CARD11 signaling determines whether antigen stimulation induces B cells to proliferate or die.
Abstract
Self-tolerance and immunity are actively acquired in parallel through a poorly understood ability of antigen receptors to switch between signaling death or proliferation of antigen-binding lymphocytes in different contexts. It is not known whether this tolerance-immunity switch requires global rewiring of the signaling apparatus or if it can arise from a single molecular change. By introducing individual CARD11 mutations found in human lymphomas into antigen-activated mature B lymphocytes in mice, we find here that lymphoma-derived CARD11 mutations switch the effect of self-antigen from inducing B cell death into T cell–independent proliferation, Blimp1-mediated plasmablast differentiation, and autoantibody secretion. Our findings demonstrate that regulation of CARD11 signaling is a critical switch governing the decision between death and proliferation in antigen-stimulated mature B cells and that mutations in this switch represent a powerful initiator for aberrant B cell responses in vivo.
Actively acquired self-tolerance and immunity are opposite processes that pose a central conundrum: how do antigen receptors switch between signaling lymphocyte death or proliferation when engaged by the same antigen in different contexts? This problem is exemplified by the surface immunoglobulin antigen receptors (BCRs) on mature, recirculating B lymphocytes. Upon binding antigen, these receptors trigger multiple rounds of B cell clonal proliferation in the context of infection or immunization but induce anergy and apoptosis when they bind continuously to antigens that are part of the body (self-antigens). Differential amplitude and duration of signaling to intracellular calcium, PI3 kinase, MAP kinases, and the transcription factors NFAT, Jun/Fos, and NF-κB correlate with differential signaling of growth or death by the B cell antigen receptor (Healy and Goodnow, 1998). However, as with most tolerance/immunity checkpoints, the nature of the switch in mature B cells remains unresolved because no individual genetic alteration of these pathways has yet been found to be sufficient to switch self-antigen–induced B cell death into proliferation.
An experiment of nature that provides clues to this problem comes from a group of common human cancers that includes non-Hodgkin’s lymphoma and chronic lymphocytic leukemia (Rui et al., 2011), in which a clone of B cells that has accumulated 30 damaging somatic mutations on average (Morin et al., 2011; Pasqualucci et al., 2011) becomes locked in an endless growth cycle. It is not yet known how these mutations individually or collectively affect the normal response to self-antigens, although multiple lines of evidence suggest that the growth of human B cell malignancies is promoted by continuous BCR stimulation by self-antigens (Cleary et al., 1986; Borche et al., 1990; Friedman et al., 1991; Kobayashi et al., 1993; Chiorazzi and Ferrarini, 2003; Rui et al., 2011; Stevenson et al., 2011). RNA interference screens in tissue culture revealed that cells from the ABC-DLBCL (activated B cell subtype of diffuse large B cell lymphoma) died when components of the antigen receptor signal transduction cascade were depleted (Ngo et al., 2006; Lenz et al., 2008; Davis et al., 2010).
One important branch of the BCR signaling cascade terminates on the transcription factor, NF-κB, which is activated by acute antigen receptor engagement but not chronic receptor engagement by self-antigens (Healy et al., 1997) through a pathway whereby PKCβ phosphorylates and activates CARD11 (also called Carma1) to form a CBM (CARD11–BCL10–MALT1) complex (Jun et al., 2003; Thome, 2004; Rawlings et al., 2006). The CBM complex in turn activates IκB kinase (IKK) to phosphorylate and degrade the inhibitor of NF-κB, IκBα, allowing NF-κB to move to the nucleus and cooperate with other transcription factors in promoting B cell survival, proliferation, and differentiation (Jun et al., 2003; Thome, 2004; Rawlings et al., 2006; Gerondakis and Siebenlist, 2010). 13% of DLBCL cases, including ABC and germinal center (GC) types, have acquired activating somatic point mutations of various amino acids in the coiled-coil regulatory domain of CARD11 that are required for the proliferation of an ABC-DLBCL lymphoma cell line in culture (Lenz et al., 2008). However, many other mutations also recur in B cell malignancy that may be needed as an ensemble to switch the way B cells respond to chronic stimulation (Rui et al., 2011), and indeed dependence on the CARD11 branch of the cascade did not extend to the GC subtype of diffuse large B cell lymphoma (Ngo et al., 2006; Lenz et al., 2008). Likewise, dysregulated NF-κB signaling on its own was insufficient to initiate B cell growth in mature, circulating B cells in transgenic mice expressing a constitutively active allele of IKK-β (Sasaki et al., 2006), and dysregulated expression of the NF-κB target gene, Bcl2, delayed self-antigen–induced death but did not result in a switch into proliferation (Cyster et al., 1994). Collectively, these results focus the question on what, if any, effects lymphoma CARD11 mutations have in isolation on the normal response to self-antigens.
In this study, we examine this question using a novel retroviral strategy to genetically manipulate a cohort of self-antigen–binding mature B cells in vivo. Using this system, all lymphoma-derived CARD11 mutations tested conferred upon CARD11 a potent ability to switch the effect of chronic binding of self-antigen from death into inducing B cell proliferation and Blimp1-mediated plasmablast differentiation and autoantibody production. We conclude that regulation of CARD11 is a central switch governing the decision between antigen-induced death and growth in mature B cells. Mutations in this switch, when paired with somatically acquired antigen receptors against self, represent a powerful initiator of aberrant B cell growth and differentiation.
RESULTS AND DISCUSSION
A human lymphoma CARD11 mutation blocks self-antigen death and drives growth
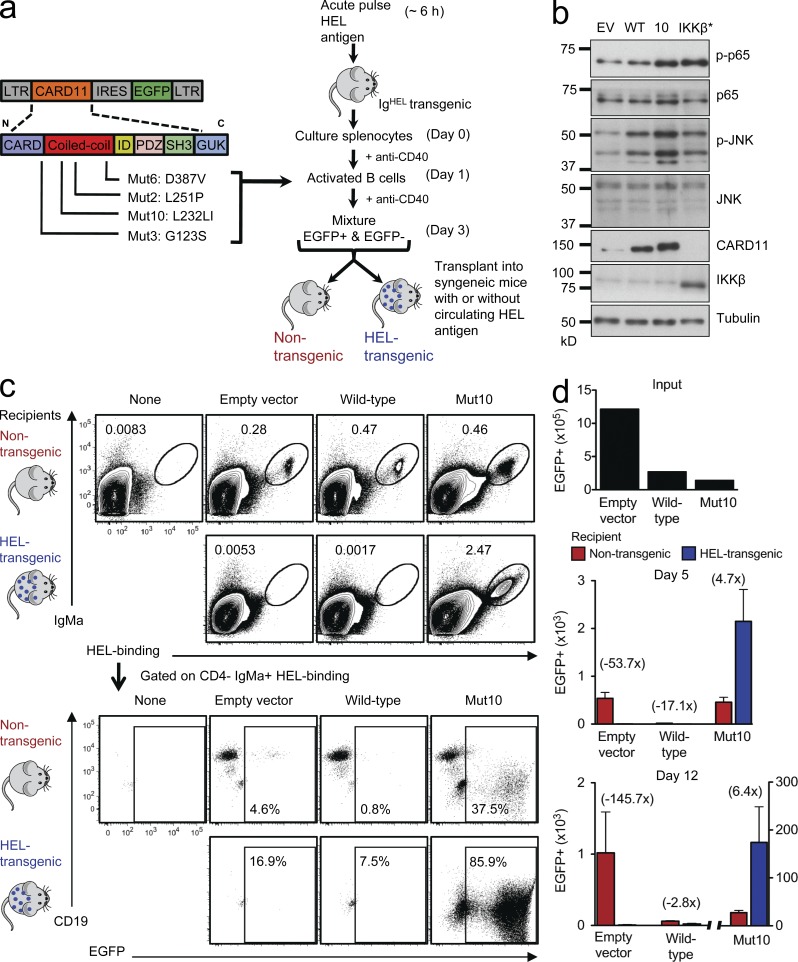
To investigate the consequences of acquiring a CARD11 somatic mutation in antigen-activated normal B cells, we developed a retroviral gene delivery system to express normal or mutant CARD11 in primary antigen-activated B cells obtained from transgenic mice (Fig. 1 a). Mature B cells bearing homogeneous antigen receptors against a protein antigen, hen egg lysozyme (HEL; Goodnow et al., 1988), were activated into proliferation by giving IgHEL transgenic mice an acute pulse of HEL antigen in vivo followed by stimulation of the B cells with antibodies to CD40 in culture for 3 d. During this time, the B cells were transduced with bicistronic vectors encoding enhanced GFP (EGFP) alone or EGFP and WT or different mutant forms of CARD11. We tested four CARD11 lymphoma mutations (Fig. 1 a): G123S (Mut3), L232LI (Mut10), L251P (Mut2), and D387V (Mut6), with the numbers in parentheses referring to the mutations as originally described (Lenz et al., 2008). After transduction, the antigen-activated B cells were transplanted back into syngeneic Rag1−/− mice that either lacked any antigen or CD40 growth stimuli or that expressed HEL protein in their circulation (Goodnow et al., 1988) so that the B cells were continuously stimulated by self-antigen (Fig. 1 a).
Figure 1.
A potent CARD11 mutation blocks self-antigen–induced B cell death and promotes extensive proliferation in vivo. (a) Experimental strategy to examine the consequences of acquiring different CARD11 coiled-coil domain mutations in normal antigen-activated B cells. (b) Immunoblot for phospho/total p65 and phospho/total JNK in cell lysates from EV, WT, Mut10, and constitutively active IKK-β (IKK-β*)–expressing B cells. Cells cultured in media without anti-CD40 for 24 h before EGFP+ cells were sorted. (c) Antigen-specific B cells transduced with the indicated vectors were transplanted into Rag1-deficient recipient mice that were either nontransgenic, in which the B cells lacked antigen or CD40 stimuli, or were HEL transgenic, in which the transplanted B cells were continuously stimulated by circulating self-antigen. Donor cells in the spleen of recipient mice were analyzed by flow cytometry 5 and 12 d after transplantation. In the top panels, the transplanted B cells were detected and enumerated as a percentage of spleen cells by their binding of HEL antigen and staining for IgMa allotype. The bottom panels are gated on these transplanted B cells and show the percentage that are EGFP+ and their expression of CD19. (d) The top panel shows the number of EGFP+ B cells that were transplanted into each mouse (input). Bottom panels show mean number ± SEM of EGFP+ B cells in the spleen of nontransgenic or HEL transgenic recipient mice on days 5 and 12 (n = 3–6 recipient mice per group and time point; one way analysis of variance [ANOVA]: P = 0.0011 and P = 0.0014, respectively). Data are representative of four independent experiments.
Because activation of NF-κB and c-Jun N-terminal kinase (JNK) by the B cell antigen receptor both depend on normal CARD11 (Jun et al., 2003), we first compared the activity of these signaling pathways in the retrovirally transduced B cells. B cells were transduced with retrovirus carrying EGFP only (empty vector [EV]), mutant (Mut10), or WT CARD11, or a vector encoding a constitutively active IKK-β (IKK-β*) as a positive control. The cells were then washed and cultured in media without anti-CD40 for 24 h before sorting EGFP+ cells and preparing cell lysates for SDS-PAGE. Compared with control B cells expressing WT CARD11 or EGFP only, CARD11 Mut10–expressing B cells consistently displayed higher phosphorylation of JNK and the p65 (RelA) NF-κB subunit, whereas IKK-β*–expressing cells induced comparably increased phosphorylation of p65 but not of JNK (Fig. 1 b). Western blotting of EGFP+ cells for CARD11 showed higher levels in cells transduced with CARD11 WT or Mut10 vectors compared with EV controls but no consistent difference in protein accumulation between cells transduced with CARD11 WT or Mut10 (also see Fig. 4 a), indicating that the effects of the mutations could not be explained by protein expression. In this context, it is notable that CARD11 copy number gains also occur in human B cell lymphomas (Morin et al., 2011). These results indicate that the lymphoma mutation in CARD11 enhanced signaling to both NF-κB and JNK pathways in primary, mature B cells.
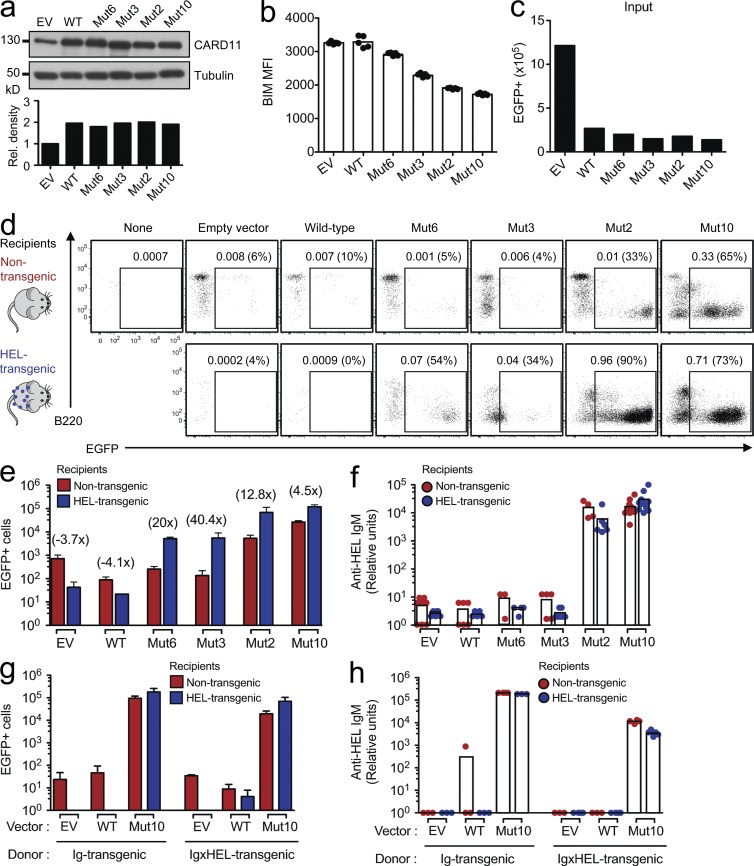
Figure 4.
A range of lymphoma CARD11 mutations switch self-antigen–induced B cell deletion into aberrant B cell growth. (a) Immunoblot and densitometry analysis of CARD11 in cell lysates from cells expressing control EV and WT CARD11 and lymphoma CARD11 mutants Mut2, Mut3, Mut6, and Mut10. Transduced B cells were cultured in media without anti-CD40 for 24 h before EGFP+ cells were sorted. (b) BIM levels expressed as mean fluorescence intensity (MFI) measured by flow cytometry analysis of transduced B cells with the indicated vectors (one-way ANOVA: P < 0.0001). (c) Number of input EGFP+ Ig transgenic B cells, transduced with vectors encoding the indicated CARD11 alleles, that were transplanted into each recipient mouse in panels d–f. (d) Flow cytometric analysis of splenocytes 12 d after transplantation of B cells, transduced with vectors encoding the indicated CARD11 alleles, into HEL transgenic or nontransgenic recipients. B220 expression and the percentage of EGFP+ B cells, either as a percentage of all splenocytes or (in parentheses) as a percentage of the transplanted B cells, are shown. (e) Mean number (±SEM; n = 3–6 mice per group) of splenic EGFP+ HEL-binding IgMa+ B cells in HEL transgenic or nontransgenic recipients 12 d after transplantation of transduced B cells (one-way ANOVA: P < 0.0001). (f) Relative units of anti-HEL IgM measured by ELISA in serum collected from nontransgenic or HEL transgenic mice 11 d after transplantation (n = 3–9 mice per group; one-way ANOVA: P < 0.0001). (g) Mean number (±SEM; n = 3–5 mice per group) of splenic EGFP+ HEL-binding IgMa+ B cells in HEL transgenic or nontransgenic recipients, 11 d after transplantation of Ig transgenic or anergic Ig × HEL double transgenic B cells (one-way ANOVA: P < 0.0001). (h) Relative units of anti-HEL IgM measured by ELISA on serum collected from nontransgenic or HEL transgenic mice 10 d after transplantation (n = 3–5 mice per group; one-way ANOVA: P < 0.0001). Data are representative of three independent experiments.
The transduced B cells were injected into the circulation of Rag1-deficient mice that either expressed HEL protein (HEL transgenic) or lacked the antigen (nontransgenic). We first tested the most potent lymphoma CARD11 mutation, Mut10, which originally occurred in a GC-type DLBCL (Lenz et al., 2008). We tracked the fate of the transplanted B cells in the spleen by flow cytometry using two unique markers of their antigen receptors, their ability to bind HEL antigen and the presence of an IgMa allotypic epitope (Fig. 1 c; Goodnow et al., 1988). EGFP fluorescence within this subset distinguished transduced from nontransduced B cells, although the expression of EGFP after CARD11 and the IRES (internal ribosome entry site) sequence was lower than from the EV control and could not be resolved above autofluorescence in some transduced cells (Fig. 1 c). In HEL transgenic Rag1−/− recipients, continuous antigen stimulation by circulating self-HEL antigen induces the proapoptotic Bim protein in mature B cells and triggers apoptosis within 3 d despite the presence of BAFF (Cyster et al., 1994; Enders et al., 2003; Lesley et al., 2004; Thien et al., 2004). Consequently, when antigen-specific B cells transduced with either EV or WT CARD11 were enumerated in the spleen 5 or 12 d after transplantation, these were readily detectable in nontransgenic recipients but had been eliminated from HEL transgenic recipients (Fig. 1, c and d). In contrast, CARD11 Mut10–expressing B cells were not eliminated in HEL transgenic recipients, but instead EGFP+ cells persisted and proliferated to very large numbers. In nontransgenic Rag1−/− mice that lacked HEL protein, the number of EGFP+ B cells expressing EGFP only or CARD11 WT exhibited little change between days 5 and 12 after transfer, whereas those expressing CARD11 Mut10 proliferated, although the proliferation of the latter was 6.4 times less than that in the presence of HEL antigen (Fig. 1, c and d). Thus, a discrete CARD11 mutation from a human lymphoma switched the effect of chronic antigen receptor stimulation, converting the normal induction of B cell death into antigen-induced, T cell–independent proliferation.
CARD11 mutation induces Blimp1-dependent plasma cell differentiation and autoantibody production
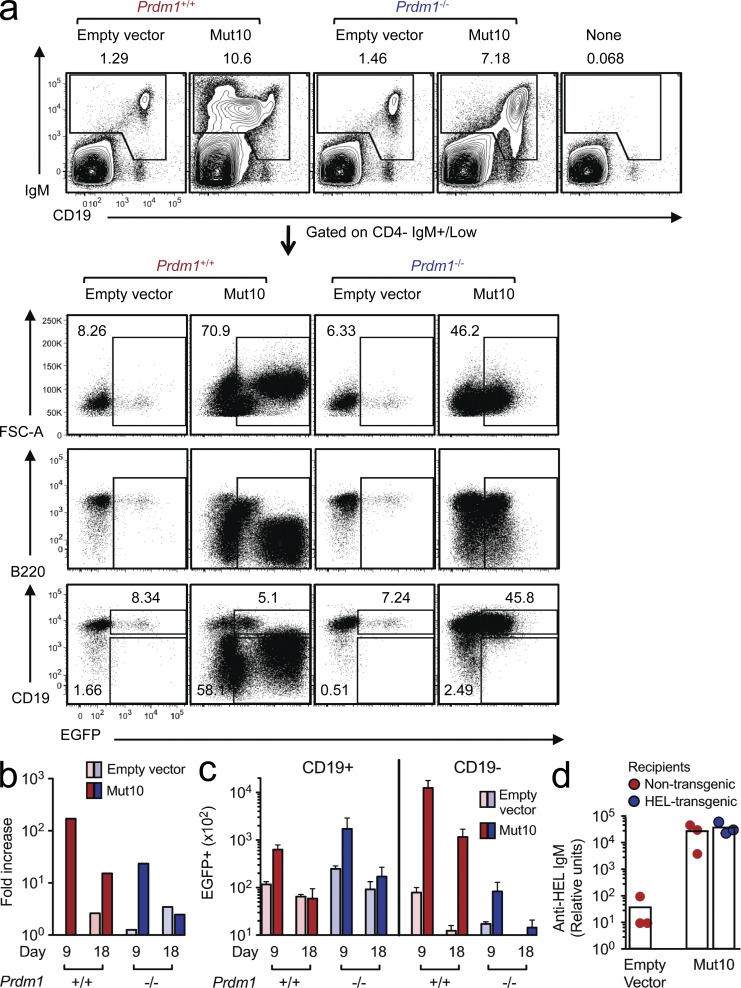
Compared with the control EGFP+ B cells, most of the EGFP+ proliferating cells expressing CARD11 Mut10 maintained surface IgM antigen receptor expression but were much larger in size (high FSC-A [forward scatter area]) and expressed much lower levels of B220 and CD19 (Figs. 1 c and 2 a), two B cell lineage markers which are repressed upon differentiation into plasma cells (Fairfax et al., 2008). This was surprising because physiological proliferation of B cells driven by foreign antigen and CD40 stimulation from helper T cells normally requires helper T cell–derived cytokines like IL-21 to stimulate differentiation into antibody-secreting plasma cells. Plasmablast differentiation requires induction of Blimp1, encoded by the Prdm1 gene (Fairfax et al., 2008; Martins and Calame, 2008). To test whether the human lymphoma CARD11 mutations did indeed induce Blimp1-dependent differentiation of mature B cells in vivo, we performed retroviral transduction and transfer experiments using activated B cells from mice with a B cell–specific deletion of the Prdm1 gene (Fig. 2, a–c). CARD11 Mut10–expressing B cells lacking Prdm1 still proliferated in vivo, and indeed three times as many CD19+ EGFP+ B cells accumulated in the absence of Prdm1 compared with the number of CD19+ cells in controls with WT Prdm1 (Fig. 2 c). Prdm1 deletion nevertheless prevented the formation of the large CD19-negative plasmablast population, which in the Prdm1+/+ controls contained more intracellular EGFP, presumably because of their larger cytoplasmic volume. Thus, aberrant proliferation of B cells induced by a lymphoma mutation in CARD11 is accompanied by efficient induction of Blimp1-mediated plasma cell differentiation.
Figure 2.
CARD11 mutation induces Blimp1-dependent plasma cell differentiation and autoantibody production. (a) Flow cytometric analysis of splenocytes 9 d after transplantation of Prdm1+/+ or Prdm1−/− B cells, or no B cells, into Rag1-deficient recipients. The top panels show the percentage of transplanted IgM+ B cells in the spleen. The bottom panels are gated on the transplanted B cells and show the percentage of total EGFP+ cells and the proportion of these cells that are either EGFP+ CD19+ B cells or EGFP+ CD19− plasmablasts. (b) Fold increase in numbers of EGFP+ B cells normalized to the number of EV-expressing Prdm1+/+ B cells detected at day 9 after transfer. (c) Mean number and SEM of EGFP+ CD19+ B cells or EGFP+ CD19− plasmablasts in the spleen (n = 3 mice per group and time) on the indicated days after transplantation (one-way ANOVA: P < 0.0001). (d) Amount of anti-HEL IgM relative to a standard reference serum from an Ig transgenic mouse, measured by ELISA in serum from transgenic and HEL transgenic mice 11 d after transplantation of HEL-specific B cells transduced with the indicated vectors (n = 3 mice per group; one-way ANOVA: P < 0.0001).
Consistent with CARD11 mutation–induced plasma cell differentiation, high concentrations of HEL-binding antibody were present in the serum of nontransgenic and HEL transgenic Rag1−/− recipients of CARD11 Mut10–expressing HEL-specific B cells (Fig. 2 d). The concentration of anti-HEL IgM was comparable with the amount in serum of Ig transgenic mice where almost all B cells are HEL specific, which was used as a reference, whereas no anti-HEL IgM antibodies were detected in the recipients of EV control B cells. These results indicate that the CARD11 lymphoma mutation was sufficient to overturn B cell tolerance not only to switch self-reactive cells from death to proliferation but also to induce secretion of autoantibodies.
Constitutively active IKK-β does not switch self-reactive B cells from death into growth
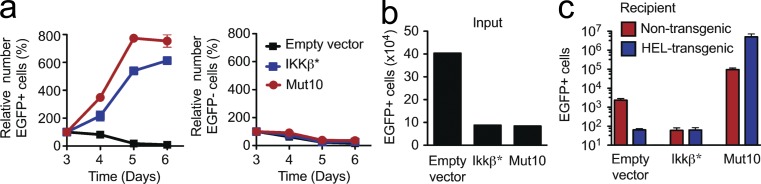
Because CARD11 is an essential scaffold for B cell antigen receptor signaling to two diverging pathways, IKK and JNK (Jun et al., 2003), we assessed whether an activating mutation in IKK itself would have the same effect as the CARD11 lymphoma mutant. Antigen-activated B cells were transduced with a retroviral vector encoding a mutant form of IKK-β bearing two amino acid substitutions in the activation loop of the kinase domain (IKK-β*) that results in a constitutively active kinase (Sasaki et al., 2006). Although EGFP+ B cells expressing IKK-β* proliferated spontaneously in tissue culture comparably with cells expressing CARD11 Mut10 (Fig. 3 a), when the IKK-β*–expressing B cells were transplanted into recipients, they did not accumulate but instead were eliminated in the presence or absence of HEL self-antigen (Fig. 3, b and c). Thus, the death to growth switch caused by lymphoma CARD11 mutations is not conferred simply by dysregulation of canonical NF-κB signaling.
Figure 3.
IKK-β activating mutation does not switch self-reactive B cell death into proliferation. (a) Number of viable EGFP+ or EGFP− B cells in tissue culture without antigen or anti-CD40 for 3 d, expressed as a percentage of the starting number on day 3. Numbers are mean ± SEM of three independent cultures, and data are representative of five independent experiments. (b) Number of EGFP+ B cells transplanted into each mouse (input). (c) Number (mean ± SEM; n = 3 per group) of EGFP+ HEL-binding IgMa+ B cells in the spleen of Rag1-deficient nontransgenic or HEL transgenic mice, 11 d after transplantation of Ig transgenic B cells transduced with vectors encoding the indicated proteins (one-way ANOVA: P = 0.0080).
A range of lymphoma CARD11 mutations switch self-antigen stimulation from death to proliferation and autoantibody secretion
Because the aforementioned in vivo findings focused on a single, particularly potent lymphoma CARD11 mutation, we next asked whether or not they were a general property of lymphoma CARD11 mutations, including weak alleles such as Mut3 whose effects on NF-κB in cell lines were augmented by antigen receptor signals (Lenz et al., 2008). Transduction and transplantation experiments were performed as before (Fig. 1, a and c), except that the antigen-activated B cells were transduced with CARD11 Mut6, Mut3, or Mut2, alongside cells transduced with Mut10 as a positive control or CARD11 WT and EV as negative controls. Western blotting of EGFP+ cells established that the expression of the different CARD11 mutant proteins was comparable with expression of CARD11 WT and approximately twofold the levels of endogenous CARD11 protein in EV EGFP+ B cells (Fig. 4 a). B cells expressing the CARD11 mutants had lower BIM levels than B cells expressing CARD11 WT or EV controls, with the magnitude of Bim down-regulation inversely proportional to the capacity of the CARD11 mutants to induce growth signals (Fig. 4, b and d). Modulation of BIM protein could therefore be one mechanism through which B cells expressing CARD11 mutant proteins avoid self-antigen–induced apoptosis.
When approximately equivalent numbers of the transduced B cells were transplanted into HEL transgenic recipients (Fig. 4 c), all four lymphoma CARD11 mutations blocked self-antigen–induced death of the transferred B cells and cooperated with chronic antigen stimulation to drive proliferation (Fig. 4, d and e) and differentiation of most of the GFP+ cells into CD19low B220low plasmablasts (Fig. 4 d). In the HEL-stimulated cells, the overall numbers of EGFP+ plasmablasts were 10–20-fold higher when they expressed CARD11 Mut2 or Mut10 than with the weaker Mut6 or Mut3 alleles (Fig. 4 e), but the presence of HEL antigen enhanced proliferation by a greater multiplier for Mut6 and Mut3 (20- and 40-fold increase in HEL transgenic compared with nontransgenic recipients, respectively) compared with Mut2 and Mut10 (13- and 4.5-fold increase, respectively). High concentrations of serum anti-HEL IgM autoantibodies were detected in HEL transgenic recipients in which Mut2- or Mut10-expressing B cells were transferred but were below the limit of detection in recipients of Mut3 or Mut6 B cells, presumably because of the lower number of plasma cells formed and antibody neutralization by an excess of circulating self-HEL antigen (Fig. 4, e and f). Without self-antigen stimulation in nontransgenic recipients, EGFP+ B cells expressing CARD11 Mut6 or Mut3 did not increase in number measurably relative to the control B cells over the 12 d, nor did these EGFP+ cells increase as a percentage of donor B cells compared with the percentage of EGFP+ at the time of transplantation (Fig. 4, c–e). Thus, two out of four lymphoma CARD11 mutations tested in this assay were insufficient to induce spontaneous B cell proliferation in vivo in the absence of ongoing antigen receptor engagement, but all were sufficient to switch the response to self-antigen from death into proliferation.
To test the ability of CARD11 mutations to break an established state of tolerance in self-reactive B cells and induce proliferation and autoantibody secretion, we repeated the aforementioned experiment with anergic B cells harvested from IgHEL × HEL double transgenic mice. The state of anergy established in IgHEL × HEL B cells is representative of anergic B cells with many different self-specificities and affinities analyzed in various Ig transgenic mice and of polyclonal anergic B cells in human peripheral blood (Goodnow and Ohashi, 2012). The self-reactive B cells were maintained in the constant presence of HEL antigen during retroviral transduction and then transferred back into HEL transgenic recipients (Fig. 4, g and h). CARD11 Mut10 nevertheless broke tolerance in the IgHEL × HEL anergic B cells, preventing their elimination in HEL transgenic recipients and causing them to proliferate, differentiate into numerous CD19low B220low GFP+ plasmablasts, and produce large amounts of anti-HEL autoantibodies.
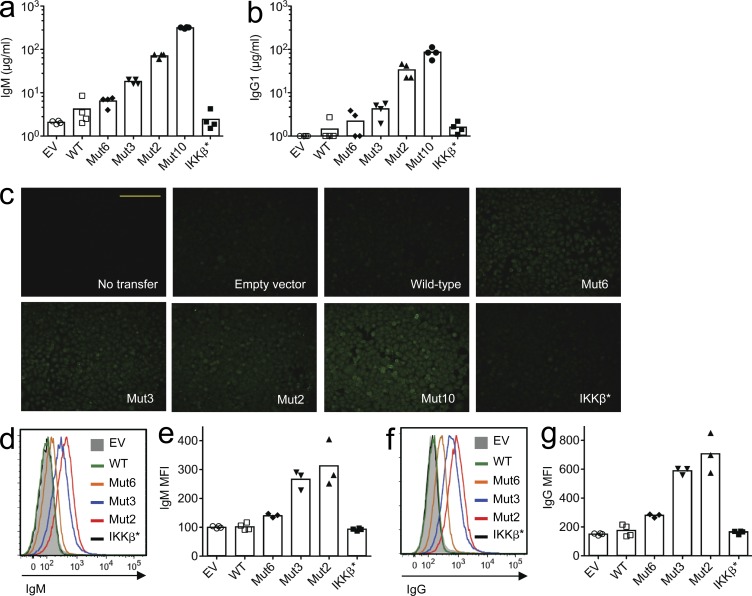
We also investigated whether the CARD11 mutations could provoke IgG autoantibody secretion by normal B cells with a diverse repertoire of specificities. B cells from nontransgenic mice were retrovirally transduced with vectors encoding mutant CARD11 or controls encoding WT CARD11, mutant IKK-β*, or EV. The B cells were transferred into Rag1−/− recipients and spleen cells, and serum was collected 12 d later. Compared with the controls, recipients of normal B cells expressing mutant CARD11 had higher concentrations of IgM and IgG1 in their sera (Fig. 5, a and b). The induction of serum IgG by the different CARD11 mutants correlated with their potency to induce plasmablast accumulation in the same animals, with Mut10 the most potent and Mut6 the least potent. IgG antibodies with a diffuse pattern of binding to the cytoplasm and nucleus of HEp-2 cells (Fig. 5 c) and IgM and IgG antibodies binding to the surface of 3T3 mouse fibroblast cells (Fig. 5, d–g) were also selectively increased in serum of recipients of B cells expressing mutant CARD11. The affinity and pathogenicity of these autoantibodies are nevertheless likely to be low because the recipients lacked T follicular helper (TFH) cells needed for affinity maturation in GCs. It will be interesting in future studies to investigate the impact of CARD11 mutations in GC B cells interacting with TFH cells.
Figure 5.
Lymphoma CARD11 mutations induce IgG autoantibody production. Normal polyclonal B cells were transduced with vectors encoding the indicated CARD11 WT or mutant proteins, constitutively active IKK-β*, or EV control. B cells were transferred into Rag1-deficient hosts, and sera were collected from the recipients after 12 d. (a and b) ELISA results show amounts of serum IgM (a; one-way ANOVA: P < 0.0001) or IgG1 (b; one-way ANOVA: P < 0.0001). (c) Representative HEp-2 staining by IgG in serum from recipients of cells expressing the indicated proteins (n = 4 recipients per transfer group). Each image was captured using an identical exposure. Bar, 500 µm. (d–g) Binding of serum IgM (d and e; one-way ANOVA: P < 0.0001) and IgG (f and g; one-way ANOVA: P < 0.0001) bound to the surface of mouse 3T3 cells (d and f: representative histogram overlays; e and g: mean fluorescence intensity [MFI]; n = 3–4 per group).
The aforementioned findings demonstrate that a range of CARD11 mutations acquired in different human lymphomas share the property of switching the mature B cell response to continuous stimulation by self-antigens from cell death into B cell proliferation, differentiation, and autoantibody secretion. In normal mature B cells, continuous self-antigen exposure desensitizes antigen receptor signaling to NF-κB and JNK while preserving signaling for calcium oscillations, NFAT, ERK, and Bim induction, and this is thought to explain the absence of proliferation and induction of apoptosis in self-reactive B cells (Healy et al., 1997; Healy and Goodnow, 1998; Enders et al., 2003). At the opposite extreme, optimal BCR cross-linking by T cell–independent type 2 antigens is sufficient to induce proliferation and plasmablast differentiation. It is not known whether a global rewiring or a single pivotal switch explains how the mature B cell response to antigen ranges from proliferation and plasma cell differentiation on the one hand to anergy and death on the other. The data here establish that regulation of a single molecule, CARD11, serves as the switch between antigen-induced signaling of death or proliferation and plasma cell differentiation.
What lies downstream of the CARD11 switch is an intriguing question for the future. Activation of NF-κB by antigen receptor signaling through CARD11 might have been predicted to be the mechanism for switching the response to self-antigen from death to growth, given that NF-κB is essential for activation of numerous B cell survival (e.g., Bcl2, BclxL, and Bfl1/A1), growth (e.g., Irf4, Myc, and Cyclin D), and differentiation genes (Irf4; Gerondakis and Siebenlist, 2010). Enforced expression of Bcl-2 nevertheless only slows the rate of self-antigen–induced death in mature B cells but does not switch them into proliferation (Cyster et al., 1994). Likewise, enforced expression of Myc using the same retroviral transduction assay used here does not inhibit self-antigen–induced death nor induce proliferation even when combined with p53 inactivation (unpublished data). Indeed, self-antigen–induced elimination of mature B cells in the assay used here remains intact even when the B cells are transduced with retrovirus containing constitutively active IKK-β* vector (Fig. 3 b). The unique ability of CARD11 to switch self-reactive B cells from death into growth and plasma cell differentiation may stem from CARD11 binding to many different signaling proteins (McCully and Pomerantz, 2008), which may change the dynamics or balance of NF-κB subunits that are activated or enlist additional signaling pathways such as JNK or p38.
Our findings also provide experimental evidence for the long-standing hypothesis that self-reactive antigen receptors, which arise frequently by VDJ rearrangements in immature B cells (Wardemann et al., 2003) and by V-region hypermutation in antigen-activated B cells (Shlomchik et al., 1990), cooperate with somatic mutations in signaling molecules to initiate neoplastic proliferation and production of anti-self antibodies (Cleary et al., 1986; Borche et al., 1990; Friedman et al., 1991; Kobayashi et al., 1993; Chiorazzi and Ferrarini, 2003; Rui et al., 2011; Stevenson et al., 2011). As opposed to binding microbial antigens, antigen receptors that bind to self-antigens provide B cells with an endless source of receptor stimulation. Given the high rate of somatic point mutation in antigen-activated B cells (Liu et al., 2008), the finding that single somatic mutations at various sites in the coiled-coil domain of CARD11 were sufficient to switch antigen receptor–induced death into growth, without requiring accumulation of numerous other mutations, identifies CARD11 as an especially weak link in the normal control of B cell malignancy and autoimmunity. The occurrence of CARD11 mutations together with self-reactive Ig V-regions can be expected to have an equally potent influence on therapeutic responses and thus should be tested in a broad range of B cell malignancies and autoimmune diseases.
MATERIALS AND METHODS
Mice.
Rag1−/− mice (Mombaerts et al., 1992) and Rag1−/− mice expressing soluble HEL (ML5 HEL transgenic strain; Goodnow et al., 1988) were used as recipients for cell transfer experiments. Donor B cells were obtained from the following: (a) antigen-specific B cells were from IgHEL transgenic mice bearing rearranged Ig transgenes encoding a HEL-specific antibody or IgHEL × ML5 HEL double transgenic mice (Goodnow et al., 1988), (b) Prdm1−/− B cells were from Prdm1Flox/Flox mice (Kallies et al., 2009) crossed to mb1-Cre transgenic mice (Hobeika et al., 2006) and littermate controls, and (c) normal B cells came from WT C57BL/6 mice. Mice were maintained on a C57BL/6 background and were housed in specific pathogen–free conditions at the Australian Phenomics Facility and the John Curtin School of Medical Research. Prdm1Flox/Flox mice crossed to mb1-Cre and littermate controls were generated and maintained in accordance with the guidelines of the Walter and Eliza Hall Institute Animal Ethics Committee. All experimental mice were used between 8 and 16 wk of age. All animals used in this study were cared for and used in accordance with protocols approved by the Australian National University Animal Experimentation Ethics Committee and the current guidelines from the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Retroviral vector and production.
Mouse Card11 and Ikbkb genes were amplified by Platinum Pfx DNA polymerase (Invitrogen) from mouse spleen cDNA and cloned into pBluescript II SK(+) vector. PCR-based site-directed mutagenesis was used to introduce gain of function mutations into mouse Card11 and Ikbkb. Amplified PCR products were purified and sequenced on a 3730xl DNA Analyzer (Applied Biosystems; at the Genome Discovery Unit–ACRF Biomolecular Resource Facility, John Curtin School of Medical Research) according to the manufacturer’s protocol. The mutations corresponded to those described by Lenz et al. (2008), and the primers used are listed in Table 1. WT and mutant Card11 and Ikbkb genes were transferred into pMXs-IRES-GFP vector (provided by T. Kitamura, The University of Tokyo, Minato-ku, Tokyo, Japan; Kitamura et al., 2003). The retroviral constructs were transfected into Phoenix ecotropic packaging cells (American Type Culture Collection) using calcium phosphate precipitation. The supernatants containing replication-defective retroviral particles were collected and frozen at −80°C until used for transduction.
Table 1.
Primers used for mutagenesis of Card11 and Ikbkb
| Primer name | Sequence | Purpose |
| CARD11 CS1 | 5′-TTTGCGGCCGCGCCGCCACCATGCCAGGAGGAGGGCCAGCTATG-3′ | Initial cloning |
| CARD11 CA1 | 5′-AAAAGCTTCAGCTGGTCCTCGTCCACCCAGATG-3′ | Initial cloning |
| CARD11 CS8 | 5′-TTTTAGATCTGCCACCATGCCAGGAGGAGGGCC-3′ | Retrovirus vector |
| CARD11 stop A2 | 5′-AAAAGCGGCCGCTCACAGCTGGTCCTCGTCC-3′ | Retrovirus vector |
| CARD11 mt2 S | 5′-GAGAGAAATCAGTCCCCCAAGCTCAAGAATGAC-3′ | L251P |
| CARD11 mt2 A | 5′-GTCATTCTTGAGCTTGGGGGACTGATTTCTCTC-3′ | L251P |
| CARD11 mt3 S | 5′-TCCACCATTGTGGTGGAGGAAAGCCATGAGGGCCTCACAC-3′ | G123S |
| CARD11 mt3 A | 5′-AGTGTGTGAGGCCCTCATGGCTTTCCTCCACCACAATGGT-3′ | G123S |
| CARD11 mt6 S | 5′-GCCTTCCACTCCCGAGTTGAGGCACAGACACAG-3′ | D387V |
| CARD11 mt6 A | 5′-CTGTGTCTGTGCCTCAACTCGGGAGTGGAAGGC-3′ | D387V |
| CARD11 mt10 S | 5′-TCCAACTCGAGATCGACCAGCTCATTAAACACCGACTGAACAAG-3′ | L232LI |
| IKK-β* S | 5′-TGAATTCGCCACCATGAGCTGGTCACCGTCCCTCCCA-3′ | S177E, S181E |
| IKK-β* A | 5′-AAAACTCGAGTCAGGCGGTTACCGTGAAGCTTCT-3′ | S177E, S181E |
Retrovirus-mediated gene transfer into mouse primary B cells.
IgHEL transgenic mice were injected intraperitoneally with 5 mg HEL (Sigma-Aldrich) in PBS (Invitrogen) to provide a pulse of antigen for activation in vivo. The spleens were yielded 6 h after the injection, and splenocytes were cultured at a density of 4 × 106 cells/ml in complete RPMI containing 10 µg/ml anti-CD40 antibody (FGK4.5; BioXCell; Fig. 1 a). B cells from IgHEL × ML5 HEL double transgenic mice or IgHEL controls were activated using 10 µg/ml anti-CD40 (FGK4.5) and 10 ng/ml IL4 (R&D Systems). Normal B cells from WT C57BL/6 mice were stimulated using 7 µg/ml anti-IgM (Jackson ImmunoResearch Laboratories, Inc.) and 10 µg/ml anti-CD40 (FGK4.5). After 24 h, the cells were spin-infected in 6-well plates at 920 g for 90 min at room temperature with the retrovirus supernatant containing 10 µl/ml DOTAP (Roche). The cells were then cultured in fresh RMPI containing 10 µg/ml anti-CD40 for 36 h. The cultured cells were washed with complete RPMI three times. The cells were resuspended at a density of 106 cells/ml. The number of live EGFP+ cells was determined by hemocytometer counting of trypan blue–negative cells in each culture combined with the results from flow cytometric analysis of the same cells.
Western blot analysis.
Day 3 (Fig. 1 a) B cell cultures were washed twice with complete RPMI and cultured in the absence of anti-CD40 for 24 h. Transduced EGFP-positive B cells were sorted using FACSAria I and II (BD) and washed with ice-cold PBS followed by lysis in SDS sample buffer (0.2 M Tris-HCl, pH 6.8, 10% SDS, 30% glycerol, 10% β-mercaptoethanol, and Bromophenol blue). Lysates were sonicated for 15 min at 5-s intervals, boiled in SDS sample buffer for 5 min at 95°C, and centrifuged for 1 min at 13,000 rpm. Proteins were resolved on 10% or 15% polyacrylamide gel and transferred onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories). Nonspecific binding to the membrane was blocked with 5% wt/vol BSA in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% vol/vol Tween 20) for 30 min followed by probing overnight at 4°C with antibody to phospho-JNK (1:2,500 dilution; Cell Signaling Technology), total JNK (1:1,000 dilution; Cell Signaling Technology), phospho–NF-κB p65 (1:1,000 dilution; Cell Signaling Technology), total NF-κB p65 (1:1,000 dilution; Cell signaling), CARD11 (1:1,000 dilution; Cell Signaling Technology), and IKK-α/β (1:250 dilution; Santa Cruz Biotechnology, Inc.). After incubation in primary antibody, membrane was washed three times in TBST and incubated with horseradish peroxidase–conjugated anti–rabbit IgG secondary antibody (1:2,500 dilution; Cell Signaling Technology) for 1 h at room temperature. Membrane was then washed five times in TBST before detection using enhanced chemiluminescence detection reagent (PerkinElmer) and developed in a dark room onto Kodak films. Membranes were reprobed with antibody to α/β-tubulin (1:5,000 dilution; Cell Signaling Technology) as a loading control.
Adoptive transfer into recipient mice.
The transduced cells were recovered 36–40 h after spin infection with retroviral particles. 5 × 106 total cultured B cells, consisting of a mixture of transduced and nontransduced B cells, were transferred into recipient mice through the lateral tail vein. The recipient mice were age-matched Rag1−/− mice (nontransgenic) and/or Rag1−/− mice expressing a soluble HEL transgene as self-antigen (HEL transgenic). The spleens of the recipients were yielded for analysis of the donor cells. Except where noted otherwise, all mice used were 8–16 wk old.
Flow cytometric analysis.
Single cell suspensions were prepared and then counted by trypan blue exclusion. Equal numbers of cells were transferred in 96-well round-bottomed plates. Cells were then incubated for 30 min at 4°C with an antibody cocktail containing the appropriate combination of antibodies, each diluted to its optimal concentration in flow cytometry buffer (PBS containing 2% [vol/vol] bovine serum and 0.1% [wt/vol] NaN3). Samples were washed twice, resuspended in flow cytometry buffer, and analyzed with an LSR II or LSR Fortessa (BD). The following antibodies from BD were used: anti-B220 (RA3-6B2), CD25 (PC61), IgMa (DS-1), CD19 (1D3), and CD4 (RM4-5). 7-AAD and Qdot 605 streptavidin conjugate from Invitrogen, CD44 (IM7) from BioLegend, and IgM (II/41) from eBioscience were also used. HyHEL9 was conjugated to Alexa Fluor 647 with a monoclonal antibody labeling kit (Molecular Probes). Staining with HyHEL9 was performed on cells that were incubated with 50 ng/ml HEL (Sigma-Aldrich; Goodnow et al., 1988). Anti-BIM antibody (clone 3C5; a gift from A. Strasser, The Walter and Eliza Hall Institute of Medical Research) was conjugated to Alexa Fluor 647 (Molecular Probes). FlowJo (Tree Star) was used for analysis of flow cytometry data.
ELISA.
96-well plates were coated with 10 µg/ml HEL (Sigma-Aldrich) for the anti-HEL ELISA or 0.5 µg/ml rat anti–mouse IgM (BD) and 0.5 µg/ml rat anti–mouse IgG1 (BD) for the WT B cell transduction experiments in Na carbonate buffer, pH 9.6, and blocked them with 1% BSA (Sigma-Aldrich). Serial dilutions of serum from recipient mice were transferred to the wells. After incubation and washing, plates were further incubated with alkaline phosphatase–conjugated goat anti–mouse IgM (SouthernBiotech) for the anti-HEL IgM ELISA or with biotin rat anti–mouse IgM (BD) followed by streptavidin alkaline phosphatase (Vector Laboratories) and alkaline phosphatase goat anti–mouse IgG1 (BD). After washing, the amount of enzyme bound to each well with the alkaline phosphatase substrate nitrophenyl phosphate was measured. The absorbance of the colored reaction product was read at 405 nm by a 96-well plate reader (THERMOmax; Molecular Devices). Control sera were used to generate standard calibration curves, and the relative units or amounts of test antibody present in sera were determined.
Detection of autoantibodies.
(a) Diluted sera (1:5 or 1:50) were incubated on HEp-2 substrate slides (INOVA), and bound antibodies were detected using goat anti–mouse IgG conjugated to Alexa Fluor 488 (Invitrogen). Slides were viewed and imaged using an IX71 microscope (Olympus). (b) 3T3 cells were incubated with 1:10 diluted sera for 30 min on ice and washed twice, and bound IgM or IgG antibodies were detected by APC rat anti–mouse IgM (BD) and Alexa Fluor 488 goat anti–mouse IgG, respectively (Molecular Probes). Cells were analyzed on an LSR II.
Acknowledgments
We thank R. Brink, A. Snow, and M. Lenardo for helpful discussions and input, C. McCrae for sequencing, H. Vohra and M. Devoy for assistance with flow cytometry, S. Daley for kindly providing advice and sharing reagents, M. Townsend and Immunogenomics laboratory members for technical support, and Animal Services for maintenance and caring of mice.
Y.S. Jeelall is supported by an Australian National University Scholarship, and J.Q. Wang is supported by an Australian Postgraduate Award. This work was supported by a Program Grant and Australia Fellowship from the National Health and Medical Research Council (NHMRC; to C.C. Goodnow), Australian government funding of the Australian Phenomics Network and the Australian National University, and Victorian State Government Operational Infrastructure Support and Australian Government NHMRC Independent Research Institute Infrastructure Support Scheme (to A. Kallies and S.L. Nutt).
The authors declare no competing financial interests.
Author contributions: Y.S. Jeelall, K. Horikawa, and C.C. Goodnow designed the experiments; Y.S. Jeelall performed most of the experiments; H.-D. Law, H. Domaschenz, and H.K.H. Fung helped with experiments; J.Q. Wang performed the Western blot experiments; A. Kallies and S.L. Nutt produced B cell–specific Pdrm1−/− mice and provided feedback on data analysis and the manuscript; and Y.S. Jeelall, K. Horikawa, and C.C. Goodnow analyzed the data and wrote the manuscript.
Footnotes
Abbreviations used:
- ANOVA
- analysis of variance
- EGFP
- enhanced GFP
- EV
- empty vector
- GC
- germinal center
- HEL
- hen egg lysozyme
- IKK
- IκB kinase
- JNK
- c-Jun N-terminal kinase
References
- Borche L., Lim A., Binet J.L., Dighiero G. 1990. Evidence that chronic lymphocytic leukemia B lymphocytes are frequently committed to production of natural autoantibodies. Blood. 76:562–569 [PubMed] [Google Scholar]
- Chiorazzi N., Ferrarini M. 2003. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annu. Rev. Immunol. 21:841–894 10.1146/annurev.immunol.21.120601.141018 [DOI] [PubMed] [Google Scholar]
- Cleary M.L., Meeker T.C., Levy S., Lee E., Trela M., Sklar J., Levy R. 1986. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell. 44:97–106 10.1016/0092-8674(86)90488-5 [DOI] [PubMed] [Google Scholar]
- Cyster J.G., Hartley S.B., Goodnow C.C. 1994. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 371:389–395 10.1038/371389a0 [DOI] [PubMed] [Google Scholar]
- Davis R.E., Ngo V.N., Lenz G., Tolar P., Young R.M., Romesser P.B., Kohlhammer H., Lamy L., Zhao H., Yang Y., et al. 2010. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 463:88–92 10.1038/nature08638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders A., Bouillet P., Puthalakath H., Xu Y., Tarlinton D.M., Strasser A. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119–1126 10.1084/jem.20030411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax K.A., Kallies A., Nutt S.L., Tarlinton D.M. 2008. Plasma cell development: from B-cell subsets to long-term survival niches. Semin. Immunol. 20:49–58 10.1016/j.smim.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Friedman D.F., Cho E.A., Goldman J., Carmack C.E., Besa E.C., Hardy R.R., Silberstein L.E. 1991. The role of clonal selection in the pathogenesis of an autoreactive human B cell lymphoma. J. Exp. Med. 174:525–537 10.1084/jem.174.3.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S., Siebenlist U. 2010. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb. Perspect. Biol. 2:a000182 10.1101/cshperspect.a000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow C., Ohashi P.2012. Immunological tolerance. In Fundamental Immunology. Seventh edition. W.E. Paul, editor. Lippincott Williams & Wilkins, Philadelphia. In press.
- Goodnow C.C., Crosbie J., Adelstein S., Lavoie T.B., Smith-Gill S.J., Brink R.A., Pritchard-Briscoe H., Wotherspoon J.S., Loblay R.H., Raphael K., et al. 1988. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 334:676–682 10.1038/334676a0 [DOI] [PubMed] [Google Scholar]
- Healy J.I., Goodnow C.C. 1998. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16:645–670 10.1146/annurev.immunol.16.1.645 [DOI] [PubMed] [Google Scholar]
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. 1997. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 6:419–428 10.1016/S1074-7613(00)80285-X [DOI] [PubMed] [Google Scholar]
- Hobeika E., Thiemann S., Storch B., Jumaa H., Nielsen P.J., Pelanda R., Reth M. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl. Acad. Sci. USA. 103:13789–13794 10.1073/pnas.0605944103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.E., Wilson L.E., Vinuesa C.G., Lesage S., Blery M., Miosge L.A., Cook M.C., Kucharska E.M., Hara H., Penninger J.M., et al. 2003. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity. 18:751–762 10.1016/S1074-7613(03)00141-9 [DOI] [PubMed] [Google Scholar]
- Kallies A., Xin A., Belz G.T., Nutt S.L. 2009. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 31:283–295 10.1016/j.immuni.2009.06.021 [DOI] [PubMed] [Google Scholar]
- Kitamura T., Koshino Y., Shibata F., Oki T., Nakajima H., Nosaka T., Kumagai H. 2003. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp. Hematol. 31:1007–1014 [PubMed] [Google Scholar]
- Kobayashi R., Rassenti L.Z., Meisenholder G., Carson D.A., Kipps T.J. 1993. Autoantigen inhibits apoptosis of a human B cell leukemia that produces pathogenic rheumatoid factor. J. Immunol. 151:7273–7283 [PubMed] [Google Scholar]
- Lenz G., Davis R.E., Ngo V.N., Lam L., George T.C., Wright G.W., Dave S.S., Zhao H., Xu W., Rosenwald A., et al. 2008. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 319:1676–1679 10.1126/science.1153629 [DOI] [PubMed] [Google Scholar]
- Lesley R., Xu Y., Kalled S.L., Hess D.M., Schwab S.R., Shu H.B., Cyster J.G. 2004. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 20:441–453 10.1016/S1074-7613(04)00079-2 [DOI] [PubMed] [Google Scholar]
- Liu M., Duke J.L., Richter D.J., Vinuesa C.G., Goodnow C.C., Kleinstein S.H., Schatz D.G. 2008. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 451:841–845 10.1038/nature06547 [DOI] [PubMed] [Google Scholar]
- Martins G., Calame K. 2008. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu. Rev. Immunol. 26:133–169 10.1146/annurev.immunol.26.021607.090241 [DOI] [PubMed] [Google Scholar]
- McCully R.R., Pomerantz J.L. 2008. The protein kinase C-responsive inhibitory domain of CARD11 functions in NF-kappaB activation to regulate the association of multiple signaling cofactors that differentially depend on Bcl10 and MALT1 for association. Mol. Cell. Biol. 28:5668–5686 10.1128/MCB.00418-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 68:869–877 10.1016/0092-8674(92)90030-G [DOI] [PubMed] [Google Scholar]
- Morin R.D., Mendez-Lago M., Mungall A.J., Goya R., Mungall K.L., Corbett R.D., Johnson N.A., Severson T.M., Chiu R., Field M., et al. 2011. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 476:298–303 10.1038/nature10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V.N., Davis R.E., Lamy L., Yu X., Zhao H., Lenz G., Lam L.T., Dave S., Yang L., Powell J., Staudt L.M. 2006. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 441:106–110 10.1038/nature04687 [DOI] [PubMed] [Google Scholar]
- Pasqualucci L., Trifonov V., Fabbri G., Ma J., Rossi D., Chiarenza A., Wells V.A., Grunn A., Messina M., Elliot O., et al. 2011. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 43:830–837 10.1038/ng.892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings D.J., Sommer K., Moreno-García M.E. 2006. The CARMA1 signalosome links the signalling machinery of adaptive and innate immunity in lymphocytes. Nat. Rev. Immunol. 6:799–812 10.1038/nri1944 [DOI] [PubMed] [Google Scholar]
- Rui L., Schmitz R., Ceribelli M., Staudt L.M. 2011. Malignant pirates of the immune system. Nat. Immunol. 12:933–940 10.1038/ni.2094 [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Derudder E., Hobeika E., Pelanda R., Reth M., Rajewsky K., Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 24:729–739 10.1016/j.immuni.2006.04.005 [DOI] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M.Z., Pisetsky D., Marshak-Rothstein A., Weigert M. 1990. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J. Exp. Med. 171:265–292 10.1084/jem.171.1.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson F.K., Krysov S., Davies A.J., Steele A.J., Packham G. 2011. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 118:4313–4320 10.1182/blood-2011-06-338855 [DOI] [PubMed] [Google Scholar]
- Thien M., Phan T.G., Gardam S., Amesbury M., Basten A., Mackay F., Brink R. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 20:785–798 10.1016/j.immuni.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Thome M. 2004. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4:348–359 10.1038/nri1352 [DOI] [PubMed] [Google Scholar]
- Wardemann H., Yurasov S., Schaefer A., Young J.W., Meffre E., Nussenzweig M.C. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377 10.1126/science.1086907 [DOI] [PubMed] [Google Scholar]