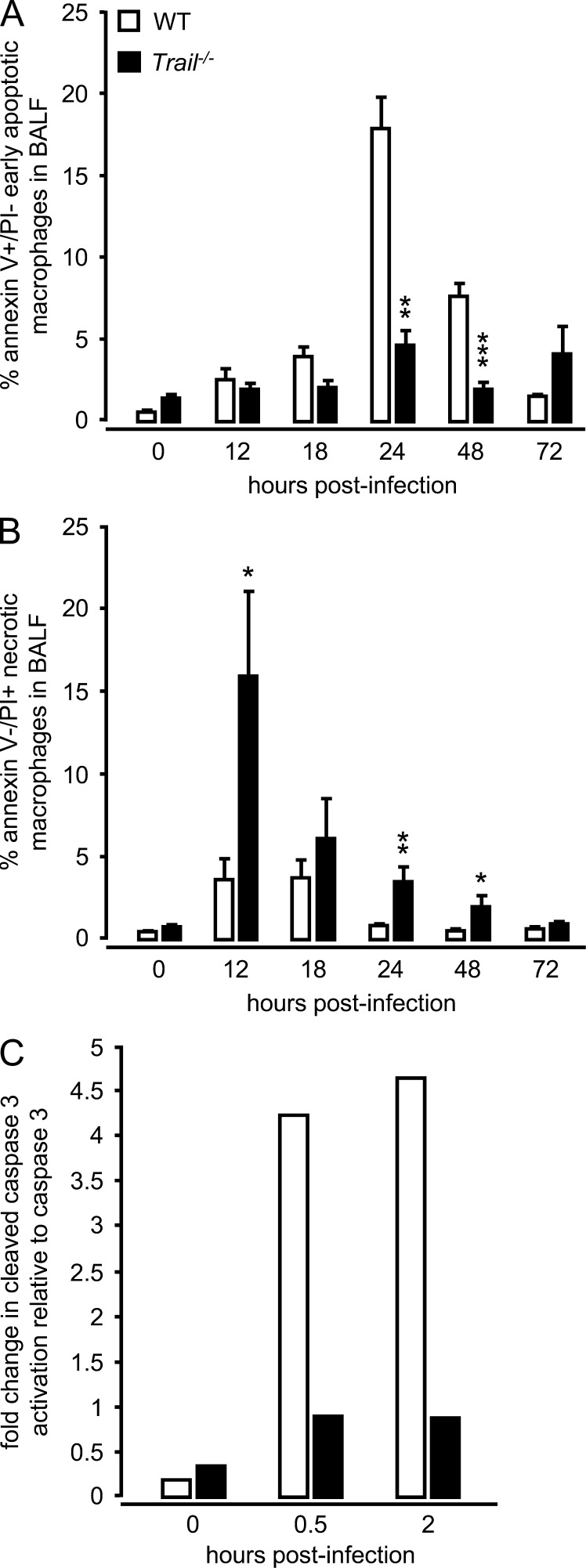
TRAIL induces apoptosis in various target cell populations via DR5 ligation (Falschlehner et al., 2009; Gonzalvez and Ashkenazi, 2010), so we next examined the kinetics of BAL fluid macrophage cell death in S. pneumoniae–infected WT and Trail−/− mice. Alveolar macrophages of WT mice primarily underwent apoptotic cell death, peaking by 24 h after infection, whereas alveolar macrophages of Trail−/− mice demonstrated a significantly reduced frequency of apoptotic cell death that did not substantially increase over the evaluated period of time after infection (Fig. 3 A). Consistent with the observed decreased apoptosis in alveolar macrophages in vivo, purified Trail−/− alveolar macrophages showed reduced caspase 3 activation after challenge with S. pneumoniae in vitro, relative to WT macrophages (Fig. 3 C). WT macrophages also responded with increased TRAIL protein production (control, 3.4 pg/ml; 0.5 h after infection, 17 ± 2 pg/ml; 2 h after infection, 21 ± 1 pg/ml; P < 0.05 versus control macrophages) after challenge with S. pneumoniae, suggesting that increased caspase 3 activation (and hence apoptosis induction) observed in WT macrophages was induced by TRAIL under these in vitro conditions.
Figure 3.
Cell death in alveolar macrophages of WT and Trail−/− mice after infection with S. pneumoniae. WT and Trail−/− mice were infected with S. pneumoniae (5 × 106 CFU/mouse). At the indicated time points, mice underwent BAL and the percentage of early apoptotic (Annexin V–positive/PI-negative; A), and necrotic (PI-positive/Annexin V–negative) alveolar macrophages (B) in BAL fluid was determined by flow cytometry. (C) Fold change in cleaved caspase 3 activation relative to caspase 3 in the alveolar macrophages of Trail−/− mice versus WT mice that were either mock-infected (0 h) or infected with S. pneumoniae (MOI 2) in vitro. Values are shown as mean ± SEM of n = 6 mice per time point and treatment group. Experiments in A and B were performed three times with similar results. Semiquantitative immunoblot analysis in C is representative of two independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, relative to WT mice.
Importantly, there was a substantially increased frequency of necrotic macrophages in the BAL fluids of Trail−/− mice as early as 12 h after pneumococcal challenge (Fig. 3 B), relative to WT mice. We did not observe differences in apoptosis and necrosis induction in alveolar neutrophils collected from WT compared with Trail−/− mice infected with S. pneumoniae (unpublished data). The similar CFU observed in the lungs of WT and Trail−/− mice at 12 h (Fig. 1 C) would suggest direct S. pneumoniae–mediated cytotoxicity was not the cause of the increased necrosis in the Trail−/− macrophages. Therefore, these data suggest that the lack of TRAIL resulted in a shift from antiinflammatory apoptosis to proinflammatory necrosis of macrophages in the lung.
TRAIL deficiency results in an excessive lung inflammatory response after pneumococcal infection
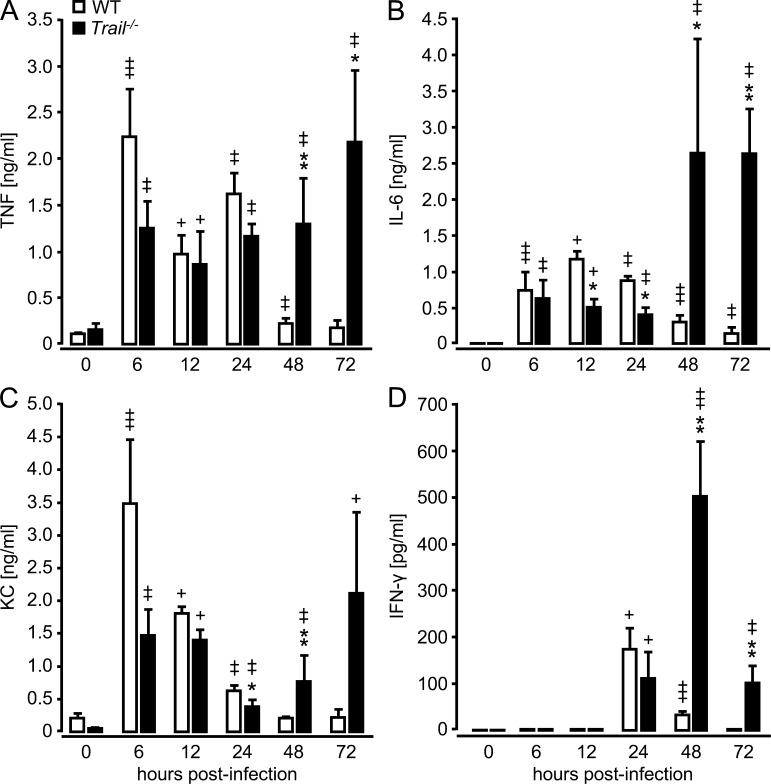
Based on the observed defects in bacterial pathogen elimination and reduced alveolar macrophage apoptosis in S. pneumoniae–infected Trail−/− mice, we hypothesized that Trail−/− mice would exhibit hyperinflammatory responses after S. pneumoniae infection. Indeed, we observed a significant increase in TNF, IL-6, KC, and IFN-γ in the lungs of Trail−/− mice relative to WT mice beginning at 48 h after infection (Fig. 4). These data support a critical role of TRAIL for regulation of proinflammatory cytokine responses during pneumococcal pneumonia in mice.
Figure 4.
Effect of TRAIL deficiency on the release of proinflammatory cytokines in the lungs of S. pneumoniae–infected mice. WT and Trail−/− mice were infected with S. pneumoniae (107 CFU/mouse), followed by BAL at the indicated time points. TNF (A), IL-6 (B), KC (C), and IFN-γ (D) were analyzed in BAL fluids by ELISA, as indicated. Values are shown as mean ± SEM of n = 5–8 mice for 6 and 12 h after infection, and n = 10–13 mice for 24–72 h after infection (n = 3 for 0 h) per treatment group. Data are representative of two independent experiments. *, P < 0.05; **, P < 0.01 relative to WT mice. +, P < 0.05; ++, P < 0.01; +++, P < 0.001 relative to baseline (0 h) values.
TRAIL is expressed on the cell surface of lung macrophages but not neutrophils in response to S. pneumoniae
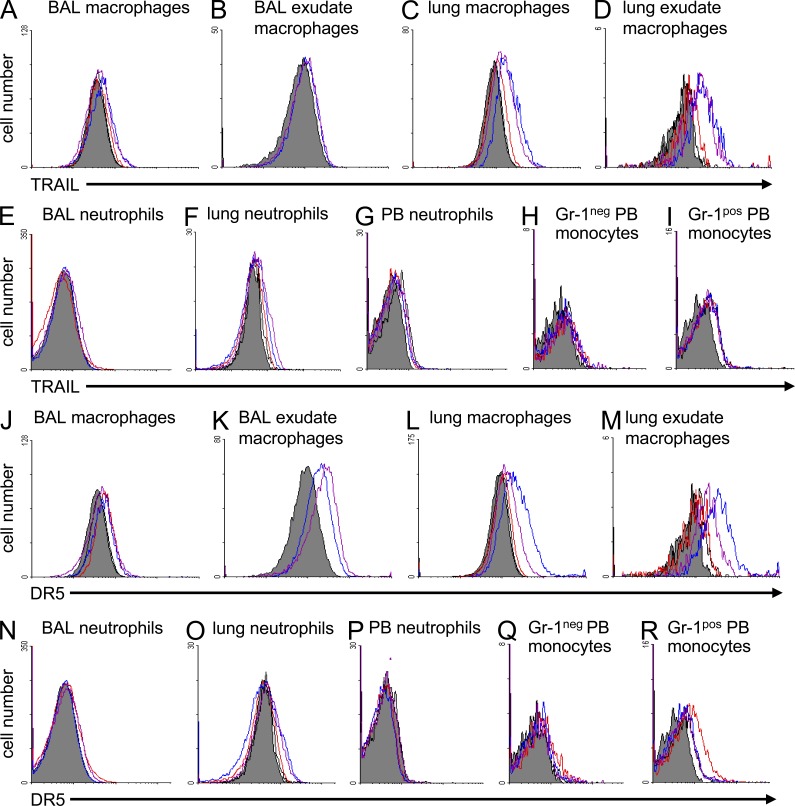
TRAIL not only exists as a membrane-bound molecule, but also as a soluble protein released upon cleavage of the membranous protein (Mariani and Krammer, 1998). Given that only macrophages, but not neutrophils, had increased necrosis induction in Trail−/− mice, we examined the relative contribution of macrophages versus neutrophils to mediate the observed TRAIL-dependent effects during pneumococcal pneumonia. TRAIL and DR5 expression was analyzed on lung professional phagocyte subsets in mock-infected and S. pneumoniae-infected WT mice. As shown in Fig. 5, resident alveolar macrophages and exudate macrophages (but not alveolar-recruited neutrophils) expressed TRAIL on their cell surface upon pneumococcal challenge. In addition, resident lung macrophages (i.e., macrophages remaining in lung tissue after BAL) and inflammatory recruited lung exudate macrophages expressed TRAIL after pneumococcal infection (Fig. 5, A–E), whereas the used anti-TRAIL antibody did not bind to lung macrophages from Trail−/− mice (unpublished data). Gr-1pos inflammatory monocytes in peripheral blood (precursors of lung exudate macrophages) up-regulated TRAIL expression after pulmonary S. pneumoniae infection, whereas circulating blood neutrophils and neutrophils in lung tissue did not (Fig. 5, F–I). Assessment of DR5 on lung professional phagocyte subsets revealed increased DR5 expression on resident alveolar macrophages and exudate macrophages, and even more so on lung macrophages and lung exudate macrophages of WT mice after infection with S. pneumoniae, whereas BAL fluid neutrophils and neutrophils in lung tissue and peripheral blood, as well as Gr-1neg blood monocytes but not Gr-1pos blood monocytes, lacked both TRAIL and DR5 on their cell surface before and after infection with S. pneumoniae (Fig. 5, J–N and O–R). Notably, the used anti-DR5 antibody did not stain lung macrophages of S. pneumoniae–infected Dr5−/− mice (unpublished data).
Figure 5.
TRAIL and DR5 expression profiles on professional phagocyte subsets of S. pneumoniae–infected mice. TRAIL and DR5 expression was analyzed on resident alveolar macrophages, inflammatory recruited alveolar exudate macrophages, or neutrophils contained in BAL, or on lung macrophages, lung exudate macrophages, and neutrophils collected from lung tissue of mock-infected or S. pneumoniae–infected WT or Trail−/− mice (5 × 106 CFU/mouse) by flow cytometry (A–F and J–O). Additionally, TRAIL and DR5 expression was analyzed on the cell surface of resident F4/80-positive/Gr-1–negative blood monocytes (H and Q), F4/80-positive/Gr-1–positive inflammatory blood monocytes (I and R), or peripheral blood (PB) neutrophils (G and P) of mock- and S. pneumoniae–infected WT mice (5 × 106 CFU/mouse) by flow cytometry. The given overlays are representative of n = 3 WT mice per treatment group, with similar results. Gray histograms (negative control) indicate baseline expression and open histograms indicate TRAIL or DR5 expression at 24 (red line), or 48 (blue line), or 72 h (purple line) after infection.
Neutrophils are an important source of secreted TRAIL, mediating macrophage apoptosis during pneumococcal pneumonia
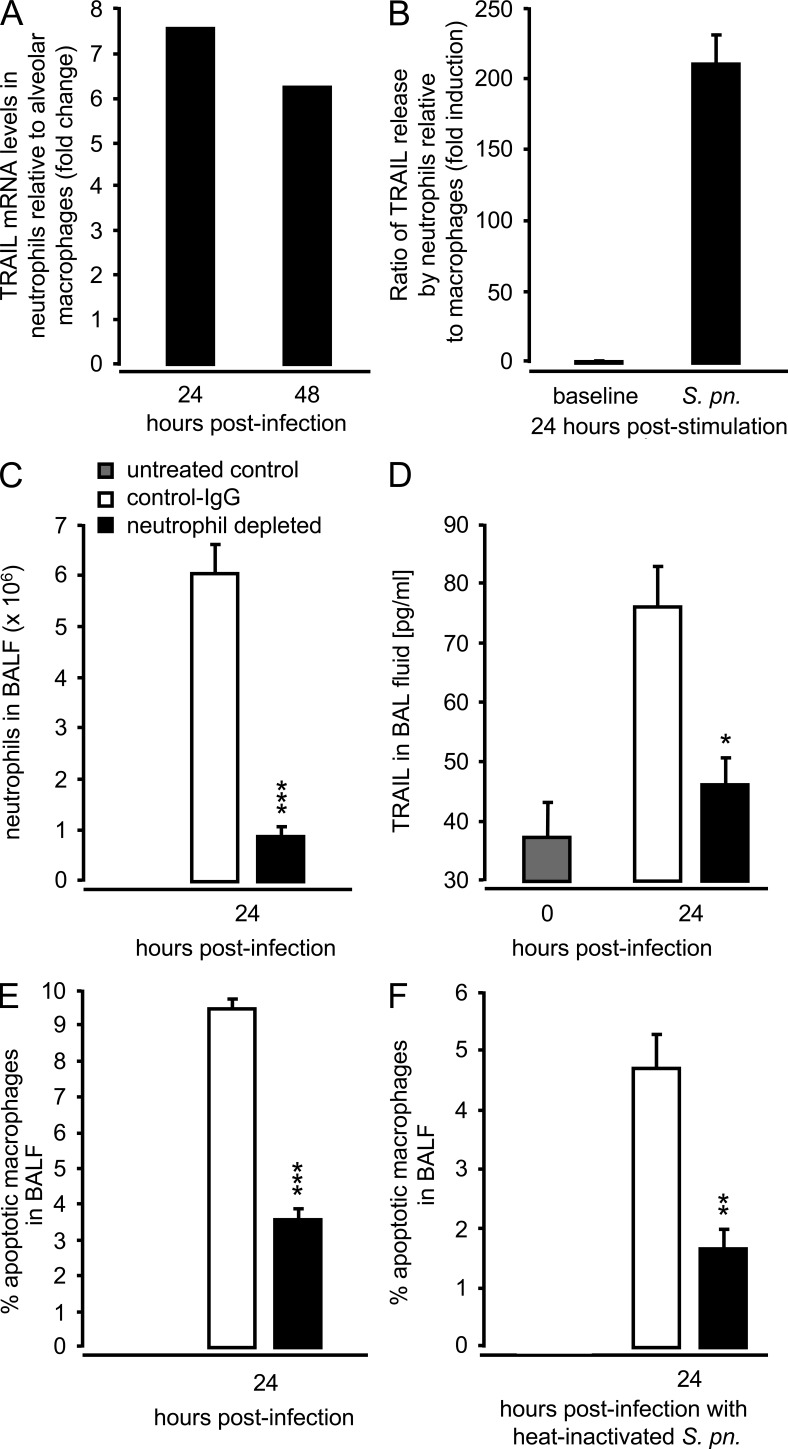
Neutrophils produce and release soluble TRAIL upon inflammatory activation (Tecchio et al., 2004; Kemp et al., 2005; Cassatella et al., 2006; Simons et al., 2007), so we examined to what extent neutrophils contributed to the TRAIL response to S. pneumoniae. Quantitative RT-PCR revealed >6-fold higher TRAIL transcript levels in flow-sorted alveolar neutrophils relative to alveolar macrophages collected 24 and 48 h after infection from the same mice (Fig. 6 A). Moreover, cell-free culture supernatants from BM-derived neutrophils challenged with S. pneumoniae in vitro contained highly elevated TRAIL protein levels compared with equally infected BM-derived macrophages (BMDM) 24 h after infection (Fig. 6 B). Together, these data suggest neutrophils are the primary source of TRAIL in the lungs of S. pneumoniae–infected mice.
Figure 6.
Effect of neutrophil depletion on macrophage apoptosis after challenge with live or heat-inactivated S. pneumoniae. WT mice were infected with S. pneumoniae (107 CFU/mouse). At 24 and 48 h after infection, mice were subjected to BAL, and sorted alveolar macrophages and alveolar-recruited neutrophils were subjected to real-time RT-PCR analysis of TRAIL mRNA levels. (A) Fold change of TRAIL mRNA in alveolar-recruited neutrophils relative to alveolar macrophages from the same mice infected with S. pneumoniae, as indicated. Due to the lack of neutrophils in untreated mice, a 0-h time point could not be included in A. (B) BM-derived neutrophils and BMDMs were either mock infected or were infected with S. pneumoniae (MOI 1). After 24 h, the amount of soluble TRAIL in culture supernatants was determined by ELISA. (C-F) Mice were pretreated with isotype control Ab or neutrophil-depleting antibody (anti–Ly-6G, clone 1A8) i.p. at −12 h and −2 h before either infection with live S. pneumoniae (5 × 106 CFU/mouse; C–E) or heat-inactivated S. pneumoniae (dose equivalent to 1.5 × 107 CFU/mouse; F). After 24 h, numbers of alveolar-recruited neutrophils were determined (C), the effect of neutrophil depletion on TRAIL protein levels in BAL fluids of mice was determined (D), and the percentage of apoptotic macrophages after infection with live (E) or heat-inactivated S. pneumoniae (F) was analyzed. Values are shown as mean ± SEM of n = 5 (C–F) mice per time point and treatment group. The results are representative of two independent experiments. *, P < 0.05; **, P < 0.001; ***, P < 0.001, relative to WT mice.
We further examined the role of neutrophil-derived TRAIL to induce apoptosis in macrophages during pneumococcal pneumonia. Depletion of neutrophils before pneumococcal challenge (Fig. 6 C) led to significantly reduced amounts of TRAIL in BAL fluids of S. pneumoniae–challenged mice (Fig. 6 D), which also significantly reduced macrophage apoptosis 24 h after infection (Fig. 6 E). Challenge of neutropenic WT mice with heat-inactivated S. pneumoniae also resulted in significantly reduced macrophage apoptosis 24 h after infection (Fig. 6 F), further supporting the concept that the reduction in amount of TRAIL protein (as a consequence of neutropenia), rather than direct cytotoxic effects of pneumococci, contributed to the observed reduced macrophage apoptosis in BAL fluids of neutropenic mice. Collectively, the data in Fig. 6 show that (a) neutrophils are an important source of soluble TRAIL in the lungs of S. pneumoniae–challenged mice, (b) neutrophil-derived TRAIL induces apoptosis in S. pneumoniae-infected alveolar macrophages, and (c) macrophages in neutropenic WT mice undergo necrotic cell death in response to infection with S. pneumoniae.
Pulmonary TRAIL therapy improves survival of mice challenged with S. pneumoniae
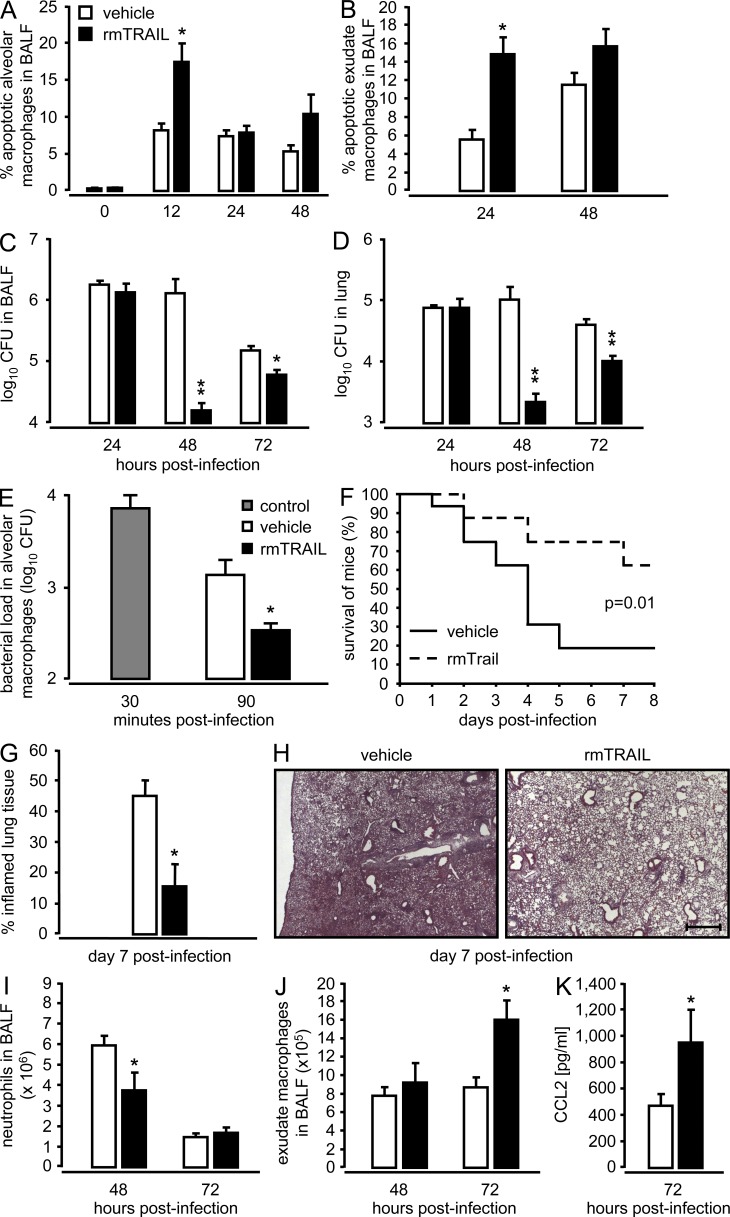
In an effort to explore the therapeutic efficacy of TRAIL as apoptosis-inducing cytokine to attenuate disease severity during pneumococcal pneumonia, WT mice were infected with S. pneumoniae and then received a single therapeutic application of recombinant murine TRAIL into the lungs 6 h after infection. There was significantly increased apoptosis of resident alveolar macrophages 12 h after infection, followed by a delayed, but significant increase in apoptotic exudate macrophages peaking at 24 h after infection (Fig. 7, A and B). This increase in TRAIL-dependent macrophage apoptosis was accompanied by significantly decreased lung bacterial loads at 48 and 72 h after infection, relative to vehicle-treated WT mice (Fig. 7, C and D). Notably, therapeutic application of recombinant TRAIL at 12 h after infection still resulted in significantly decreased bacterial loads in BAL fluids of mice at 48 h after infection (vehicle-treated: 2.4 ± 0.4 × 104 CFU versus TRAIL-treated mice: 4 ± 2 × 103; n = 4; P < 0.03; representative of two independent experiments performed). Moreover, alveolar macrophages exhibited a significantly increased pneumococcal killing in the presence of recombinant TRAIL (Fig. 7 E), demonstrating that TRAIL-induced macrophage apoptosis directly results in the killing of phagocytosed pneumococci. Even more important was the finding that a single therapeutic instillation of recombinant TRAIL into the lungs 6 h after high-dose challenge with S. pneumoniae (1.5 × 107 CFU/mouse) resulted in dramatically improved survival relative to vehicle-treated mice (Fig. 7 F). Histopathological examination of lung tissue sections of S. pneumoniae–infected WT mice receiving TRAIL therapy revealed substantially reduced consolidated infiltrates and inflamed lung tissue compared with vehicle-treated mice at day 7 after infection (Fig. 7, G and H). Moreover, therapeutic application of TRAIL strongly promoted the lung resolution/repair process, as judged by (a) decreased neutrophil counts in the lungs of mice at 48 h after infection and (b) significantly increased recruitment of exudate macrophages observed at 72 h after infection coinciding with (c) significantly increased levels of the monocyte chemoattractant protein, CCL2 in BAL fluids of WT mice (Fig. 7, I–K). Collectively, these data show that TRAIL therapy regulates alveolar macrophage apoptosis and early bacterial killing, with major beneficial effects on severity and outcome of pneumococcal pneumonia.
Figure 7.
Effect of therapeutic application of recombinant murine TRAIL on apoptosis induction in lung macrophages, and bacterial pathogen elimination and survival of mice infected with S. pneumoniae. (A–D) Mice were infected with S. pneumoniae (5 × 106 CFU/mouse), followed by therapeutic intratracheal application of either vehicle (white bars) or recombinant murine (rm) TRAIL (2.5 µg/mouse, 6 h after infection; filled bars). At the indicated time points, the percentage of apoptotic alveolar macrophages (A) and exudate macrophages (B) was analyzed, and bacterial loads were determined in BAL fluids (C) and lung tissue (D) of mice. Values are shown as mean ± SEM of n = 5–9 mice per time point and treatment group. Data are representative of two independent experiments with similar results. *, P < 0.05; **, P < 0.01 relative to vehicle-treated WT mice. (E) In vitro killing activity of alveolar macrophages infected with S. pneumoniae (MOI 50) in the presence (filled bar) or absence (open bar) of rmTRAIL (10 ng/ml) analyzed at 90 min after infection. The gray bar represents the loading of untreated macrophages with S. pneumoniae determined at 30 min after inoculation. Values in E are shown as mean ± SEM of triplicate determinations, with independent experiments repeated two times. *, P < 0.05; **, P < 0.01, relative to vehicle-treated macrophages. (F–K) Effect of therapeutic treatment with vehicle (solid line in F; open bars in G and I–K) or single rmTRAIL (2.5 µg/mouse, applied at 6 h after infection; dashed line in F; filled bars in G and I–K) on survival of mice infected with S. pneumoniae (high-dose challenge, 1.5 × 107 CFU/mouse). Survival was monitored during an observation period of 8 d (n = 16 mice per treatment group). For histopathological assessment of lung injury, the percentage of inflamed lung tissue was calculated in HE-stained lung tissue sections of mice of the two treatment groups (n = 3 mice per group) at day 7 after infection (G and H). Representative photographs are shown at 2.5× original magnification (Bar, 500 µm) using an Axiovert 200 M microscope. (I and J) At the indicated time-points, mice were subjected to BAL for enumeration of alveolar-recruited neutrophils (I) and recruited exudate macrophages (J) and for determination of BAL fluid CCL2 levels (K). Data in F–K are representative of two independent experiments with similar results. Values in I–K are shown as mean ± SEM of n = 5–6 mice per time point and treatment group. *, P < 0.05, relative to vehicle-treated WT mice.
Therapeutic application of agonistic anti-DR5 antibody MD5-1 improves survival of intact and neutrophil-depleted mice challenged with S. pneumoniae
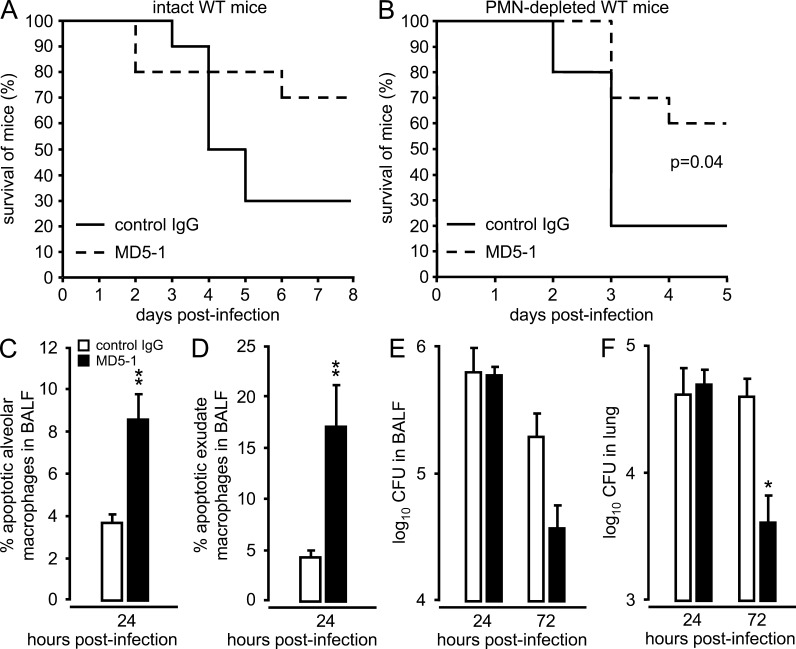
The data presented to this point support our hypothesis that alveolar-recruited neutrophils are a major source of intraalveolar released TRAIL, which directs infected macrophages toward apoptotic cell death during pneumococcal pneumonia. With this in mind, we examined the efficacy of an agonistic anti-DR5 mAb (MD5-1), instead of recombinant TRAIL, to exert protective effects in S. pneumoniae–infected WT mice with a normal hematopoietic system, but also in neutropenic WT mice. Single therapeutic application of agonistic MD5-1 mAb substantially improved survival of intact WT mice given a high-dose challenge with S. pneumoniae, but also significantly improved survival of S. pneumoniae–challenged neutropenic mice (Fig. 8, A and B). MD5-1 therapy of S. pneumoniae–infected WT mice recapitulated the effects on apoptosis induction in resident and exudate macrophages as reported for recombinant TRAIL therapy, accompanied by significantly reduced bacterial loads in lungs of WT mice at 72 h after infection (Fig. 8, C–F). Collectively, these data show the therapeutic efficacy of MD5-1 dependent activation of the TRAIL–DR5 axis in neutropenic hosts, acting as a compensatory tool for the lack of neutrophil-derived TRAIL. This strategy may thus be a valuable therapeutic approach for the treatment of neutropenic, immunocompromised patients.
Figure 8.
Effect of agonistic anti-DR5 mAb therapy on survival and alveolar macrophage apoptosis of mice challenged with S. pneumoniae. Wild-type mice were either left untreated (A) or were made neutropenic by i.p. injection of neutrophil-depleting 1A8 antibody (B) applied at −12 h and −2 h before infection with S. pneumoniae (107 CFU/mouse and 3–4 × 106 CFU/mouse, respectively). 6 h after infection, mice received a single intratracheal application of control IgG or agonistic anti-DR5 mAb (MD5-1; 75 µg/mouse), and survival (n = 10 mice per group) was recorded over time. Data in A and B are representative of two to three independent experiments. (C–F) WT mice were infected with S. pneumoniae (5 × 106 CFU/mouse). 6 h after infection, mice either received a single intratracheal instillation of control IgG (white bars) or agonistic anti-DR5 antibody MD5-1 (75 µg/mouse; black bars). At 24 h after infection, induction of apoptosis was analyzed in resident alveolar macrophages (C) and exudate macrophages (D) by flow cytometry, and respective bacterial loads were determined at 24 and 72 h after infection both in BAL fluids (E) and lung tissue (F), as indicated. The data in (C-F) are shown as mean ± SEM of n = 3 mice per time point and treatment group and are representative of two independent experiments with similar results. *, P < 0.05; **, P < 0.01, relative to control IgG.
DISCUSSION
In this study, we investigated the importance of the TRAIL–TRAIL receptor system in lung macrophage apoptosis and its impact on lung protective immunity against the prototypic lung pathogen S. pneumoniae. Trail−/− mice showed significantly increased mortality compared with WT mice upon infection with S. pneumoniae. Moreover, lung macrophages from Trail−/− mice underwent necrotic death after infection with S. pneumoniae, which was in contrast to WT mice that had lung sentinel cells undergo apoptotic death after bacterial challenge. This scenario of increased necrotic cell death in Trail−/− mice was accompanied by increased bacterial burden and pro-inflammatory cytokines in their lungs. Examination of TRAIL expression by lung professional phagocyte subsets revealed that in addition to TRAIL-expressing alveolar and lung macrophages, alveolar-recruited neutrophils in S. pneumoniae–infected mice were identified as a major source of secreted TRAIL within the bronchoalveolar compartment. Based on these data, we believe that soluble TRAIL released from alveolar accumulating neutrophils is largely responsible for inducing apoptotic cell death in alveolar and lung macrophages in response to S. pneumoniae to limit both bacterial spread and proinflammatory responses. In support of this hypothesis, we show for the first time that therapeutic application of recombinant murine TRAIL or agonistic anti-DR5 mAb substantially improved the survival of both normal and neutropenic WT mice after high-dose challenge with S. pneumoniae. Therefore, activation of the TRAIL–DR5 axis may represent a novel therapeutic avenue for the treatment of life-threatening pneumococcal pneumonia particularly, but not exclusively, in immunocompromised patients.
A prominent feature of pneumococcal lung infection is the rapid apoptotic cell death of lung macrophages in the early phase of infection, which is considered an important step in limiting infection-induced lung inflammation and acute lung injury (Paton, 1996; Dockrell et al., 2003; Maus et al., 2004, 2007; Marriott et al., 2006; Winter et al., 2007; Taut et al., 2008; Hahn et al., 2011b). TRAIL induces apoptosis in various leukocyte subsets, as well as lung barrier cells (Katsikis et al., 1997; Renshaw et al., 2003; Zheng et al., 2004; Lum et al., 2005; McGrath et al., 2011; Zhu et al., 2011). Therefore, we hypothesized that TRAIL might underlie the observed apoptotic death of lung macrophages after infection with S. pneumoniae. Several observations of the current study support this concept. First, when TRAIL was present, alveolar macrophages died predominately by apoptosis in response to S. pneumoniae, whereas Trail−/− macrophages primarily underwent necrotic cell death. Second, consistent with this finding, lung macrophages of WT mice responded with increased DR5 expression after S. pneumoniae infection, presumably becoming more responsive to TRAIL-mediated apoptosis. Third, caspase 3 cleavage acting downstream of DR5 signaling was induced primarily in WT macrophages after S. pneumoniae challenge in vitro, again supporting the view that absence of TRAIL during pneumococcal pneumonia fails to trigger apoptosis in macrophages that cannot be compensated by other death receptor signaling pathways, such as the Fas–Fas ligand axis (Ali et al., 2003; Matute-Bello et al., 2005). We did not observe any differences in the apoptosis/necrosis rate of alveolar-recruited neutrophils in S. pneumoniae–infected WT and Trail−/− mice, as well as WT mice receiving TRAIL therapy, which is consistent with the finding that neutrophils express little to no DR5.
In contrast to WT mice, S. pneumoniae–infected Trail−/− mice were characterized by: macrophages undergoing necrotic cell death, significantly increased levels of proinflammatory cytokines in BAL fluids, increased lung tissue damage, increased bacterial burdens, and increased mortality. These data lead us to conclude that TRAIL directs the fate of S. pneumoniae–infected alveolar macrophages toward apoptotic cell death, thereby favoring a diminished viability of phagocytosed bacteria and their subsequent elimination from distal lung air spaces after pneumococcal challenge. This concept is strongly supported by the reported therapeutic efficacy profile of both recombinant TRAIL and agonistic MD5-1 antibody, both of which induced increased apoptotic cell death of macrophages, concomitantly reduced lung bacterial loads, and significantly improved survival of mice after high-dose challenge with S. pneumoniae.
Host cell apoptosis has been identified as a protective mechanism in various viral and (myco-) bacterial infection models, as targeted apoptosis induction in infected host cells hinders intracellular spread of bacterial and viral pathogens (Clem and Miller, 1993; Antoni et al., 1995; Leemans et al., 2001; Behar et al., 2011). Studies on macrophage apoptosis in lung protective immunity against inhaled bacterial pathogens have widely used liposomal encapsulated clodronate as an experimental tool. However, clodronate liposomes deplete virtually all lung macrophage subsets, and as such are deleterious for the lung host defense against extracellular pathogens such as Klebsiella pneumoniae or S. pneumoniae (Broug-Holub et al., 1997; Knapp et al., 2003). In contrast, the current study illustrates that a defined and limited macrophage apoptosis induction in the lungs of mice, as induced by treatment with TRAIL or agonistic anti-DR5 mAb had a major therapeutic effect on the survival of lethal pneumococcal pneumonia. Thus, the timing and mode of apoptosis induction in lung sentinel cells are two critical determinants affecting the survival of severe pneumonia. Our study is also the first to report that even neutropenic hosts could survive fatal pneumococcal lung infection upon activation of the TRAIL–DR5 axis. In addition, we provide evidence of a novel TRAIL–DR5-dependent neutrophil–macrophage cross talk that further expands the nonoxidative antibacterial repertoire of neutrophils in lung bacterial infections.
In the current study, we observed that TRAIL therapy increased CCL2 in BAL fluids of mice, along with increased alveolar recruitment of exudate macrophages and an improved inflammation resolution subsequent to pneumococcal challenge. Inflammatory exudate macrophage recruitment is regulated by the CCL2–CCR2 axis and critically contributes to the resolution/repair phase after pneumococcal challenge (Winter et al., 2007), making it likely that increased numbers of CCL2-elicited exudate macrophages contributed to the observed improved resolution/repair process and survival of WT mice treated with recombinant TRAIL. Mechanistically, CCR2-expressing macrophages regulate intraalveolar CCL2 levels during lung inflammation (Maus et al., 2005). Therefore, the observed increase in intraalveolar CCL2 and concomitant increase in exudate macrophage recruitment observed in S. pneumoniae–infected, TRAIL-treated mice were most likely caused by TRAIL-dependent apoptosis of CCR2-expressing resident alveolar macrophages that led to increased intraalveolar CCL2 release and subsequent lung exudate macrophage recruitment. Hence, we believe the improved lung resolution/repair process observed in response to TRAIL therapy is most likely an indirect consequence of the current treatment regimen.
Previous studies demonstrated TRAIL-inducing apoptosis in human and murine neutrophils in various models, including LPS-induced acute lung injury and zymosan-induced peritonitis (McGrath et al., 2011); however, we did not observe any differences in neutrophil apoptosis induction in WT and Trail−/− mice infected with S. pneumoniae. Moreover, we were unable to detect either TRAIL or DR5 on the cell surface of peripheral blood neutrophils or alveolar-recruited neutrophils of S. pneumoniae–infected mice, contrary to the observed TRAIL and DR5 expression on Gr-1pos inflammatory monocyte subsets in peripheral blood. It appears unlikely that inflammatory activation of elicited neutrophils triggered TRAIL cleavage or DR5 down-regulation on alveolar neutrophils in WT mice, as both of these molecules were detectable on macrophages collected from the same inflammatory microenvironment. We cannot exclude the possibility that DR5 expression on alveolar-recruited neutrophils was below detectable levels measured by flow cytometry, so a role for TRAIL in mediating neutrophil apoptosis during pneumococcal pneumonia remains unknown. By comparison, our data suggests neutrophils were a major source for soluble TRAIL released within the alveolar space, as alveolar-recruited neutrophils demonstrated more than sevenfold increased TRAIL mRNA levels relative to alveolar macrophages by 24 h after infection. Equal cell numbers of BM-derived neutrophils released significantly more TRAIL into cell culture supernatants relative to BMDMs infected with S. pneumoniae, and depletion of neutrophils substantially decreased the amount of soluble TRAIL in BAL fluids of S. pneumoniae–infected mice and substantially reduced TRAIL-mediated macrophage apoptosis. Together, these data also suggest a previously unrecognized TRAIL–DR5-dependent neutrophil-macrophage cross talk, which offers promising perspectives for therapeutic interventions even in immunocompromised, neutropenic patients.
In summary, we show that apoptosis of lung macrophages in pneumococcal pneumonia is mediated by neutrophil-derived TRAIL, which exerts important antiinflammatory effector functions in pneumococcal pneumonia in mice. Moreover, we for the first time show that therapeutic engagement of the TRAIL–DR5 axis by either application of recombinant TRAIL or agonistic anti-DR5 antibody exerts major protective effects on survival of mice subjected to severe pneumococcal pneumonia. These findings may direct the development of novel antibacterial treatment options for neutropenic patients.
MATERIALS AND METHODS
Mice.
Trail−/− mice, backcrossed >10 generations onto the C57BL/6 (The Jackson Laboratory) background, have been previously characterized (Sedger et al., 2002; Griffith et al., 2011). As a reference strain, WT C57BL/6 mice purchased from Charles River were used. In selected experiments designed to assess the specificity of the anti-DR5 antibody used, Dr5−/− mice (Finnberg et al., 2005; Gurung et al., 2011) backcrossed for >10 generations onto the C57BL/6 background were used. Mice were kept under conventional conditions with free access to food and water, and were used for the described experiments at 8–12 wk of age. This study was performed in accordance with the guidelines of the Animal Care and Use Committee of the Central Animal Facility at Hannover School of Medicine. Animal experiments were approved by the Lower Saxony State Office for Consumer Protection and Food Safety, Hannover, Germany.
Reagents.
Rabbit anti-cleaved caspase 3 (clone 5A1) and anti–caspase 3 antibody were purchased from Cell Signaling Technologies. Mouse anti–β-actin antibody (clone AC-15) was purchased from Sigma-Aldrich. Peroxidase-conjugated donkey anti–rabbit polyclonal IgG (H+L) and peroxidase-conjugated goat anti–mouse polyclonal IgG (H+L) were purchased from Jackson ImmunoResearch Laboratories. All antibodies used for flow cytometry, including anti-CD11c PE-Cy5.5, anti-CD11b PE-Cy7, anti-Ly-6G/Ly-6C (Gr-1) PE-Cy7, and anti-Ly6G/Ly-6C (Gr-1) FITC, were purchased from BD. Anti-F4/80 FITC and anti-F4/80 APC were purchased from Serotec. Anti-TRAIL PE and anti-DR5 PE were purchased from eBioscience. The MACS kit and CD11c beads for purification of CD11c+ cells from lung parenchymal tissue were purchased from Miltenyi Biotec. In selected experiments, neutrophil-specific mouse anti–Ly-6G antibody (clone 1A8) was used for in vivo neutrophil depletion, according to recent protocols (Hahn et al., 2011a). Rat IgG2a isotype antibody (clone 2A3) was used as control (both from BioXCell). Recombinant murine TRAIL was purchased from R&D Systems. Agonistic anti-DR5 mAb MD5-1 was purified from hybridoma culture supernatants. Isotype control IgG for in vivo studies was purchased from eBioscience.
Culture, quantification, and infection of mice with S. pneumoniae.
Throughout this study, we used a capsular group 19 S. pneumoniae strain EF3030. In selected experiments, a virulent serotype 2 S. pneumoniae strain D39 was used, as indicated (Briles et al., 2003; Winter et al., 2007; Henken et al., 2010b). Bacteria were grown in Todd-Hewitt broth (Oxoid) supplemented with 20% FCS, and aliquots were snap-frozen in liquid nitrogen and stored at −80°C. S. pneumoniae stocks were quantified by plating serial dilutions on sheep blood agar plates (BD), followed by incubation at 37°C/5% CO2 for 18 h and subsequent determination of CFU (Maus et al., 2007; Winter et al., 2007; Henken et al., 2010b).
Intratracheal infection with S. pneumoniae at the respective infection doses was performed essentially as previously described (Henken et al., 2010b; Steinwede et al., 2011; Weber et al., 2012). Mock-infected mice received intratracheal instillations of PBS only. After infection, mice were returned to their cages and monitored daily for disease symptoms. Survival of S. pneumoniae–infected mice was recorded daily during an observation period of 14 d. In some experiments, mice received intratracheal applications of heat-inactivated (65°C, 30 min) pneumococci at a predefined multiplicity of infection (MOI), as indicated.
Determination of bacterial loads.
Bacterial loads in BAL fluids of S. pneumoniae–infected mice were determined by plating 10-fold serial dilutions of aliquots from the respective BAL fluid samples of each mouse on sheep blood agar plates (BD), followed by incubation at 37°C/5% CO2 for 18 h. Subsequent to collection of BAL fluid, lung tissue was homogenized in 2 ml of HBSS without supplements using a tissue homogenizer (IKA), and 10-fold serial dilutions of lung tissue homogenates were plated on sheep blood agar plates for CFU determination.
Therapeutic treatment of mice with recombinant murine TRAIL or MD5-1.
After infection with S. pneumoniae, mice received a single intratracheal application of either recombinant murine TRAIL (rmTRAIL, 2.5 µg/mouse, dissolved in 50 µl PBS/0.1% HSA) or vehicle (50 µl PBS/0.1% HSA), or agonistic anti-DR5 antibody MD5-1 or control IgG (75 µg/mouse in PBS) under desfluran anesthesia (Baxter), as recently described (Steinwede et al., 2011).
BAL and determination of BAL fluid leukocyte differentials.
Total leukocyte numbers in BAL fluids were determined from whole-lung washes of S. pneumoniae–infected mice. In brief, mice were euthanized with an overdose of Isoflurane (Baxter), and tracheas were exposed and cannulated with a shortened 20-gauge needle that was firmly fixed to the trachea. 300-µl aliquots of cold PBS supplemented with EDTA (Biochrom) were instilled, followed by careful aspiration, until a BAL fluid volume of 1.5 ml was collected. BAL was then continued until an additional BAL fluid volume of 4.5 ml was collected. Whole-lung washes were subjected to centrifugation at 1,400 rpm (4°C, 10 min), and cell pellets were pooled to determine total numbers of BAL fluid leukocytes. Quantification of BAL fluid leukocyte subsets was done on differential cell counts of Pappenheim-stained cytocentrifuge preparations, using overall morphological criteria, including cell size and shape of nuclei and subsequent multiplication of those values with the respective absolute BAL cell counts (Maus et al., 2002, 2007; Taut et al., 2008). Quantification of resident or recruited mononuclear phagocyte subsets (alveolar macrophages and exudate macrophages) and neutrophils recovered by BAL from the lungs of mice of the various treatment groups was done using flow cytometric–based differences in their cell surface antigen expression profiles of F4/80, CD11b, CD11c, MHC II, and Gr-1, as outlined below.
Isolation and immunophenotypic analysis of leukocyte subsets collected from lung tissue and peripheral blood.
Lungs of S. pneumoniae– or mock-infected mice were subjected to BAL, followed by perfusion in situ via the right ventricle with HBSS until the lungs were visually free of blood. Lung lobes were carefully removed and digested with collagenase A and DNase I. Leukocyte subsets contained in lung homogenates were further purified using a CD11c MACS kit following the instructions of the manufacturer (Miltenyi Biotec), as previously described (Srivastava et al., 2007; Taut et al., 2008). The purity of isolated CD11c+ cells was reproducibly ∼90%.
CD11c+ mononuclear phagocyte subsets and neutrophils isolated from lung tissue and BAL fluids were immunophenotypically analyzed according to their cell surface antigen expression profiles. In brief, after preincubation with Octagam, 2–5 × 105 cells were stained with various combinations of fluorochrome-conjugated antibody directed against the corresponding cell surface markers for 20 min at 4°C. Subsequently, cells were washed twice with FACS buffer (PBS/1% bovine serum albumine/0.2% sodium azide), and cell acquisition was performed on a FACSCanto flow cytometer (BD). First, CD11c+ mononuclear phagocyte subsets purified from lung homogenates and BAL fluids of S. pneumoniae-infected mice were gated according to their forward scatter (FSC)/side scatter (SSC) and FSC/autofluorescence properties. Highly autofluorescent cells were further characterized as alveolar macrophages (contained in BAL fluid) or lung macrophages (remaining in lung tissue after BAL) according to their F4/80+/CD11c+/CD11b- expression profile or as autofluorescent exudate macrophages in BAL fluid or lung tissue based on their additional CD11b expression (F4/80+/CD11c+/CD11b+). Neutrophils were gated according to their FSC/SSC and Gr-1 cell surface expression. In addition, cell surface expression of TRAIL or DR5 was analyzed on alveolar and lung macrophages, exudate macrophages, and neutrophils. To verify binding specificity of the used anti-TRAIL and –DR5 antibodies, in selected experiments, macrophages collected from the lungs of S. pneumoniae–infected (5 × 106 CFU/mouse, 48 h) Trail−/− and Dr5−/− mice were stained with PE-conjugated anti-TRAIL and anti-DR5 antibodies. Data analysis and after acquisition compensation of spectral overlaps between the various fluorescence channels was performed using BD FACSDiva software.
Depletion of neutrophils.
Neutrophil depletion was achieved by i.p. injection of 250 µg anti-Ly-6G Ab (clone 1A8; BioXCell) at −12 h and −2 h before infection of mice with S. pneumoniae. Control mice received equal amounts of isotype control Ab (clone 2A3). In selected experiments, neutropenic mice were infected with S. pneumoniae (3 × 106 CFU/mouse), followed by a single intratracheal application of agonistic MD5-1 or control IgG at 6 h after infection.
Determination of lung permeability.
Mice received an intravenous injection of FITC-labeled human albumin (1 mg/mouse in 100 µl saline; Sigma-Aldrich) 1 h before sacrifice. Subsequently, undiluted BAL fluid samples and serum samples (diluted 1/10 in saline) were placed in a 96-well microtiter plate, and fluorescence intensities were measured using a fluorescence spectrometer (FLx800 microplate fluorescence reader; Bio-Tek) operating at 485 nm absorbance and 528 ± 20 nm emission wavelengths. The lung permeability index is defined as the ratio of fluorescence signals of undiluted BAL fluid samples relative to fluorescence signals of 1/100 diluted serum samples (Henken et al., 2010a).
Analysis of apoptosis/necrosis induction in macrophages and neutrophils.
Analysis of apoptosis and necrosis induction in resident alveolar macrophages and inflammatory recruited exudate macrophages and neutrophils was done by incubation of BAL cells with APC-labeled Annexin V and propidium iodide for 15 min at room temperature, according to the manufacturer’s instructions (BD). Subsequently, alveolar macrophages were gated according to their FSC/SSC, FSC/F4/80-FITC, and CD11b-PE-Cy7 cell surface expression, and neutrophils were gated according to their FSC/SSC and Gr-1-PE-Cy7 cell surface expression profiles followed by determination of the percentage of early apoptotic resident alveolar macrophages and alveolar exudate macrophages (Annexin V+/PI−), and necrotic alveolar macrophages and alveolar exudate macrophages (PI+/Annexin V−), as previously described (Taut et al., 2008; Steinwede et al., 2011).
Determination of TRAIL and caspase 3 activation in lysates of S. pneumoniae–infected alveolar macrophages.
Alveolar macrophages isolated by BAL from untreated mice were seeded in 24-well plates (106 cells/well). After 1 h, nonadherent cells were removed by two washing steps with cell culture medium and adherent alveolar macrophages were then either mock-infected or infected with S. pneumoniae (MOI = 2) for 30 min or 2 h in RPMI 1640 medium/10% FCS/1% glutamine at 37°C/5% CO2. At indicated time points, cell culture supernatants were collected for quantification of TRAIL protein by ELISA, and after washing, alveolar macrophages were lysed in PBS containing protease inhibitor cocktail (Roche) by sonication for 10 s on ice (UW 2200; Bandelin Electronics) or with ice-cold lysis buffer containing protease inhibitors, as described previously (Srivastava et al., 2008). Subsequently, cell lysates of mock-infected or S. pneumoniae–infected alveolar macrophages were used for Western blot analysis of native caspase 3, cleaved caspase 3, and β-actin using the antibody as specified in the Reagent section (Srivastava et al., 2008; Steinwede et al., 2011). Expression of immunogenic proteins was determined by evaluation of enhanced chemiluminescence signals (ECL Plus; GE Healthcare) using a Vilber/Lourmat Chemismart 5000, and cleavage of caspase 3 was further quantified using the Bio1D software package (Vilber/Lourmat Deutschland GmbH).
Isolation of BM-derived cells and stimulation with S. pneumoniae.
Femurs and tibias were removed aseptically from untreated donor mice and flushed with RPMI 1640/10% FCS. BM cell suspensions were filtered through a 40-µm nylon mesh to remove residual cell aggregates. Purification of neutrophils from BM cells was performed using an anti-Ly-6G MicroBead kit (Miltenyi Biotec) according to the manufacturer’s instructions. For generation of BMDMs, BM cells were incubated overnight in cell culture dishes (Greiner Bio-One) in RPMI 1640/ 10% FCS plus glutamine (2 mM), 1% penicillin/streptomycin at 37°C/5% CO2. After 24 h, nonadherent cells were centrifuged and resuspended in RPMI 1640. and then cultured in the presence of M-CSF (50 ng/ml) to differentiate into BMDM. Medium was replaced with fresh medium containing M-CSF on day 4. BMDM were harvested for further experiments on day 6 of culture. BMDM and isolated neutrophils were washed and resuspended in cell culture medium without antibiotics. Cells were seeded in 24-well plates (2 × 106 cells/well) and incubated for 24 h in medium alone (mock) or medium containing S. pneumoniae (MOI 1). Subsequently, culture supernatants were collected for quantification of TRAIL protein by ELISA.
Phagocytosis and bacterial killing assay.
Alveolar macrophages (2 × 105 cells/well) were infected with S. pneumoniae (MOI 50) for 30 min in RPMI 1640/10% FCS plus 1% glutamine at 37°C/5% CO2. Subsequently, nonphagocytosed pneumococci were removed by four washing steps with HBSS, and either vehicle (PBS/ 0.1% HSA) or 10 ng/ml rmTRAIL dissolved in PBS/0.1% HSA was added to each well, followed by incubation at 37°C/5% CO2. After 90 min, alveolar macrophages were washed and lysed with 0.1% saponin in HBSS to release intracellular pneumococci. Subsequently, CFU were quantified by plating 10-fold serial dilutions of cell lysates on sheep agar plates followed by incubation of the plates at 37°C/5% CO2 for 18 h. Control experiments included the addition of gentamicin (15 min) to kill nonphagocytosed, extracellular bacteria.
Flow sorting of alveolar macrophages and neutrophils.
A high-speed FACSAria II flow cytometer (BD) equipped with an aerosol management system was used for sorting alveolar macrophages and neutrophils from S. pneumoniae–infected mice. In brief, after preincubation with Octagam, 2–5 × 105 BAL fluid cells were stained with fluorochrome-conjugated antibody directed against the corresponding cell surface markers for 20 min at 4°C. Cells were then washed two times with FACS buffer and resuspended for sorting in RPMI. Alveolar macrophages and neutrophils were gated according to their FSC/SSC and FSC/autofluorescence properties. Alveolar macrophages were further gated according to their F4/80+/CD11c+/CD11b− profile, and neutrophils were gated according to their Gr-1+/CD11b+ expression. Cells were sorted aseptically at a flow rate ∼10,000 particles/sec, using an 85-µm nozzle and switched on sample agitation. The complete sorting process was performed at a constant temperature of 4°C, followed by resort analysis to verify sort purities that routinely exceeded 98% purity (Srivastava et al., 2007; Steinwede et al., 2011).
Total cellular RNA isolation, cDNA synthesis, and real-time RT-PCR.
Total cellular RNA was isolated from BAL cells, lung tissue, or flow-sorted alveolar macrophages and neutrophils from mock- or S. pneumoniae–infected mice at 24 h or 48 h after infection using an RNeasy Micro-kit (QIAGEN). 150,000 sorted alveolar macrophages or neutrophils were lysed, and 100 ng of purified total RNA were used for cDNA synthesis. Real-time RT-PCR analysis was performed as recently described (Srivastava et al., 2007). Primers used for determination of TRAIL mRNA in BAL cells, lung tissue, sorted alveolar macrophages or neutrophils were as follows: TRAIL, forward primer, 5′-GAAGACCTCAGAAAGTGGC-3′, and reverse primer: 5′-GACCAGCTCTCCATTCCTA-3′; β-actin forward primer, 5′-CCACAGCTGAGAGGGAAATC-3′, and reverse primer, 5′-TCTCCAGGGAGGAAGAGGAT-3′. Mean fold-changes were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001; Srivastava et al., 2007).
Lung histopathology.
Mice were euthanized and nonlavaged whole lungs were harvested and immediately submitted to immersion fixation in buffered formalin for 24 h at room temperature. After routine embedding, two whole-lung sections from each lung lobe were cut into 3-µm sections and stained with hematoxylin and eosin. The percentage of inflamed lung tissue areas was determined as mean values of affected areas per left and right lung lobes of individual mice using a Zeiss Axiovert 200 M microscope (Carl Zeiss).
ELISA.
Cytokine concentrations in BAL fluids of S. pneumoniae–infected mice were determined using Duo-Set ELISA kits for IL-6, KC, and TNF, and Quantikine ELISA kit for IFN-γ (R&D Systems). TRAIL ELISA kit was purchased from Cusabio. Detection limits of the used ELISA kits were: IFN-γ, 9.4 pg/ml; IL-6, 15.6 pg/ml; KC, 15.6 pg/ml; CCL2, 15.6 pg/ml; TNF, 31.2 pg/ml; TRAIL, 3.15 pg/ml.
Statistics.
All data are given as mean ± SEM. Significant differences between treatment groups were analyzed using Mann-Whitney-U test. Survival curves were compared by log-rank test. Statistically significant differences between treatment groups were assumed when P values were <0.05.
Acknowledgments
U.A. Maus and T. Welte are members of the German Center for Lung Research.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchoalveolar lavage
- BMDM
- BM-derived macrophage
- FSC
- forward scatter
- MOI
- multiplicity of infection
- SSC
- side scatter
- TRAIL
- TNF-related apoptosis-inducing ligand
References
- Ali F., Lee M.E., Iannelli F., Pozzi G., Mitchell T.J., Read R.C., Dockrell D.H. 2003. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcgamma receptors correlates with intracellular bacterial load. J. Infect. Dis. 188:1119–1131 10.1086/378675 [DOI] [PubMed] [Google Scholar]
- Antoni B.A., Sabbatini P., Rabson A.B., White E. 1995. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J. Virol. 69:2384–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A., Dixit V.M. 1999. Apoptosis control by death and decoy receptors. Curr. Opin. Cell Biol. 11:255–260 10.1016/S0955-0674(99)80034-9 [DOI] [PubMed] [Google Scholar]
- Behar S.M., Martin C.J., Booty M.G., Nishimura T., Zhao X., Gan H.X., Divangahi M., Remold H.G. 2011. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 4:279–287 10.1038/mi.2011.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D.E., Hollingshead S.K., Paton J.C., Ades E.W., Novak L., van Ginkel F.W., Benjamin W.H., Jr 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339–348 10.1086/376571 [DOI] [PubMed] [Google Scholar]
- Brincks E.L., Katewa A., Kucaba T.A., Griffith T.S., Legge K.L. 2008. CD8 T cells utilize TRAIL to control influenza virus infection. J. Immunol. 181:4918–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincks E.L., Gurung P., Langlois R.A., Hemann E.A., Legge K.L., Griffith T.S. 2011. The magnitude of the T cell response to a clinically significant dose of influenza virus is regulated by TRAIL. J. Immunol. 187:4581–4588 10.4049/jimmunol.1002241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broug-Holub E., Toews G.B., van Iwaarden J.F., Strieter R.M., Kunkel S.L., Paine R., III, Standiford T.J. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbo E., Garau J. 2010. Of mice and men: innate immunity in pneumococcal pneumonia. Int. J. Antimicrob. Agents. 35:107–113 10.1016/j.ijantimicag.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Cassatella M.A., Huber V., Calzetti F., Margotto D., Tamassia N., Peri G., Mantovani A., Rivoltini L., Tecchio C. 2006. Interferon-activated neutrophils store a TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) intracellular pool that is readily mobilizable following exposure to proinflammatory mediators. J. Leukoc. Biol. 79:123–132 10.1189/jlb.0805431 [DOI] [PubMed] [Google Scholar]
- Clem R.J., Miller L.K. 1993. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J. Virol. 67:3730–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cziupka K., Busemann A., Partecke L.I., Pötschke C., Rath M., Traeger T., Koerner P., von Bernstorff W., Kessler W., Diedrich S., et al. 2010. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) improves the innate immune response and enhances survival in murine polymicrobial sepsis. Crit. Care Med. 38:2169–2174 10.1097/CCM.0b013e3181eedaa8 [DOI] [PubMed] [Google Scholar]
- Dockrell D.H., Marriott H.M., Prince L.R., Ridger V.C., Ince P.G., Hellewell P.G., Whyte M.K. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380–5388 [DOI] [PubMed] [Google Scholar]
- Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101:890–898 10.1172/JCI1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falschlehner C., Schaefer U., Walczak H. 2009. Following TRAIL’s path in the immune system. Immunology. 127:145–154 10.1111/j.1365-2567.2009.03058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnberg N., Gruber J.J., Fei P., Rudolph D., Bric A., Kim S.H., Burns T.F., Ajuha H., Page R., Wu G.S., et al. 2005. DR5 knockout mice are compromised in radiation-induced apoptosis. Mol. Cell. Biol. 25:2000–2013 10.1128/MCB.25.5.2000-2013.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F., Ashkenazi A. 2010. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 29:4752–4765 10.1038/onc.2010.221 [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Brincks E.L., Gurung P., Kucaba T.A., Ferguson T.A. 2011. Systemic immunological tolerance to ocular antigens is mediated by TRAIL-expressing CD8+ T cells. J. Immunol. 186:791–798 10.4049/jimmunol.1002678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P., Rai D., Condotta S.A., Babcock J.C., Badovinac V.P., Griffith T.S. 2011. Immune unresponsiveness to secondary heterologous bacterial infection after sepsis induction is TRAIL dependent. J. Immunol. 187:2148–2154 10.4049/jimmunol.1101180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn I., Klaus A., Janze A.K., Steinwede K., Ding N., Bohling J., Brumshagen C., Serrano H., Gauthier F., Paton J.C., et al. 2011a. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect. Immun. 79:4893–4901 10.1128/IAI.05593-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn I., Klaus A., Maus R., Christman J.W., Welte T., Maus U.A. 2011b. Dendritic cell depletion and repopulation in the lung after irradiation and bone marrow transplantation in mice. Am. J. Respir. Cell Mol. Biol. 45:534–541 10.1165/rcmb.2010-0279OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henken S., Bohling J., Martens-Lobenhoffer J., Paton J.C., Ogunniyi A.D., Briles D.E., Salisbury V.C., Wedekind D., Bode-Böger S.M., Welsh T., et al. 2010a. Efficacy profiles of daptomycin for treatment of invasive and noninvasive pulmonary infections with Streptococcus pneumoniae. Antimicrob. Agents Chemother. 54:707–717 10.1128/AAC.00943-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henken S., Bohling J., Ogunniyi A.D., Paton J.C., Salisbury V.C., Welte T., Maus U.A. 2010b. Evaluation of biophotonic imaging to estimate bacterial burden in mice infected with highly virulent compared to less virulent Streptococcus pneumoniae serotypes. Antimicrob. Agents Chemother. 54:3155–3160 10.1128/AAC.00310-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbold W., Maus R., Hahn I., Ding N., Srivastava M., Christman J.W., Mack M., Reutershan J., Briles D.E., Paton J.C., et al. 2010. Importance of CXC chemokine receptor 2 in alveolar neutrophil and exudate macrophage recruitment in response to pneumococcal lung infection. Infect. Immun. 78:2620–2630 10.1128/IAI.01169-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann O., Priller J., Prozorovski T., Schulze-Topphoff U., Baeva N., Lunemann J.D., Aktas O., Mahrhofer C., Stricker S., Zipp F., Weber J.R. 2007. TRAIL limits excessive host immune responses in bacterial meningitis. J. Clin. Invest. 117:2004–2013 10.1172/JCI30356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E., Nakazawa M., Yoshinari M., Minami M. 2005. Role of tumor necrosis factor-related apoptosis-inducing ligand in immune response to influenza virus infection in mice. J. Virol. 79:7658–7663 10.1128/JVI.79.12.7658-7663.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Torres-Roca J.F., Tijoe I.M., Smith C.A., Herzenberg L.A., Herzenberg L.A. 1997. Interleukin-1β converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365–1372 10.1084/jem.186.8.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp T.J., Ludwig A.T., Earel J.K., Moore J.M., Vanoosten R.L., Moses B., Leidal K., Nauseef W.M., Griffith T.S. 2005. Neutrophil stimulation with Mycobacterium bovis bacillus Calmette-Guerin (BCG) results in the release of functional soluble TRAIL/Apo-2L. Blood. 106:3474–3482 10.1182/blood-2005-03-1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S., Leemans J.C., Florquin S., Branger J., Maris N.A., Pater J., van Rooijen N., van der Poll T. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171–179 10.1164/rccm.200207-698OC [DOI] [PubMed] [Google Scholar]
- Leemans J.C., Juffermans N.P., Florquin S., van Rooijen N., Vervoordeldonk M.J., Verbon A., van Deventer S.J., van der Poll T. 2001. Depletion of alveolar macrophages exerts protective effects in pulmonary tuberculosis in mice. J. Immunol. 166:4604–4611 [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lum J.J., Bren G., McClure R., Badley A.D. 2005. Elimination of senescent neutrophils by TNF-related apoptosis-inducing [corrected] ligand. J. Immunol. 175:1232–1238 [DOI] [PubMed] [Google Scholar]
- Mariani S.M., Krammer P.H. 1998. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur. J. Immunol. 28:973–982 [DOI] [PubMed] [Google Scholar]
- Marriott H.M., Ali F., Read R.C., Mitchell T.J., Whyte M.K., Dockrell D.H. 2004. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 18:1126–1128 [DOI] [PubMed] [Google Scholar]
- Marriott H.M., Bingle C.D., Read R.C., Braley K.E., Kroemer G., Hellewell P.G., Craig R.W., Whyte M.K., Dockrell D.H. 2005. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J. Clin. Invest. 115:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott H.M., Hellewell P.G., Cross S.S., Ince P.G., Whyte M.K., Dockrell D.H. 2006. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 177:6480–6488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G., Liles W.C., Frevert C.W., Dhanireddy S., Ballman K., Wong V., Green R.R., Song H.Y., Witcher D.R., Jakubowski J.A., Martin T.R. 2005. Blockade of the Fas/FasL system improves pneumococcal clearance from the lungs without preventing dissemination of bacteria to the spleen. J. Infect. Dis. 191:596–606 10.1086/427261 [DOI] [PubMed] [Google Scholar]
- Maus U.A., Koay M.A., Delbeck T., Mack M., Ermert M., Ermert L., Blackwell T.S., Christman J.W., Schlöndorff D., Seeger W., Lohmeyer J. 2002. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 282:L1245–L1252 [DOI] [PubMed] [Google Scholar]
- Maus U.A., Srivastava M., Paton J.C., Mack M., Everhart M.B., Blackwell T.S., Christman J.W., Schlöndorff D., Seeger W., Lohmeyer J. 2004. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J. Immunol. 173:1307–1312 [DOI] [PubMed] [Google Scholar]
- Maus U.A., Wellmann S., Hampl C., Kuziel W.A., Srivastava M., Mack M., Everhart M.B., Blackwell T.S., Christman J.W., Schlöndorff D., et al. 2005. CCR2-positive monocytes recruited to inflamed lungs downregulate local CCL2 chemokine levels. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L350–L358 10.1152/ajplung.00061.2004 [DOI] [PubMed] [Google Scholar]
- Maus U.A., Backi M., Winter C., Srivastava M., Schwarz M.K., Rückle T., Paton J.C., Briles D., Mack M., Welte T., et al. 2007. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am. J. Respir. Crit. Care Med. 175:958–966 10.1164/rccm.200610-1533OC [DOI] [PubMed] [Google Scholar]
- McGrath E.E., Marriott H.M., Lawrie A., Francis S.E., Sabroe I., Renshaw S.A., Dockrell D.H., Whyte M.K. 2011. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J. Leukoc. Biol. 90:855–865 10.1189/jlb.0211062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J.C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4:103–106 10.1016/0966-842X(96)81526-5 [DOI] [PubMed] [Google Scholar]
- Renshaw S.A., Parmar J.S., Singleton V., Rowe S.J., Dockrell D.H., Dower S.K., Bingle C.D., Chilvers E.R., Whyte M.K. 2003. Acceleration of human neutrophil apoptosis by TRAIL. J. Immunol. 170:1027–1033 [DOI] [PubMed] [Google Scholar]
- Rubins J.B., Charboneau D., Fasching C., Berry A.M., Paton J.C., Alexander J.E., Andrew P.W., Mitchell T.J., Janoff E.N. 1996. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 153:1339–1346 [DOI] [PubMed] [Google Scholar]
- Schneider P., Olson D., Tardivel A., Browning B., Lugovskoy A., Gong D., Dobles M., Hertig S., Hofmann K., Van Vlijmen H., et al. 2003. Identification of a new murine tumor necrosis factor receptor locus that contains two novel murine receptors for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J. Biol. Chem. 278:5444–5454 10.1074/jbc.M210783200 [DOI] [PubMed] [Google Scholar]
- Sedger L.M., Glaccum M.B., Schuh J.C., Kanaly S.T., Williamson E., Kayagaki N., Yun T., Smolak P., Le T., Goodwin R., Gliniak B. 2002. Characterization of the in vivo function of TNF-alpha-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur. J. Immunol. 32:2246–2254 [DOI] [PubMed] [Google Scholar]
- Simons M.P., Moore J.M., Kemp T.J., Griffith T.S. 2007. Identification of the mycobacterial subcomponents involved in the release of tumor necrosis factor-related apoptosis-inducing ligand from human neutrophils. Infect. Immun. 75:1265–1271 10.1128/IAI.00938-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Meinders A., Steinwede K., Maus R., Lucke N., Bühling F., Ehlers S., Welte T., Maus U.A. 2007. Mediator responses of alveolar macrophages and kinetics of mononuclear phagocyte subset recruitment during acute primary and secondary mycobacterial infections in the lungs of mice. Cell. Microbiol. 9:738–752 10.1111/j.1462-5822.2006.00824.x [DOI] [PubMed] [Google Scholar]
- Srivastava M., Steinwede K., Kiviranta R., Morko J., Hoymann H.G., Länger F., Buhling F., Welte T., Maus U.A. 2008. Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis. Respir. Res. 9:54 10.1186/1465-9921-9-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary G., Klein I., Kohlhofer S., Koszik F., Scherzer T., Müllauer L., Quendler H., Kohrgruber N., Stingl G. 2009. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 114:3854–3863 10.1182/blood-2009-04-217927 [DOI] [PubMed] [Google Scholar]
- Steinwede K., Tempelhof O., Bolte K., Maus R., Bohling J., Ueberberg B., Länger F., Christman J.W., Paton J.C., Ask K., et al. 2011. Local delivery of GM-CSF protects mice from lethal pneumococcal pneumonia. J. Immunol. 187:5346–5356 10.4049/jimmunol.1101413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taut K., Winter C., Briles D.E., Paton J.C., Christman J.W., Maus R., Baumann R., Welte T., Maus U.A. 2008. Macrophage turnover kinetics in the lungs of mice infected with Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 38:105–113 10.1165/rcmb.2007-0132OC [DOI] [PubMed] [Google Scholar]
- Tecchio C., Huber V., Scapini P., Calzetti F., Margotto D., Todeschini G., Pilla L., Martinelli G., Pizzolo G., Rivoltini L., Cassatella M.A. 2004. IFNalpha-stimulated neutrophils and monocytes release a soluble form of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2 ligand) displaying apoptotic activity on leukemic cells. Blood. 103:3837–3844 10.1182/blood-2003-08-2806 [DOI] [PubMed] [Google Scholar]
- Weber M., Lambeck S., Ding N., Henken S., Kohl M., Deigner H.P., Enot D.P., Igwe E.I., Frappart L., Kiehntopf M., et al. 2012. Hepatic induction of cholesterol biosynthesis reflects a remote adaptive response to pneumococcal pneumonia. FASEB J. 26:2424–2436 10.1096/fj.11-191957 [DOI] [PubMed] [Google Scholar]
- Welte T., Torres A., Nathwani D. 2012. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 67:71–79 10.1136/thx.2009.129502 [DOI] [PubMed] [Google Scholar]
- Winter C., Taut K., Srivastava M., Länger F., Mack M., Briles D.E., Paton J.C., Maus R., Welte T., Gunn M.D., Maus U.A. 2007. Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: role of the CCL2-CCR2 axis. J. Immunol. 178:5828–5838 [DOI] [PubMed] [Google Scholar]
- Wu G.S., Burns T.F., Zhan Y., Alnemri E.S., El-Deiry W.S. 1999. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 59:2770–2775 [PubMed] [Google Scholar]
- Zheng S.J., Jiang J., Shen H., Chen Y.H. 2004. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J. Immunol. 173:5652–5658 [DOI] [PubMed] [Google Scholar]
- Zhu D.M., Shi J., Liu S., Liu Y., Zheng D. 2011. HIV infection enhances TRAIL-induced cell death in macrophage by down-regulating decoy receptor expression and generation of reactive oxygen species. PLoS ONE. 6:e18291 10.1371/journal.pone.0018291 [DOI] [PMC free article] [PubMed] [Google Scholar]