Neuropilin 1 mediates anti-tumor control by promoting regulatory T cell infiltration.
Abstract
Infiltration of Foxp3+ regulatory T (T reg) cells is considered to be a critical step during tumor development and progression. T reg cells supposedly suppress locally an effective anti-tumor immune response within tumor tissues, although the precise mechanism by which T reg cells infiltrate the tumor is still unclear. We provide evidence that Neuropilin 1 (Nrp-1), highly expressed by Foxp3+ T reg cells, regulates the immunological anti-tumor control by guiding T reg cells into the tumor in response to tumor-derived vascular endothelial growth factor (VEGF). We demonstrate for the first time that T cell–specific ablation of Nrp-1 expression results in a significant breakdown in tumor immune escape in various transplantation models and in a spontaneous, endogenously driven melanoma model associated with strongly reduced tumor growth and prolonged tumor-free survival. Strikingly, numbers of tumor-infiltrating Foxp3+ T reg cells were significantly reduced accompanied by enhanced activation of CD8+ T cells within tumors of T cell–specific Nrp-1–deficient mice. This phenotype can be reversed by adoptive transfer of Nrp-1+ T reg cells from wild-type mice. Thus, our data strongly suggest that Nrp-1 acts as a key mediator of Foxp3+ T reg cell infiltration into the tumor site resulting in a dampened anti-tumor immune response and enhanced tumor progression.
Tumor progression is a complex process which involves tumor–host interactions through multiple cellular and molecular factors of the tumor microenvironment. Evidence has amassed that diverse stromal, vascular, and inflammatory cells, which make up the tumor microenvironment, are essential for various aspects of macroscopic tumor growth, maintenance, invasion, and angiogenesis. Thereby, multiple cell populations have the capability to influence the initial responses to therapy, tumor recurrence, and drug resistance (Dave et al., 2004; Galon et al., 2006; Andreu et al., 2010). However, tumors are able to create an immunosuppressive microenvironment to escape immune surveillance and promote tumor development (Coussens and Werb, 2002; Balkwill and Coussens, 2004). It has been reported that CD4+CD25+ regulatory T (T reg) cells, which express the T reg cell–specific transcription factor Foxp3—thereby defined as CD4+CD25+ T reg cells of Foxp3+ T reg cells in this study—participate in anti-tumor immune responses by dampening T cell immunity to tumor-associated antigens and to be the main obstacle tempering successful immunotherapy and active vaccination. In different mouse models, but also in patients with various cancers, large numbers of CD4+CD25+ T reg cells have been found in the circulation or in the tumor microenvironment (Zou, 2006). Importantly, the number of CD4+CD25+ T reg cells present in tumors and, in particular, decreased ratios of CD8+ T cells to T reg cells, correlate with poor prognosis in patients with breast, gastric, and ovarian cancer (Nishikawa and Sakaguchi, 2010). Furthermore, depletion of CD4+CD25+ T reg cells by administration of anti-CD25 antibodies inhibits tumor growth, demonstrating that T reg cells indeed promote tumorigenesis (Onizuka et al., 1999; Shimizu et al., 1999) and potentially modulate the clinical course of the disease. A direct link between T reg cells and reduced tumor immunity was provided by adoptive transfer experiments. Tumor-specific CD8+ T cells were transferred with either CD4+ T cells lacking the CD4+CD25+ compartment or CD4+CD25+ T reg cells to melanoma-bearing mice. In mice that received T reg cells, but not in mice that received CD4+CD25− T cells, CD8+ T cell immunity against tumor antigens was abolished (Turk et al., 2004; Antony et al., 2005). Collectively, these studies clearly demonstrate the importance of CD4+CD25+ T reg cells in tumor immunity. However, by which mechanism CD4+CD25+ T reg cells infiltrate into the tumor to locally suppress an effective anti-tumor immune response remains elusive. To develop better immunotherapeutic strategies, it is important to identify the mechanisms underlying the recruitment and interactions between tumor cells and cells of the immune system.
Recently, we have identified the type I transmembrane protein Neuropilin 1 (Nrp-1) to be highly expressed by CD4+CD25+ T reg cells and showed that CD4+Nrp-1+ T cells are able to suppress proliferation of naive T cells upon stimulation in vitro in contrast to CD4+Nrp-1− T cells (Bruder et al., 2004). Overexpression of the T reg cell–specific transcription factor Foxp3 led to the induction of Nrp-1 expression in CD4+CD25− T cells, suggesting that Nrp-1 expression is regulated by Foxp3 (Bruder et al., 2004; Loser et al., 2005). Moreover, it was proposed that Nrp-1 expressed by CD4+CD25+ T reg cells plays a crucial role in the formation of long-lasting interactions of T reg cells with immature DCs (Sarris et al., 2008), and Tordjman et al. (2002) described Nrp-1 as player in the establishment of cellular contacts between naive T cells and DCs involved in T cell activation. Originally, Nrp-1 was described to be expressed in the developing Xenopus laevis nervous system (Fujisawa et al., 1995), but Nrp-1 expression has also been observed in other cells like DCs, endothelial cells, and tumor cells (Kawakami et al., 2002; Tordjman et al., 2002; Bielenberg et al., 2006). The absence of a functional Nrp-1 receptor leads to embryonic lethality because of impaired heart development and defective endothelial cell migration, resulting in vessel enlargement and defects in vascular sprouting (Kitsukawa et al., 1997; Kawasaki et al., 1999; Jones et al., 2008). In addition, it has been demonstrated that Nrp-1 acts as a receptor for the vascular endothelial growth factor (VEGF) to regulate vascular development during embryogenesis (Soker et al., 1998). VEGF and its receptors are also essential during postnatal formation of new blood vessels (Chen et al., 2005), and VEGF expression is associated with increased angiogenesis and advanced-stage disease in a variety of solid tumor types (Takahashi et al., 1995; Price et al., 2001). The role of Nrp-1 in VEGF signaling was originally studied using porcine aortic endothelial cells. Co-expression of Nrp-1 and VEGF receptor 2 (VEGFR2) in these cells enhances VEGF binding and chemoattraction toward VEGF (Soker et al., 1998). Subsequent investigations using human endothelial cells demonstrated that Nrp-1 alone, without VEGFR2 expression, can mediate migration toward VEGF and endothelial cell adhesion to extracellular matrix (Wang et al., 2003; Murga et al., 2005; Pan et al., 2007). It has been demonstrated that the interplay between VEGF and Nrp-1 is also involved in the correct migration of the somata of facial motor neurons (Schwarz et al., 2004). Thus, a fundamental function of Nrp-1 is the regulation of cell motility toward a VEGF gradient.
In the present study, we demonstrate in several transplantation models, but also in a spontaneous, endogenously driven murine melanoma, that knockdown of Nrp-1 expression in T reg cells resulted in delayed tumor formation and progression. This reduced tumor outgrowth was strongly dependent on a decreased infiltration of Foxp3+ T reg cells into the tumor site which, in turn, is mediated by tumor-derived VEGF attraction. Importantly, the impaired tumor growth in T cell–specific Nrp-1–deficient mice could be restored by adoptive transfer of Nrp-1+ T reg cells from WT mice. These results highlight the relevance of Nrp-1 on Foxp3+ T reg cells as a key mediator of the immunological anti-tumor control.
RESULTS
Delayed tumor growth in Nrp-1flox/flox × CD4-cre mice
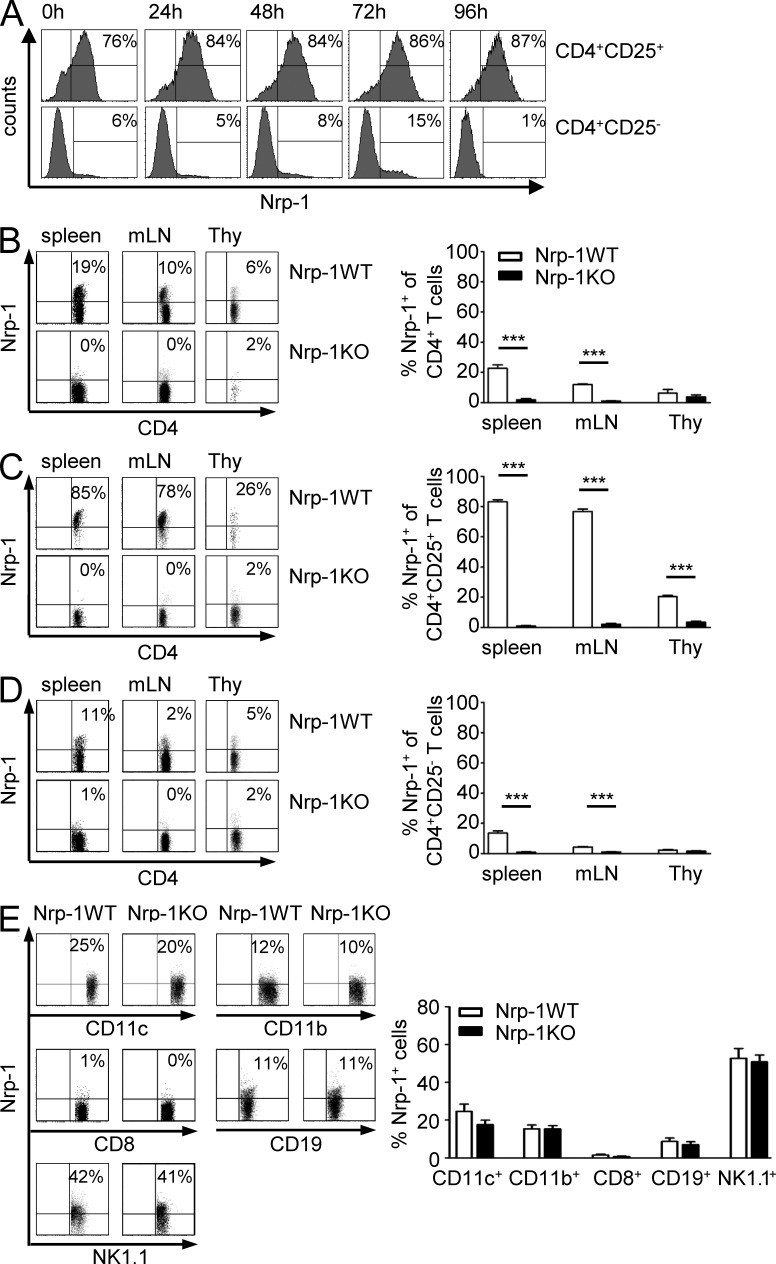
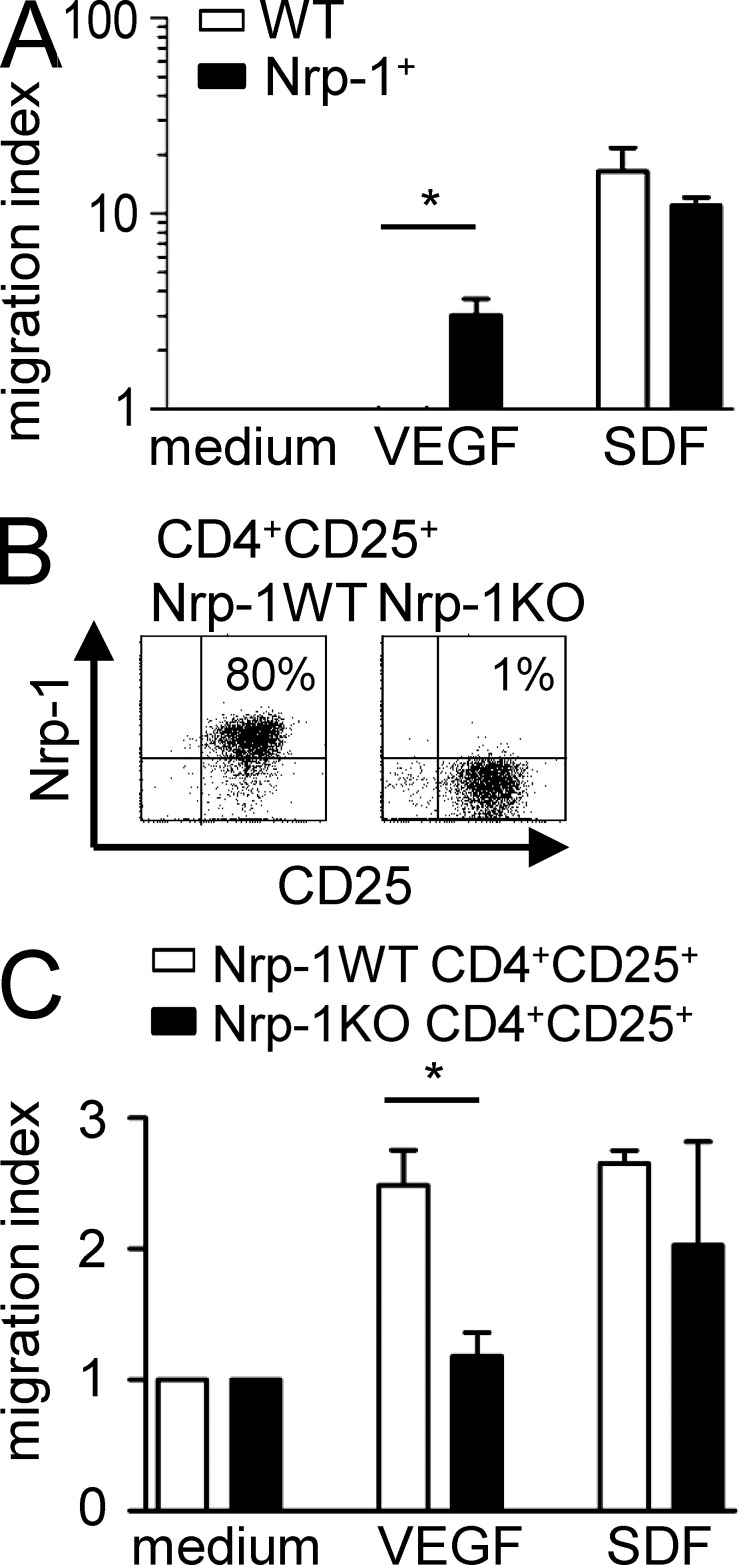
CD4+Foxp3+ T reg cells play a critical role in tumor immunity by suppression of an appropriate anti-tumor immune response. A few years ago, we identified Nrp-1 to be specifically expressed by CD4+CD25+ T reg cells in contrast to CD4+CD25− naive T cells and in vitro activated T cells (Bruder et al., 2004; Fig. 1 A). To define how Nrp-1 expression contributes to T reg cell function during tumorigenesis, we made use of Nrp-1flox/flox mice allowing selective ablation of Nrp-1 expression in T cells by crossing them with CD4-cre mice. Nrp-1 expression in CD4+ T cells of secondary lymphoid organs was significantly reduced from 23% in spleen and 14% in mesenteric LNs (mLN) of Nrp-1WT mice to <1% in Nrp-1flox/flox × CD4-cre mice (Nrp-1KO; Fig. 1 B). Depletion of Nrp-1 expression using CD4-cre mainly affects CD4+CD25+ T reg cells, as ∼80% of CD4+CD25+ T reg cells from secondary lymphoid organs of Nrp-1WT mice expressed Nrp-1 but only 1–2% of T reg cells from Nrp-1KO mice (Fig. 1 C). Nrp-1 expression was also significantly reduced in CD4+CD25− T cells from 13 or 4% in spleen or mLN, respectively, to 1% in Nrp-1KO mice (Fig. 1 D). Ablation of Nrp-1 expression in Nrp-1KO mice was specifically restricted to CD4+ T cells, as only a minor population of CD8+ T cells (1%) from Nrp-1WT mice expressed Nrp-1 and Nrp-1 expression on CD11c+, CD11b+, CD8+, CD19+, and NK1.1+ splenocytes was not affected in Nrp-1flox/flox × CD4-cre mice (Fig. 1 E). These results show that crossing of CD4-cre mice with Nrp-1flox/flox mice leads to specific ablation of Nrp-1 expression in CD4+ T cells, in particular CD4+CD25+ T reg cells.
Figure 1.
CD4+ T cell–specific ablation of Nrp-1 expression in Nrp-1flox/flox × CD4-cre mice. (A) Sorted CD4+CD25+ T reg cells and CD4+CD25− T cells were stimulated in vitro with anti-CD3, anti-CD28, and IL-2 for 24, 48, 72, and 96 h or left untreated (0 h) before analysis of Nrp-1 expression by flow cytometry. Representative histograms from one experiment out of three with similar results are shown. (B–D) Percentages of Nrp-1–expressing CD4+ T cells (B), CD4+CD25+ T cells (C), or CD4+CD25− T cells (D) were determined in spleen, mLN, and thymus (Thy) of Nrp-1flox/flox × CD4-cre mice (black bars; Nrp-1KO) and WT mice (white bars; Nrp-1WT) by flow cytometry. Representative dot plots from one experiment are depicted on the left and mean values ± SEM from n = 7–11 mice analyzed in three independent experiments are shown on the right. (E) Nrp-1 expression on CD11c+, CD11b+, CD8+, CD19+, and NK1.1+ splenocytes isolated from Nrp-1KO mice (black bars) and Nrp-1WT mice (white bars) analyzed by flow cytometry. Results from two independent experiments (n = 7–8 mice) are depicted as mean ± SEM. ***, P < 0.001 (Student’s t test).
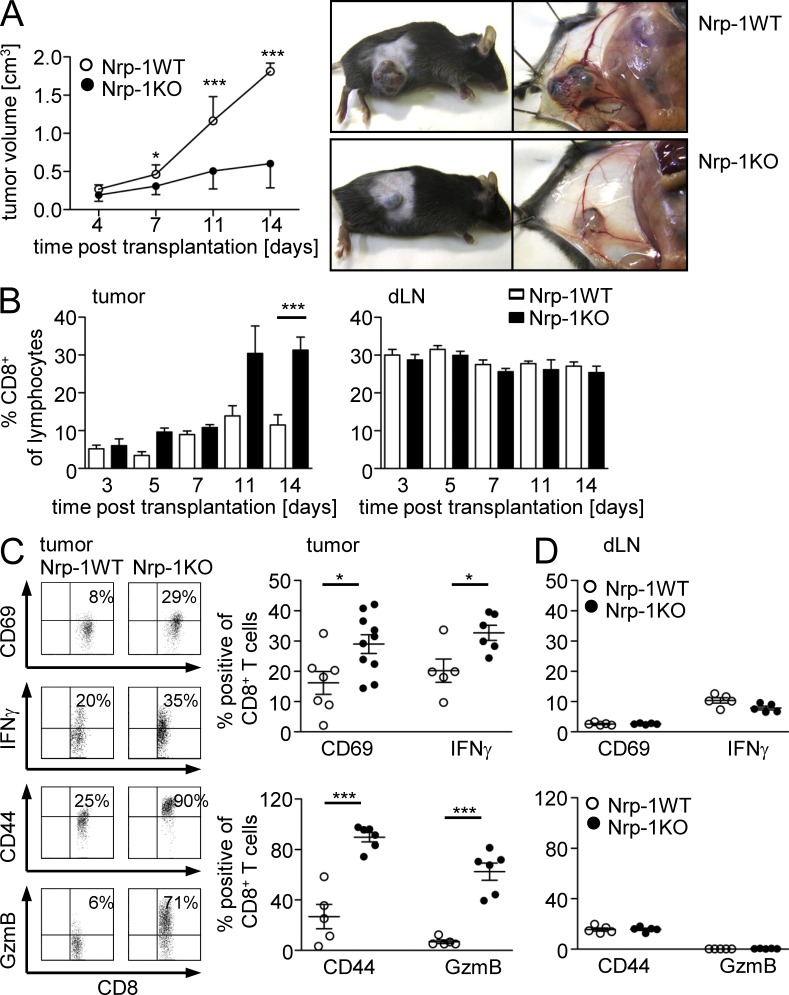
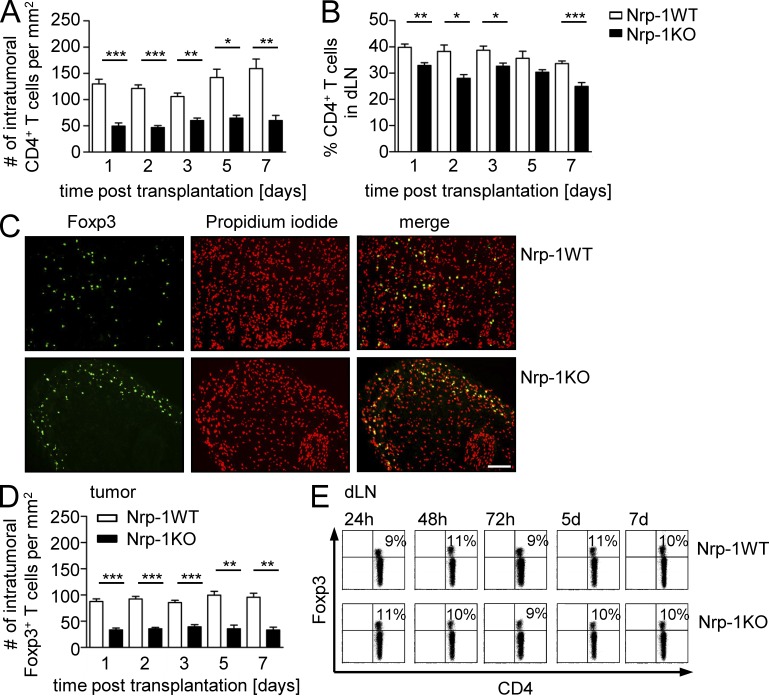
Next, we asked whether ablation of Nrp-1 expression in T reg cells impairs tumor development in vivo. Therefore, we transplanted the established mouse metallothionein I promoter-enhancer (MT)/rearranged during transfection (ret) melanoma cell line (Nasarre et al., 2009) into Nrp-1WT and Nrp-1KO mice and measured tumor growth at indicated time points (Fig. 2 A). As shown in Fig. 2 A, tumor growth was significantly reduced in Nrp-1KO mice in comparison to Nrp-1WT mice (Fig. 2 A). When we compared the amount of CD8+ effector T cells within the tumors, we noticed an increase at day 11 and a significant up-regulation at day 14 post transplantation (p.t.) in Nrp-1KO mice (Fig. 2 B, left) but no alteration within the draining LNs (dLNs; Fig. 2 B, right). Importantly, the percentage of activated CD8+ T cells determined by CD69, CD44, Granzyme B, and IFN-γ expression was significantly enhanced at day 14 p.t. in tumors of Nrp-1KO mice (Fig. 2 C), whereas the activation status of CD8+ T cells in the dLN was not affected (Fig. 2 D).
Figure 2.
Delay in tumorigenesis of transplanted tumors to T cell–specific Nrp-1–deficient mice is accompanied by activation of intratumoral CD8+ T cells. (A) Tumor growth curves of either Nrp-1flox/flox × CD4-cre mice (Nrp-1KO; black circles) or WT mice (Nrp-1WT; white circles) at days 4, 7, 11, and 14 after s.c. transplantation of MT/ret–derived tumor cells are shown as mean ± SEM from two independent experiments (n = 4–5 mice per experiment; left). Representative pictures are shown of individual tumor-bearing Nrp-1WT and Nrp-1KO mice at 14 d p.t. using MT/ret cells (right). (B) The amount of CD8+ T cells within TILs (left) or dLN cells (right) was determined at days 3, 5, 7, 11, and 14 p.t. of MT/ret tumor cells in Nrp-1KO mice (black bars) or Nrp-1WT mice (white bars) by flow cytometry. Results are shown as mean ± SEM of n = 3–10 mice analyzed in one or two independent experiments, respectively. (C and D) The amount of CD69, IFN-γ, CD44, and granzyme B (GzmB) expressing CD8+ tumor-infiltrating T cells (C) or CD8+ dLN cells (D) was analyzed 14 d p.t. of MT/ret tumor cells in Nrp-1KO mice (black circles) or Nrp-1WT mice (white circles) by flow cytometry. Representative dot plots from flow cytometric analysis of CD8+ T cells from tumor tissues of Nrp-1WT and Nrp-1KO are shown (C, left). Each data point represents one animal analyzed in one or two (CD69) experiments, respectively. Error bars represent ±SEM. Horizontal bars show the mean. *, P < 0.05; ***, P < 0.001 (Student’s t test).
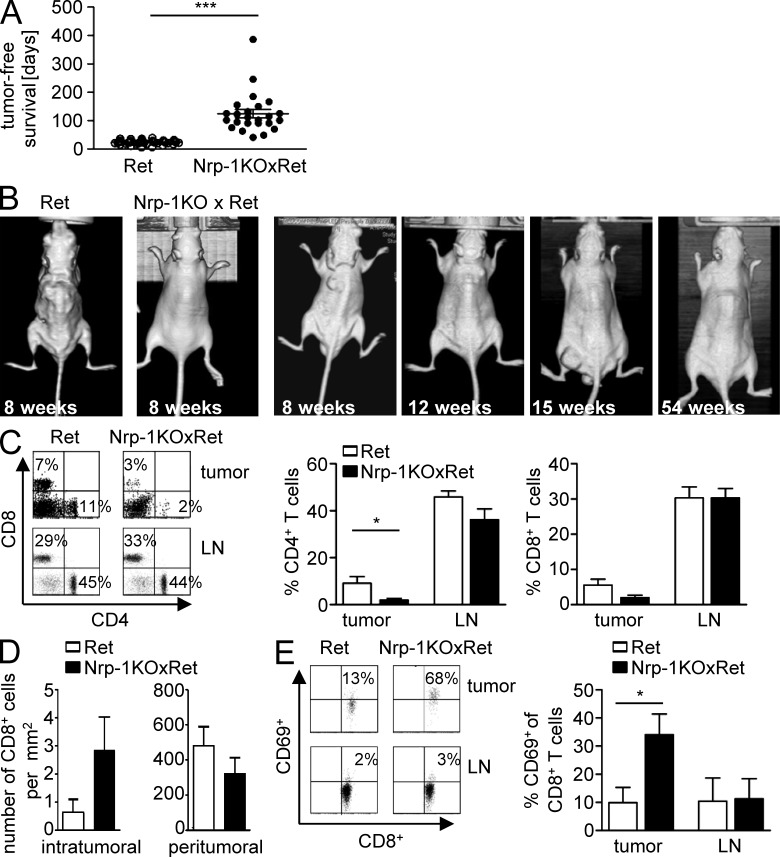
Subcutaneous transplantation of tumor cells is a well established and commonly used method in cancer research. However, transplantation of tumor cells does not reflect the complex developmental and growth characteristics of spontaneously arising tumors and also triggers inflammatory processes caused by injection per se. Therefore, we additionally analyzed the effect of T cell–specific Nrp-1 ablation in MT/ret (Ret) transgenic mice, which develop spontaneously cutaneous malignant melanoma and metastases in distant organs (Kato et al., 1998; Helfrich et al., 2010). Remarkably, the onset of tumor development in Nrp-1KO mice crossed with MT/ret mice (Nrp-1KOxRet) was significantly delayed (mean = 121 d) in contrast to Ret transgenic mice (mean = 24 d; Fig. 3 A). Furthermore, Nrp-1KOxRet mice developed many fewer tumors over time (Fig. 3 B). Flow cytometry analysis of tumor-infiltrating lymphocytes (TILs) revealed that the percentage of CD4+ TIL was significantly reduced within tumors of Nrp-1KOxRet mice (Fig. 3 C). The frequency of CD8+ T cells within the whole tumor tissue did not significantly differ between Ret and Nrp-1KOxRet mice (Fig. 3 C), but a more detailed analysis using immunohistochemistry revealed an increase in intratumoral, but not peritumoral, CD8+ T cells (Fig. 3 D). The amount of CD4+ and CD8+ T cells within the LNs did not change (Fig. 3 C). Similar to the results obtained from transplantation experiments (Fig. 2), the delay in tumor development and the reduction of tumor incidence correlated with a significant increase in activated CD8+ T cells within tumors, but not LNs, of Nrp-1KOxRet mice as determined by the expression of CD69 (Fig. 3 E). Thus, T cell–specific ablation of Nrp-1 expression impairs the development of tumors by reestablishing the CD8+ T cell–mediated anti-tumor immune response.
Figure 3.
Prolonged tumor-free survival and reduced tumor development of MT/ret mice deficient in T cell–specific Nrp-1 expression. Nrp-1flox/flox × CD4-cre mice were crossed with MT/ret mice. (A) Scatter plot for the age of mice until melanomas were palpable in either MT/ret (white circles; Ret) or Nrp-1flox/flox × CD4-cre mice × MT/ret (black circles; Nrp-1KOxRet). Each data point represents one animal (n = 42 Ret, n = 26 Nrp-1KOxRet mice). Error bars represent ±SEM. (B) Representative pictures for the assessment of tumor development during life in Ret and Nrp-1KOxRet mice at indicated time points using CT. (C) The percentage of CD4+ and CD8+ T cells on gated lymphocytes within the tumor and LN was determined by flow cytometry. Representative dot plots are shown on the left and summarized results from n = 4–5 mice analyzed in four independent experiments are shown as mean ± SEM in the middle (CD4+ T cells) and on the right (CD8+ T cells). (D) Immunofluorescence-based quantification for the number of intratumoral and peritumoral CD8+ T cells within Ret (n = 6) and Nrp-1KOxRet (n = 8) mice. A total of five regions of interest (ROI) per tumor were evaluated. Means were calculated for all ROI. Data are summarized as mean ± SEM of two independent experiments. (E) CD69-expressing cells on gated CD8+ T cells were determined in tumors (n = 5 mice) and LN (n = 3 mice) of Ret- and Nrp-1KOxRet-transgenic mice by flow cytometry. Representative dot plots are shown on the left, and summarized results as mean ± SEM of n = 3–5 mice analyzed in three independent experiments are on the right. *, P < 0.05; ***, P < 0.001 (Student’s t test).
Depletion of Foxp3+ T reg cells results in impaired tumor growth similar to Nrp-1flox/flox × CD4-cre mice
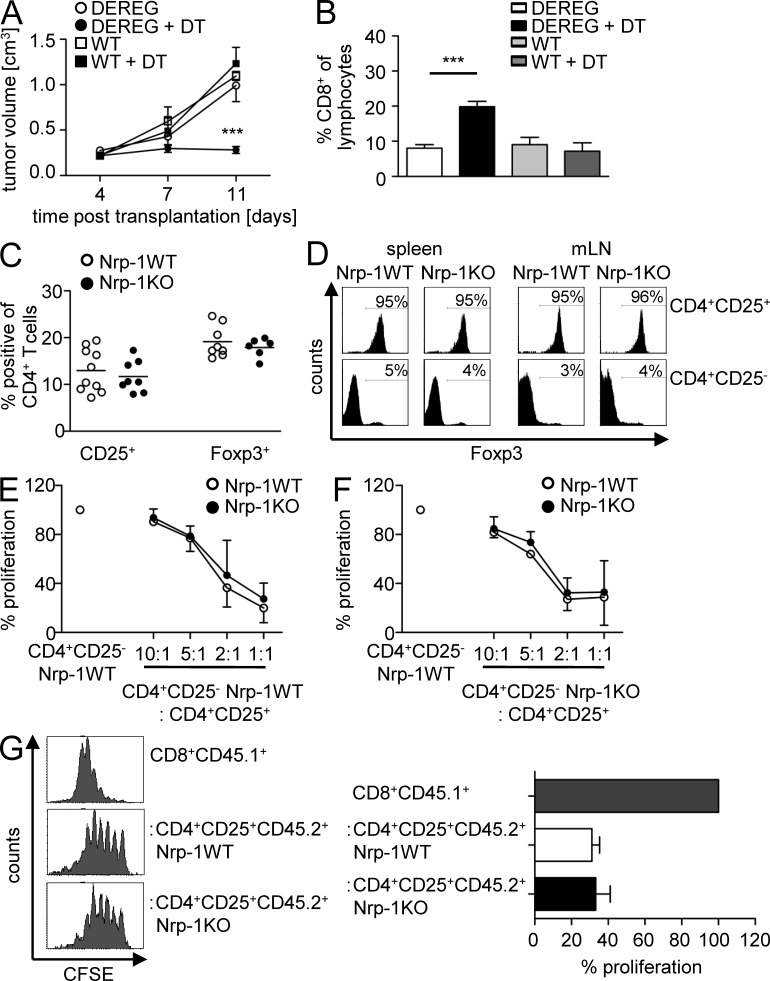
T cell–specific ablation of Nrp-1 by using Nrp-1flox/flox × CD4-cre mice mainly affects Foxp3+ T reg cells as only a minor population of CD4+CD25− T cells from WT mice expresses Nrp-1 (Fig. 1). Therefore, we hypothesized that the impaired tumor growth observed in Nrp-1flox/flox × CD4-cre was based on modulation in the biological properties of Foxp3+ T reg cells. Most recently, it has been demonstrated that tumor growth of transplanted B16 melanoma cells could be reduced by depletion of Foxp3+ T reg cells after tumor transplantation using transgenic depletion of T reg cells (DEREG) mice, which express a diphtheria toxin (DT) receptor under control of the foxp3 locus (Klages et al., 2010). To analyze whether this finding corresponds to our MT/ret melanoma transplantation model, we depleted Foxp3+ T reg cells from DEREG mice by application of DT and transplanted MT/ret cells. As shown in Fig. 4 A, Foxp3+ T reg cell–deficient mice (DT-treated DEREG mice) exhibited a significantly impaired tumor growth rate at day 11 p.t. In addition, we detected a significant expansion of tumor-infiltrating CD8+ T cells (Fig. 4 B). This effect was specifically caused by the depletion of Foxp3+ T reg cells as application of DT to WT mice had no influence on tumor development or the frequency of CD8+ TILs (Fig. 4, A and B). These results highlighted in accordance to our MT/ret melanoma cell transplantation model the crucial role of Foxp3+ T reg cells for tumor growth and progression. More importantly, depletion of Foxp3+ T reg cells resulted in an impaired tumor growth of transplanted MT/ret melanoma cells to a similar extent as in mice with T cell–specific ablation in Nrp-1 expression.
Figure 4.
Foxp3+ T reg cells contribute to tumor growth, but Nrp-1 expression by Foxp3+ T reg cells is dispensable for their development and immunosuppressive function. (A and B) DT-treated (black circles) or untreated (white circles) DEREG mice and DT-treated (black squares) or untreated (white squares) WT mice were transplanted s.c. with MT/ret melanoma cells. Tumor volume was measured at days 3, 7, and 11 p.t. (A) and the percentage of CD8+ T cells within the tumors was assessed by flow cytometry at day 11 p.t. (B). Results from two independent experiments are summarized as mean ± SEM (n = 2–5 mice per group and experiment). (C) Percentages of CD25 and Foxp3-expressing CD4+ splenocytes of Nrp-1flox/flox × CD4-cre mice (black circles; Nrp-1KO) and WT mice (white circles; Nrp-1WT) determined by flow cytometry. Each data point represents one animal analyzed in three independent experiments. Horizontal bars show the mean. (D) Percentages of Foxp3-expressing CD4+CD25+ (top) and CD4+CD25− T cells (bottom) isolated from spleen or mLN of Nrp-1KO mice and Nrp-1WT mice were analyzed by flow cytometry. Representative data from one experiment out of three with similar results are shown. (E and F) Sorted CD4+CD25− T cells from Nrp-1WT mice (n = 3–4; E) or Nrp-1KO mice (n = 3–4; F) were cultured alone or co-cultured with increasing numbers of CD4+CD25+ T reg cells from Nrp-1KO mice (black circles) and Nrp-1WT mice (white circles) in the presence of anti-CD3 and irradiated splenocytes as APCs. Proliferation was measured by 3[H] incorporation. Results from two to three independent experiments performed in triplicate were summarized with respect to CD4+CD25− WT T cells (set as 100%) ± SEM. (G) CD8+ T cells were sorted from CD45.1+ WT mice, labeled with CFSE, and either cultured alone (gray bars) or co-cultured with CD45.2+CD4+CD25+ T reg cells from Nrp-1WT (white bars) or Nrp-1KO (black bars) in the presence of anti-CD3 and irradiated splenocytes. Proliferation was assessed at day 3 by loss of CFSE on gated CD45.1+CD8+ responder T cells. Histograms of one out of two independent experiments are shown on the left and data are summarized as mean ± SEM with respect to CD8+ responder T cells (set as 100%; right). ***, P < 0.001 (Students t test).
Nrp-1 is not essential for the suppressive activity of CD4+CD25+ T reg cells in vitro
Based on these results, we wondered whether T cell–specific ablation of Nrp-1 expression influences their development and/or suppressive phenotype, leading to CD8+ T cell activation and impaired tumor growth as observed in our tumor models. Analysis of CD4+CD25+ and Foxp3+ T reg cells revealed no differences in their overall percentage in Nrp-1KO and Nrp-1WT mice (Fig. 4 C). Moreover, CD4+CD25+ and CD4+CD25− T cells from Nrp-1KO and Nrp-1WT mice expressed similar amounts of Foxp3 as analyzed by flow cytometry (Fig. 4 D). In addition, we detected no differences in CD4+ and CD8+ T cell distribution in thymus and secondary lymphoid organs of both mouse strains (unpublished data). To analyze the inhibitory capacity of Nrp-1–deficient CD4+CD25+ T reg cells, we performed co-culture experiments with sorted CD4+CD25− T cells isolated from either Nrp-1WT (Fig. 4 E) or Nrp-1KO mice (Fig. 4 F) and sorted CD4+CD25+ T reg cells from Nrp-1WT mice and Nrp-1KO mice at different ratios. CD4+CD25+ T reg cells from Nrp-1WT mice led to decreased proliferative responses of CD4+CD25− T cells both isolated from Nrp-1WT and Nrp-1KO mice. Noteworthy, we did not observe any differences in the suppressive activity of Nrp-1–deficient CD4+CD25+ T reg cells compared with WT CD4+CD25+ T reg cells (Fig. 4, E and F). Moreover, CD4+CD25− T cells and CD4+CD25+ T reg cells from spleens of Nrp-1WT and Nrp-1KO mice exhibited similar proliferative activity upon stimulation in vitro and produced equal amounts of the proinflammatory cytokines IL-2, IFN-γ, and TNF upon stimulation in vitro (unpublished data). As we observed an increased activation of CD8+ TILs in tumor-bearing Nrp-1KO mice (Fig. 2 C), we asked whether Nrp-1–deficient T reg cells are able to suppress the proliferation of CD8+ T cells. For this purpose, we performed co-culture experiments with CFSE-labeled congenic CD8+ T cells from CD45.1+WT mice and CD45.2+CD4+CD25+ T reg cells isolated either from Nrp-1WT or from Nrp-1KO mice (Fig. 4 G). By this approach, we could demonstrate that Nrp-1–deficient CD4+CD25+ T reg cells inhibited the proliferation of CD8+ T cells to a similar extent as CD4+CD25+ T reg cells from WT mice (Fig. 4 G). These results demonstrate that Nrp-1 expression by CD4+ effector T cells does not modulate their susceptibility to T reg cell–mediated suppression and, more importantly, Nrp-1 expression by CD4+CD25+ T reg cells is not required for their inhibitory function in vitro. This finding was somehow surprising, as we observed an increased activation of CD8+ T cells within transplanted tumors from Nrp-1KO mice in comparison to Nrp-1WT mice (Fig. 2), which suggests differences in the immunosuppressive function of WT T reg cells and Nrp-1–deficient T reg cells. Therefore, Nrp-1 expression seems to contribute to the complex biological properties of T reg cells not directly through a modulation of their suppressive capacity.
Tumor-derived VEGF contributes to Nrp-1+Foxp3+ T reg cell infiltration into the tumor
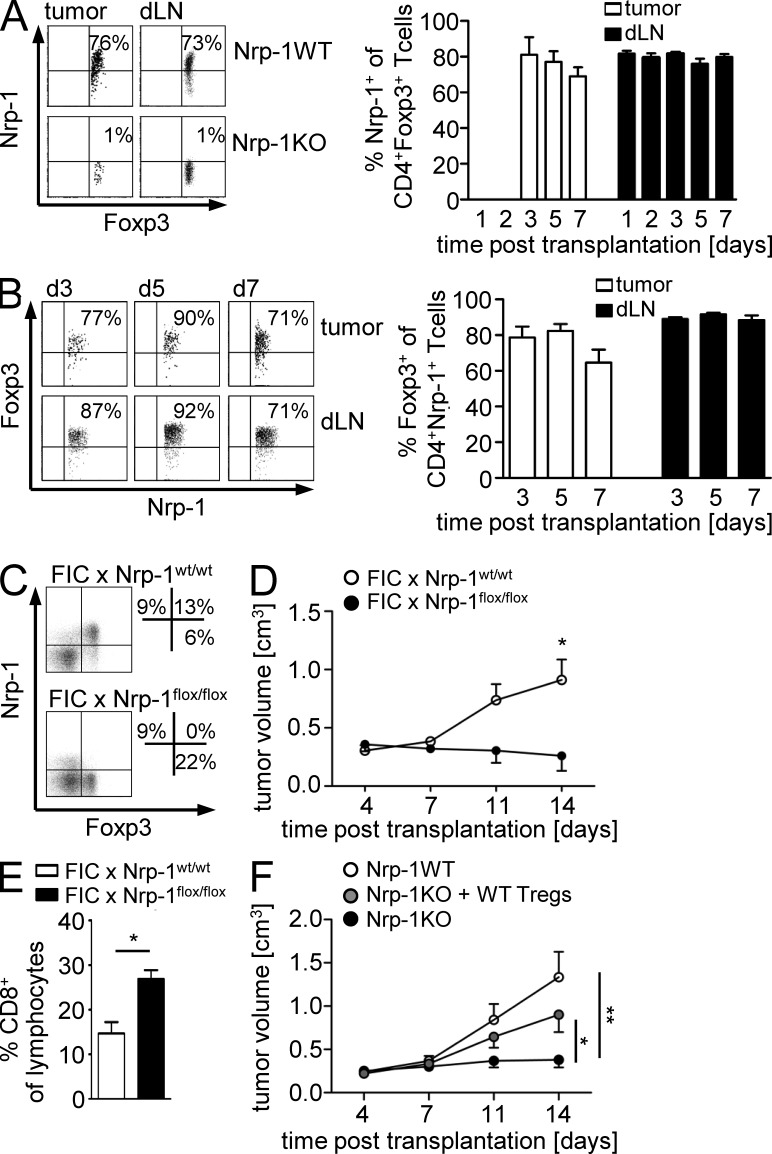
An effective immunosuppression in vivo requires an appropriate colocalization of suppressor and effector cells, and increased numbers of CD4+CD25+ T reg cells is a characteristic feature of several tumor entities (Zou, 2006). Because Nrp-1 regulates the directed migration of various cell types (Wang et al., 2003; Schwarz et al., 2004; Murga et al., 2005), we asked whether Nrp-1 is involved in the migratory potential of T reg cells with respect to the Nrp-1 ligand VEGF. Therefore, we performed an in vitro transwell migration assay with an Nrp-1–deficient T cell hybridoma line (Weber et al., 1992) that served as WT and an Nrp-1–expressing mutant (Nrp-1+) that was generated by transduction of a Nrp-1 encoding retroviral vector (Fig. 5 A). Both WT and Nrp-1+ cells were able to migrate through transwell in response to stromal cell–derived factor (SDF; positive control) to the same degree, but only Nrp-1+ cells exhibited migratory activity toward recombinant VEGF, whereas Nrp-1 deficiency completely abolished the migration of T cells in response to VEGF (Fig. 5 A). To further corroborate these results, we isolated CD4+CD25+ T reg cells from Nrp-1WT and Nrp-1KO mice by cell sorting. The majority (∼80%) of sorted CD4+CD25+ T reg cells from Nrp-1WT mice expressed Nrp-1, whereas we detected only 1% of Nrp-1+ cells within the sorted CD4+CD25+ T reg cells from Nrp-1KO mice (Fig. 5 B). As shown in Fig. 5 C, both cells types migrate toward SDF (positive control), but Nrp-1–expressing CD4+CD25+ T reg cells from Nrp-1WT mice exhibited a significantly increased migratory activity toward VEGF compared with Nrp-1–deficient CD4+CD25+ T reg cells. Thus, we concluded that Nrp-1 is required for T cell migration toward VEGF in the in vitro situation.
Figure 5.
Nrp-1+ cells migrate toward VEGF in vitro. (A and C) The Nrp-1 deficient hybridoma cell line 16.2.11 was transduced with an Nrp-1 encoding retroviral vector (Nrp-1+). Nrp-1− (WT; white bars; A) or Nrp-1+ cells (black bars; A), or freshly isolated CD4+CD25+ T reg cells from Nrp-1WT mice (white bars; C) or Nrp-1KO mice (black bars; C) were seeded in the upper chamber of a transwell system. The lower chamber contained medium alone (negative control), recombinant VEGF, or recombinant SDF as positive control. The migration index was calculated with regard to cell number counted in the lower chamber of negative control (set as 1). Results from two to three independent experiments are depicted as mean ± SEM. *, P < 0.05 (Student’s t test). (B) Expression levels of CD25 and Nrp-1 on isolated CD4+CD25+ T reg cells from Nrp-1WT and Nrp-1KO mice were determined by flow cytometry.
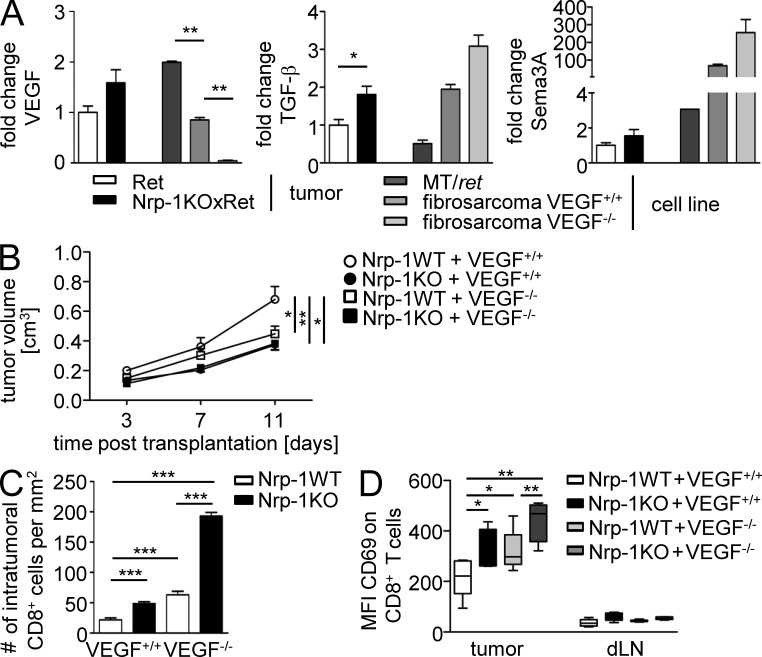
To analyze the impact of the VEGF–Nrp-1 signaling axis on the infiltration of Nrp-1–expressing Foxp3+ T reg cells into tumors in vivo, we addressed the impact of tumor cell–derived VEGF during this process. Therefore, we made use of a murine fibrosarcoma cell line with a homozygous deletion of the vegfa gene (VEGF−/−), as well as the corresponding VEGF-expressing WT fibrosarcoma cells (VEGF+/+; Stockmann et al., 2008). First, we analyzed the VEGF mRNA expression level in tumor tissues from Ret and Nrp-1KOxRet mice, as well as in the MT/ret tumor cell line, the WT (VEGF+/+), and the VEGF-deficient fibrosarcoma cells (VEGF−/−). We observed no significant differences in VEGF expression in Nrp-1KOxRet tumors compared with Ret tumors and identified the established MT/ret cell line as a source of high VEGF expression compared with the VEGF+/+ fibrosarcoma cell line (Fig. 6 A, left). As expected, VEGF−/− fibrosarcoma cells did not express VEGF. Besides VEGF, TGF-β and Semaphorin 3A (Sema 3A) are also known to interact with Nrp-1 and, thereby, might contribute to the impaired tumor growth in mice with T cell–specific Nrp-1 ablation. Interestingly, we detected a significant increase in TGF-β mRNA expression in tumors from Nrp-1KOxRet mice compared with tumors from Ret mice, but no significant differences in the TGF-β expression in VEGF+/+ fibrosarcoma cells when compared with VEGF−/− fibrosarcoma cells (Fig. 6 A, middle). Additionally, similar levels of Sema 3A were expressed in tumor tissues from Nrp-1KOxRet mice compared with Ret-transgenic tumors, and in both fibrosarcoma cell lines (Fig. 6 A, right).
Figure 6.
VEGF ablation in tumor cells resembles a similar phenotype as T cell–specific deletion of Nrp-1 expression. (A) VEGF (left), TGF-β (middle), and Sema 3A (right) mRNA expression levels were determined in tumor tissues from Ret mice (white bars) and Nrp-1KOxRet mice (black bars), as well as in the MT/ret melanoma cell line (dark gray bars), VEGF+/+ (gray bars), and VEGF−/− (light gray bars) fibrosarcoma cell lines by real-time PCR. Fold changes in expression levels were calculated with regard to expression level in tumors from Ret mice (set as 1). Results from two to three independent experiments are summarized as ± SEM (n = 2 tumors). (B) VEGF+/+ (circles) or VEGF−/− (squares) fibrosarcoma cells were injected s.c. either to Nrp-1flox/flox × CD4-cre mice (black symbols; Nrp-1KO) or to WT mice (white symbols; Nrp-1WT). Volumes of transplanted tumors to Nrp-1WT mice and Nrp-1KO mice were measured at days 3, 7, and 11 p.t. Results from two independent experiments are summarized as mean ± SEM (n = 3–4 mice per group). (C) Immunohistochemical assessment of intratumoral CD8+ T cells from Nrp-1WT (n = 6) and Nrp-1KO mice (n = 4) at day 11 p.t. with VEGF+/+ or VEGF−/− tumor cells. Data are summarized as mean ± SEM of two independent experiments. (D) The CD69 expression level (mean fluorescence intensity = MFI) on gated CD8+ T cells within the tumor tissue and the dLN of Nrp-1WT or Nrp-1KO mice that received VEGF+/+ or VEGF−/− fibrosarcoma cells was determined by flow cytometry at day 11 p.t. Results from two independent experiments with n = 2–3 mice per group and experiment are depicted as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test).
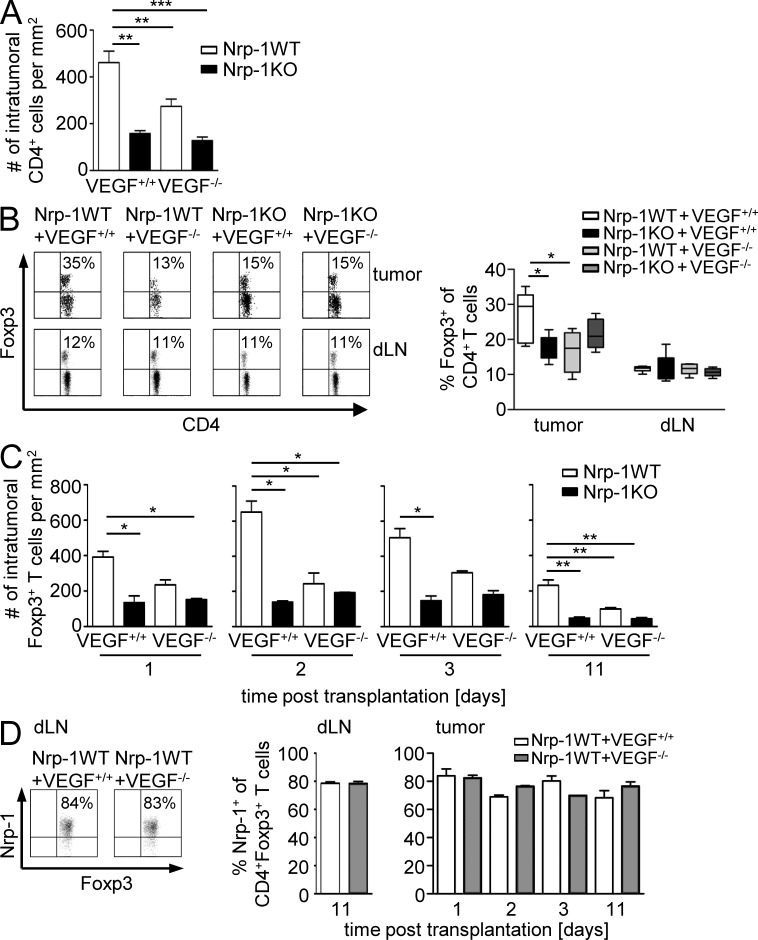
As we have demonstrated that Nrp-1–expressing CD4+CD25+ T reg cells from WT mice have the capacity to migrate toward VEGF in contrast to Nrp-1–deficient CD4+CD25+ T reg cells in vitro, we hypothesized that Nrp-1 expressed by the majority of WT CD4+CD25+ T reg cells is involved in T reg cell tumor infiltration in dependency on tumor-derived VEGF. To analyze this in more detail, we subcutaneously transplanted VEGF+/+ or VEGF−/− fibrosarcoma cells to Nrp-1KO and Nrp-1WT mice. As assumed, transplantation of VEGF+/+ cells into Nrp-1WT mice resulted in the highest tumor volumes (Fig. 6 B). However, we observed a similar reduction in tumor growth in Nrp-1KO mice transplanted with VEGF+/+ cells or VEGF−/− cells and Nrp-1WT mice transplanted with VEGF−/− cells compared with Nrp-1WT mice that received VEGF+/+ cells (Fig. 6 B). Moreover, the absence of VEGF expression in tumor cells and ablation of Nrp-1 in T cells of the host had no additive effect on tumor growth retardation. Consistent with the tumor growth rates, deletion of tumor-derived VEGF (VEGF−/−) or ablation of T cell–specific Nrp-1 expression in the host (Nrp-1KO) resulted in increased numbers of intratumoral CD8+ T cells that exhibited a more pronounced activation status as determined by the expression of CD69 in comparison to the Nrp-1WT mice transplanted with VEGF+/+ fibrosarcoma cells (Fig. 6, C and D). Interestingly, we detected an increase in the number of intratumoral CD8+ T cells in Nrp-1KO mice after transplantation of VEGF−/− cells accompanied with a strong activated phenotype compared with the corresponding Nrp-1WT mice (Fig. 6, C and D). CD8+ T cells isolated from the dLNs of the various fibrosarcoma transplantation models did not exhibit an activated phenotype (Fig. 6 D). To analyze whether this effect is indeed based on impaired T reg cell infiltration in dependency on VEGF expression by tumor cells and Nrp-1 expression by host-derived T cells, we determined the amount of CD4+ T cells and Foxp3+ T reg cells within the tumors and dLNs of Nrp-1WT and Nrp-1KO mice that received either VEGF+/+ cells or VEGF−/− cells at day 11 p.t. The number of CD4+ T cells within tumors of mice with either Nrp-1–deficient T cells (Nrp-1KO) or tumors that do not produce VEGF (VEGF−/−), or both, was significantly lower than in VEGF-producing (VEGF+/+) tumors from Nrp-1WT mice (Fig. 7 A). More importantly, the percentages of Foxp3+ T reg cells within the tumors are significantly higher in Nrp-1WT mice that received VEGF+/+ fibrosarcoma cells than in Nrp-1KO mice transplanted with VEGF+/+ fibrosarcomas or Nrp-1 WT mice harboring VEGF-deficient tumors (VEGF−/−; Fig. 7 B). The amount of Foxp3+ T reg cells within the dLNs of tumor-bearing mice did not change in dependency on tumor-produced VEGF or Nrp-1 expression in T cells from the host (Fig. 7 B). To verify whether the number of Foxp3+ T reg cells within the tumors is only affected during tumor progression (day 11 p.t.) or also modulated at early time points of tumor development, we additionally analyzed tumor tissues at days 1, 2, and 3 p.t. As shown in Fig. 7 C, the number of intratumoral Foxp3+ T cells of Nrp-1KO mice bearing either VEGF+/+ or VEGF−/− fibrosarcoma cell–derived tumors as well as from Nrp-1WT mice that received VEGF−/− fibrosarcoma cells was also significantly reduced at least at day 11 p.t. to a similar level across genotypes in comparison to Nrp-1WT mice that were transplanted with VEGF+/+ cells (Fig. 7 C). Moreover, the majority of tumor-infiltrating Foxp3+ T reg cells from Nrp-1WT mice expressed Nrp-1 to a similar extent as Foxp3+ T reg cells within the dLNs at different time points p.t. (Fig. 7 D). The mRNA expression analysis of the Nrp-1 ligands VEGF, TGF-β, and Sema 3A (Fig. 6 A) revealed that only VEGF was differentially expressed in VEGF+/+ and VEGF−/− fibrosarcomas; therefore, it is unlikely that TGF-β or Sema 3A contribute to the observed phenotype. Thus, our results strongly suggest that tumor-derived VEGF serves as a chemoattractant for Foxp3+ T reg cells and that Nrp-1 expression on T reg cells is required for their infiltration into tumors. Importantly, disruption of VEGF/Nrp-1 signaling between tumor cells and T reg cells by ablating either the tumor-derived VEGF signal or the Nrp-1 receptor on T reg cells prevents T reg cell infiltration into the tumor and impairs tumorigenesis.
Figure 7.
Host T cell–expressed Nrp-1 and tumor-derived VEGF modulate Foxp3+ T reg cell infiltration into tumors. (A) Immunohistochemical assessment of intratumoral CD4+ T cells into Nrp-1WT (white bars, n = 6) and Nrp-1KO mice (black bars, n = 4) at day 11 p.t. with VEGF+/+ or VEGF−/− tumor cells. Data are summarized as mean ± SEM of two independent experiments. (B) Percentages of Foxp3-expressing CD4+ T cells within tumor tissues and dLN from Nrp-1WT of Nrp-1KO mice that were transplanted with either VEGF+/+ or VEGF−/− tumor cells were analyzed by flow cytometry at day 11 p.t. Results from one out of two independent experiments with n = 2–3 mice (per group and experiment) are shown (left) and summarized as mean ± SEM (right). (C) Immunohistochemically based quantification for tumor-infiltrating Foxp3+ T cells in Nrp-1WT mice (white bars) and Nrp-1KO mice (black bars) at days 1, 2, 3, and 11 after s.c. transplantation of VEGF+/+ or VEGF−/− tumor cells. Data are summarized as mean ± SEM (n = 2–6 mice per group per indicated time point). (D) The frequency of Nrp-1–expressing Foxp3+ T reg cells from Nrp-1WT mice that were transplanted with either VEGF+/+ (white bars) or VEGF−/− (gray bars) tumor cells was determined within tumors by immunohistochemistry (n = 2–6) and in dLN by flow cytometry on gated CD4+Foxp3+ cells. Representative dot plots from one flow cytometric analysis out of two independent experiments with n = 2–4 mice (per group and experiment; left) and summarized data are shown as mean ± SEM (right). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test).
Impaired melanoma development is paralleled by the reduction of tumor-infiltrating Foxp3+ T reg cells
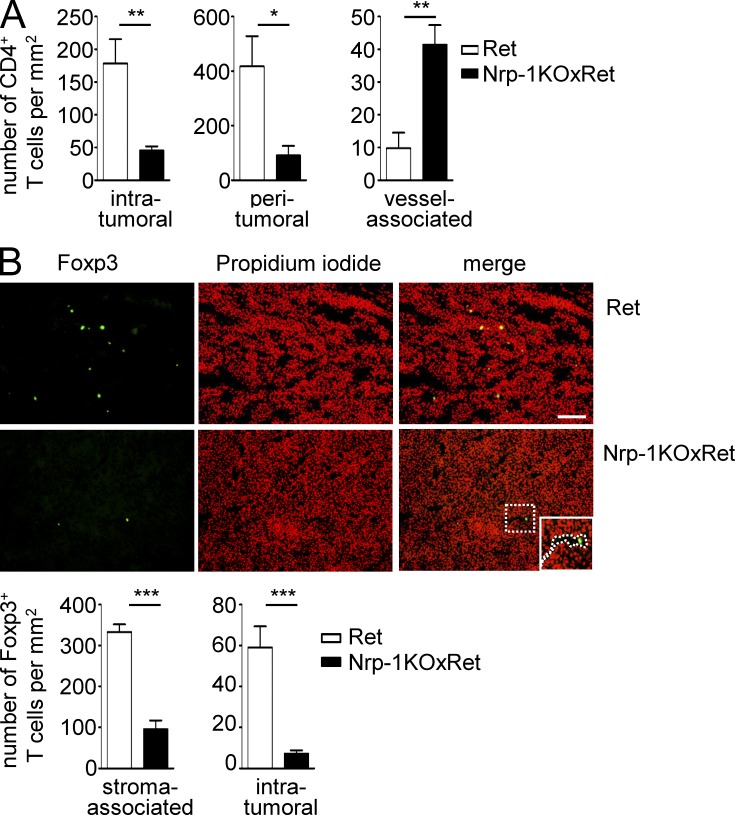
Next, we asked whether the Nrp-1–dependent infiltration of T reg cells into tumor tissues is restricted to the chosen fibrosarcoma model or a more common mechanism. Therefore, we went back to our melanoma model and studied infiltration and localization of CD4+ T cells and Foxp3+ T reg cells in spontaneously developing tumors of Nrp-1KOxRet mice. The CD4+ T cell number was significantly reduced in the intra- and peritumoral area of tumor-bearing Nrp-1KOxRet mice, and intratumoral CD4+ T cells were exclusively associated with vascular structures (Fig. 8 A). Along with this finding, we detected an impaired infiltration of Foxp3+ T reg cells into tumors of Nrp-1KOxRet mice in comparison to Ret mice (Fig. 8 B). The number of stroma-associated, as well as intratumoral Foxp3+ T reg cells, was significantly reduced in Nrp-1KOxRet tumor-bearing mice. Moreover, intratumoral Foxp3+ T reg cells from Nrp-1KOxRet mice were exclusively located in the lumen of tumor-associated vessels (Fig. 8 B). Immunohistochemistry-based analysis of Ret-transgenic tumors revealed that the majority (56.7 ± 9.1%) of intratumoral CD4+ T cells expressed Nrp-1 (unpublished data). In summary, these results provide evidence that Nrp-1, expressed by Foxp3+ T reg cells, regulates the infiltration of T reg cells into tumors, thereby enabling a dampened anti-tumor immune response that allows unrestricted tumor progression.
Figure 8.
Impaired Foxp3+ T reg cell tumor infiltration in MT/ret mice deficient in T cell–specific Nrp-1 expression. Nrp-1flox/flox × CD4-cre mice were crossed with MT/ret mice. (A) Immunohistochemically based quantification of intratumoral, peritumoral, and vessel-associated CD4+ T cells in MT/ret (Ret; n = 6 tumors of four mice) and Nrp-1flox/flox × CD4-cre mice × MT/ret (Nrp-1KOxRet; n = 8 tumors of four mice) summarized as mean ± SEM. (B) Representative picture of immunohistologically based detection of Foxp3+ T reg cell infiltration (green) into Ret and Nrp-1KOxRet tumors. Nuclei were counterstained using propidium iodide. Quantification for the number of Foxp3+ T cells recruited to the tumor-associated stroma or into the center of Ret (white bars) or Nrp-1KOxRet (black bars) tumors (intratumoral). Data are shown as mean ± SEM from three independent experiments (n = 9–12 tumors from three mice, Ret; n = 6–9 tumors from three mice, Nrp-1KOxRet). Bar, 100 µm. Dotted line: intratumoral vessel. *, P < 0.05; **, P < 0.001; ***, P < 0.0001 (Student’s t test).
To characterize the influence of T cell–expressed Nrp-1 on the infiltration and localization of CD4+ T cells and Foxp3+ T reg cells at early time points of tumor progression, we analyzed the T cells recruited into the tumors and dLN cells of Nrp-1WT and Nrp-1KO mice transplanted with MT/ret cells at days 1, 2, 3, 5, and 7 p.t. in more detail. The number of tumor-infiltrating CD4+ T cells (Fig. 9 A) and Foxp3+ T reg cells (Fig. 9 D) and the frequency of CD4+ T cells within the dLNs (Fig. 9 B) were significantly reduced in Nrp-1KO mice compared with Nrp-1WT mice. Strikingly, whereas Nrp-1WT mice showed a homogenous distribution of Foxp3+ T cells across the tumor, the localization of Foxp3+ T cells within tumors from Nrp-1KO mice was restricted to the outer rim of the tumor with almost complete absence of Foxp3+ T cells in the tumor center (Fig. 9 C). Within the dLNs, we observed no differences in the percentages of Foxp3+ T reg cells at any time point analyzed (Fig. 9 E). To verify that tumor-infiltrating Foxp3+ T reg cells coexpress Nrp-1 also in our MT/ret transplantation model, we determined the percentages of Nrp-1+ on Foxp3+ T reg cells. As shown in Fig. 10 A, ∼70–80% of Foxp3+ T reg cells from tumors and dLNs of Nrp-1WT mice transplanted with MT/ret cells expressed Nrp-1 at days 3, 5, and 7 p.t., whereas we did not detect Nrp-1–expressing Foxp3+ T reg cells in Nrp-1KO mice, as expected (Fig. 10 A). However, by using the Nrp-1flox/flox × CD4-cre (Nrp-1KO) mouse model, we not only ablated Nrp-1 expression in Foxp3+ T reg cells but also in the small population of Nrp-1+Foxp3− T cells, which might have an influence on tumor growth and progression. Flow cytometry analysis of Nrp-1+CD4+ T cells from tumors and dLN of MT/ret transplanted Nrp-1WT mice revealed that ∼10–30% of Nrp-1+CD4+ T cells did not coexpress Foxp3 and thereby did not represent T reg cells (Fig. 10 B). To elucidate the effect of Nrp-1 ablation in these CD4+Nrp-1+Foxp3− effector T cells, we made use of an additional transgenic mouse model. We crossed Nrp-1flox/flox mice with Foxp3–IRES–cre recombinase (FIC) mice, expressing the cre recombinase under control of the foxp3 locus (Wing et al., 2008). In these FIC × Nrp-1flox/flox mice, Nrp-1 expression is specifically ablated in Foxp3+ T reg cells but not in CD4+Foxp3− T cells (Fig. 10 C). Transplantation of tumor cells resulted in a significant delay in tumor growth at day 14 p.t. accompanied by an expansion of tumor-infiltrating CD8+ T cells in these T reg cell–specific Nrp-1–deficient mice (FIC × Nrp-1flox/flox) in comparison to FIC × Nrp-1wt/wt mice (Fig. 10, D and E). Thus, this phenotype resembles the impaired tumor growth that we also observed in mice with T cell–specific Nrp-1 ablation (Nrp-1KO), suggesting that Nrp-1 expression in Foxp3+ T reg cells, but not in Foxp3− T cells, is responsible for this phenotype. Finally, to corroborate our observation that Nrp-1 is important for the infiltration of Foxp3+ T reg cells into VEGF-producing tumor tissues, groups of Nrp-1WT mice and Nrp-1KO mice were transplanted with MT/ret cells and, additionally, WT T reg cells were adoptively transferred to one group of Nrp-1KO mice. As expected, transplanted Nrp-1KO mice that did not receive any cells exhibited a significantly impaired tumor growth compared with Nrp-1WT mice. Importantly, Nrp-1KO mice that were reconstituted with Nrp-1–expressing WT T reg cells showed a significant increase in tumor growth compared with untreated Nrp-1KO mice and similar tumor volumes as Nrp-1WT mice at day 14 p.t. (Fig. 10 F). These results further strengthen our conclusion that Nrp-1 highly expressed by T reg cells controls infiltration of Foxp3+ T reg cells into tumor tissues in dependency on tumor-derived VEGF.
Figure 9.
Decreased number of intratumoral CD4+ and Foxp3+ T cells in T cell–specific Nrp-1–deficient mice upon transplantation of MT/ret cells. (A and B) Immunohistochemically based quantification of intratumoral CD4+ T cells (A) and percentages of CD4+ T cells within the dLN (B) analyzed by flow cytometry of Nrp-1flox/flox × CD4-cre mice (black bars; Nrp-1KO) or WT mice (open bars; Nrp-1WT) at days 1, 2, 3, 5, and 7 d after s.c. transplantation of MT/ret cells are shown as mean ± SEM (n = 2–6 mice per time point and group of at least two independent experiments at days 3, 5, and 7). (C) Representative pictures of Foxp3+ T reg cell infiltration into tumors of Nrp-1WT and Nrp-1KO mice 24 h p.t. of MT/ret cells by immunohistochemistry (green). Nuclei were counterstained using propidium iodide (red). Bar, 100 µm. Data are representative of at least two independent experiments. (D) Immunohistochemically based quantification of Foxp3+ T cells in tumor sections of Nrp-1KO mice (black bars) or Nrp-1WT mice (white bars) at days 1, 2, 3, 5, and 7 d p.t. of MT/ret cells shown as mean ± SEM (n = 2 to 6 mice per time point and group of at least two independent experiments at days 3, 5, and 7). (E) Percentages of Foxp3+ T reg cells in dLN of Nrp-1WT or Nrp-1KO mice that received MT/ret cells analyzed by flow cytometry on gated CD4+ T cells. Representative dot plots from one experiment out of two with similar results (n = 2–5 per time point and group) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student’s t test).
Figure 10.
Foxp3-specific ablation of Nrp-1 expression results in impaired tumor growth and adoptive transfer of Nrp-1+ T reg cells reverses the phenotype of tumor-bearing Nrp-1KO mice. (A) Nrp-1 expression on gated CD4+Foxp3+ T reg cells isolated from tumors (white bars) and dLN (black bars) of WT mice (Nrp-1WT) and Nrp-1flox/flox × CD4-cre mice (Nrp-1KO) mice that received MT/ret cells at different time points p.t. as indicated. Representative results from one experiment (day 5 p.t.) are shown (left) and data from one to three experiments with n = 2–3 Nrp-1WT mice (per time point and experiment) are summarized as mean ± SEM (right). (B) Percentages of Foxp3-expressing Nrp-1+CD4+ T cells were analyzed by flow cytometry at days 3, 5, and 7 p.t. in tumors (white bars) and dLN (black bars) of Nrp-1WT mice that were transplanted with MT/ret cells. Representative dot plots (left) and results from two independent experiments (n = 3 mice per experiment) are summarized as mean ± SEM (right). (C) Foxp3 and Nrp-1 expression on gated CD4+ T cells from spleen of FIC × Nrp-1flox/flox and FIC × Nrp-1wt/wt mice were analyzed by flow cytometry. (D) FIC × Nrp-1flox/flox mice (black circles) and FIC × Nrp-1wt/wt mice (white circles) were transplanted with CT-26 tumor cells s.c. Tumor volumes were monitored at days 4, 7, 11, and 14 p.t. and are shown as mean ± SEM from one out of two independent experiments (n = 3–5 mice). (E) The percentages of tumor-infiltrating CD8+ T cells were determined in MT/ret transplanted FIC × Nrp-1flox/flox mice (black bars) and FIC × Nrp-1wt/wt mice (white bars) at day 14 p.t. by flow cytometry and summarized as mean ± SEM from two independent experiments (n = 3–5 mice). (F) Nrp-1WT and Nrp-1KO mice were transplanted with MT/ret cells and one group of transplanted Nrp-1KO mice were additionally injected with WT T reg cells. Tumor volumes were monitored at days 4, 7, 11, and 14. Results from four independent experiments with n = 8–11 mice (per group in total) are shown as mean ± SEM. *, P < 0.05; **, P < 0.01 (Student’s t test).
DISCUSSION
Tumor cells are dependent on their local microenvironment for the supply of oxygen, nutrients, survival factors, and supporting stroma. Moreover, tumor growth requires a complex interplay between the expanding tissues and several accessory cells, including immune cells. It has been shown that CD4+CD25+ regulatory T cells are increased in the peripheral blood and tumor microenvironment in different types of cancer (Strauss et al., 2007; Li et al., 2009; Ohara et al., 2009), suggesting that T reg cells worsen the clinical prognosis. Here, we could demonstrate that depletion of Foxp3+ T reg cells from tumor-bearing mice indeed significantly reduced tumor growth. The infiltration of Foxp3+ T reg cells into the tumor site to locally suppress an effective anti-tumor response is supposed to be one of the main obstacles in regulating tumor immune escape. However, the precise mechanism by which T reg cells infiltrate into the tumor remains unknown as well as its exact functional role in modulating the interaction between immune system and tumor.
Recently, we have identified the VEGF co-receptor Nrp-1 as a suitable surface marker for the identification of CD4+CD25+ T reg cells, whose expression is regulated by the T reg cell–specific transcription factor Foxp3 (Bruder et al., 2004; Loser et al., 2005). To gain mechanistic insights regarding Nrp-1 function on T reg cells in particular during tumorigenesis, we made use of Nrp-1flox/flox × CD4-cre mice (Nrp-1KO) to specifically ablate Nrp-1 expression in T cells. By using these transgenic mice, we could show for the first time that knockdown of Nrp-1 expression in T cells, mainly Foxp3+ T reg cells, results in a significant breakdown of tumor immune escape in several murine melanoma models and a fibrosarcoma model accompanied by reduced tumor growth and, interestingly, prolonged tumor-free survival in a T cell–specific Nrp-1–deficient spontaneous mouse melanoma model. We observed altered immune cell recruitment associated with decreased numbers of infiltrating Foxp3+ T reg cells into the tumor site by a disconnection in Nrp-1–dependent migration in response to tumor-derived VEGF. This entire process resulted in a compensatory enhanced activation of recruited CD8+ T cells within these tumors, suggesting that Nrp-1 ablation in Foxp3+ T reg cells contributes to a more effective anti-tumor response. Analysis of Foxp3+ T reg cells in the peripheral lymphoid organs revealed no differences in the amount of Foxp3+ T reg cells in naive and tumor-bearing Nrp-1KO (Nrp-1flox/flox × CD4-cre mice) and WT mice. Consistent with these results, Solomon et al. (2011) also detected similar amounts of Foxp3+ T reg cells in both Nrp-1flox/flox × CD4-cre mice and WT mice, suggesting that the observed differences in tumorigenesis are not a result of overall reduced numbers of Foxp3+ T reg cells in these mice.
Most recently, it was proposed that Nrp-1 promotes long interactions between T reg cells and immature DCs (iDCs; Sarris et al., 2008), which are thought to play a crucial role in the maintenance of self-tolerance by presenting self-antigens and inducing unresponsiveness of T cells. However, upon maturation of DCs by LPS interactions of naive T cells to mature DCs were comparable to those of T reg cells (Sarris et al., 2008). This observation suggests that Nrp-1 confers a relatively high adhesive property on T reg cells in their interaction with iDCs under steady state, but T reg cells loose this advantage over naive T cells under inflammatory conditions (Mizui and Kikutani, 2008). Thus, during tumorigenesis it is unlikely that the proposed function of Nrp-1 on T reg cells by Sarris et al. (2008) is the main underlying mechanism of delayed tumor growth in Nrp-1KO mice. Therefore, we investigated the effect of Nrp-1 ablation on the intrinsic immune-suppressive function of T reg cells in vitro. Our co-culture experiments revealed no differences in the inhibitory capability of Nrp-1–expressing T reg cells and Nrp-1–deficient T reg cells on neither CD4+ nor CD8+ responder cells. Consistent with our results, human Nrp-1+ T reg cells exhibited suppressive activity in vitro, but the addition of blocking anti–Nrp-1 antibodies did not alter their inhibitory capability (Battaglia et al., 2008). Although Nrp-1 does not seem to be critical for the intrinsic immune-suppressive function of T reg cells in our hands, we observed a striking phenotypic difference in Nrp1-KO mice when studying tumorigenesis. In contrast to the fixed microenvironment in the in vitro culture system, immunosuppression in vivo requires the appropriate colocalization of suppressor and effector T cells (von Boehmer, 2005); therefore, in vivo T reg cells must be able to migrate into tissues to physically interact directly with effector cells or indirectly via antigen-presenting cells. As Nrp-1 has been described to be involved in migration and/or adhesion of endothelial cells (Wang et al., 2003; Murga et al., 2005), we hypothesized that Nrp-1 expressed by Foxp3+ T reg cells contributes to migration of T reg cells toward tumor-derived VEGF.
It is well established that CD4+CD25+ T reg cells play a crucial role in the regulation of anti-tumor immune responses (Zou, 2006), and increased numbers of T reg cells were detected in different tumors (Wada et al., 2009). Interestingly, Battaglia et al. (2008) have demonstrated that the amount of Nrp-1+ T reg cells in dLNs of cervical cancer patients directly correlated with the disappearance of the primary tumor mass upon chemoradiation therapy. Furthermore, data from a patient with ovarian cancer during therapy revealed a direct correlation between the amount of T reg cells and VEGF concentration in malignant effusions (Wada et al., 2009). Gupta et al. (2007) likewise described a positive, although not significant correlation of intratumoral T reg cells with VEGF in breast cancer. Vice versa, blocking of VEGF by administration of anti-VEGF antibodies significantly reduced the amount of Foxp3+ T reg cells in an inflammatory breast cancer model (Roland et al., 2009). Together, these studies suggest that tumor-derived VEGF chemoattracts Foxp3+ T reg cells via Nrp-1. Indeed, in an in vitro migration assay, we could demonstrate that Nrp-1+ T reg cells from WT mice, but not Nrp-1–deficient T reg cells from Nrp-1KO mice, exhibited migratory activity toward VEGF. Moreover, T cell–specific ablation of Nrp-1 expression resulted in delayed tumor growth in a tumor transplantation model as well as in our spontaneous MT/ret melanoma model accompanied by reduced numbers of Foxp3+ TILs in contrast to tumor-developing WT mice. Thus, our detailed analysis of Nrp-1–deficient T reg cells in vitro argues that Nrp-1 expression is not directly involved in their intrinsic suppressive mode of action but confers migratory activity into inflamed tissue sites that release VEGF. This mechanism is then exploited by inflammatory tumors to attract immune-inhibitory cells like Nrp-1–expressing Foxp3+ T reg cells into the tumor and to overcome anti-tumor immunity.
Besides VEGF two other molecules, namely TGF-β and Sema 3A, have been described to interact with the Nrp-1 receptor (Takahashi et al., 1998; Glinka and Prud’homme, 2008). As different tumors also produce TGF-β and Sema 3A (Catalano et al., 2006; Busse and Keilholz, 2011), one might speculate that both molecules contribute to the attraction of Nrp-1–expressing Foxp3+ T reg cells into the tumor tissue. However, we could demonstrate that ablation of VEGF in fibrosarcoma cells (VEGF−/−) resembles a similar phenotype, including reduced tumor growth, increased activation of CD8+ TILs, and reduced numbers of intratumoral Foxp3+ T reg cells as observed in T cell–specific Nrp-1–deficient mice transplanted with WT (VEGF+/+) fibrosarcoma cells. Real-time PCR analysis revealed no significant differences in the expression of TGF-β and Sema 3A in VEGF-deficient (VEGF−/−) and VEGF WT (VEGF+/+) fibrosarcoma cells. In addition, we detected decreased numbers of Foxp3+ T reg cells within tumors from Nrp-1KOxRet mice in contrast to Ret mice and again Sema 3A mRNA expression levels were not altered in tumors from both mouse lines. TGF-β mRNA expression was significantly increased in tumor tissues isolated from Nrp-1KOxRet mice compared with Ret mice. However, if one assumes that TGF-β contributes to Foxp3+ T reg cell infiltration into the tumor in dependency on Nrp-1, one would expect more but not fewer numbers of tumor-infiltrating Foxp3+ T reg cells in T cell–specific Nrp-1–deficient Ret (Nrp-1KOxRet) mice. Thus, from our results it is unlikely that Foxp3+ T reg cell infiltration into the tumor is directly dependent on tumor-expressed Sema 3A and TGF-β but rather is regulated by tumor-derived VEGF.
Nrp-1 is not only highly expressed by Foxp3+ T reg cells because Nrp-1 expression has been detected in various human carcinoma cell lines and primary tumors (Staton et al., 2007), including tumor cells from melanoma patients themselves. Overexpression of Nrp-1 promotes tumor cell growth in vitro whereas antibody-mediated Nrp-1 blockage resulted in tumor growth inhibition (Pan et al., 2007). Moreover, Straume and Akslen (2003) demonstrated that an increased expression of VEGF receptors, including Nrp-1, was associated with glomeruloid microvascular proliferation, representing an aggressive angiogenic phenotype in patients suffering from malignant melanoma. With regard to the Nrp-1 ligand VEGF, it was shown that overexpression of VEGF in melanoma cells that express its receptors, including Nrp-1, led to increased growth and survival of melanoma cells through MAPK and PI3K signaling (Graells et al., 2004). Whether Nrp-1–VEGF interaction in Foxp3+ T reg cells activates this pathway and thereby contributes to an increased survival of intratumoral T reg cells has to be clarified in further studies. However, results from our study provide evidence that Nrp-1 expressed by Foxp3+ T reg cells contributes to their migration into tumor tissues in dependency of tumor-derived VEGF leading to local suppression of an adequate anti-tumor immune response. Thus, Nrp-1–VEGF interaction affects different processes that are important during tumorigenesis including angiogenesis, tumor cell growth, and survival and, as demonstrated in this study, chemoattraction of immunosuppressive Foxp3+ T reg cells.
In further studies, it has to be clarified whether Nrp-1–mediated migration of Foxp3+ T reg cells is a special mechanism active during tumorigenesis or a fundamental process also occurring during other VEGF-dependent disease settings. It is well known that VEGF expression is extensively increased in mice suffering from experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis (MS). Furthermore, the level of VEGF expression positively correlates with the clinical score in the EAE model (Roscoe et al., 2009) as well as in patients with acute and chronic MS (Proescholdt et al., 2002). Most recently, Solomon et al. (2011) observed an increase in the clinical score of EAE diseased Nrp-1fl/fl × CD4-cre (Nrp-1KO) mice in contrast to WT mice, owing at least in part to a decreased intrinsic T reg cell functionality. However, one might also speculate about disrupted VEGF-dependent chemoattraction of Nrp-1–deficient Foxp3+ T reg cells to the central nervous system, leading to uncontrolled proinflammatory immune responses and thereby contributing to a more exacerbated EAE severity in Nrp-1KO mice.
In summary, our results provide for the first time evidence that Nrp-1 expression is critically required for the infiltration of Foxp3+ T reg cells into VEGF-producing tumors and that blocking this process reestablishes the anti-tumor immune response, resulting in impaired tumor progression. This might open up new therapeutic strategies in oncology that aim to interfere with the chemoattraction of Nrp-1+ T reg cells toward VEGF not only during tumorigenesis but possibly also in various other VEGF-dependent diseases like MS.
MATERIALS AND METHODS
Mice.
Nrp-1flox/flox mice (provided by D.D. Ginty, John Hopkins University, Baltimore, MD; Gu et al., 2003), CD4-cre mice (provided by W. Müller, University of Manchester, Manchester, UK), FIC mice (Wing et al., 2008), DEREG mice (Lahl et al., 2007), C57/BL6 mice (Harlan), and MT/ret mice (provided by M. Kato, Chubu University, Aichi, Japan) were crossed and maintained under specific pathogen-free conditions at the Animal Facility of the University Hospital Essen. Heterozygous MT/ret mice express the human ret proto-oncogene under the control of MT/ret and develop spontaneously cutaneous malignant melanoma with metastases to distant organs that resemble human melanoma in many aspects of histopathology and clinical development (Kato et al., 1998). All animal experiments were performed in accordance with government and institute guidelines and regulations. Animal procedures were approved by the state authorities for Ethics in Animal Experiments of North-Rhine Westphalia, Germany.
Tumor transplantation.
Immortalized and transformed VEGF+/+ and VEGF−/− mouse fibrosarcoma cells and MT/ret-derived tumor cells were generated as described previously (Stockmann et al., 2008; Nasarre et al., 2009). VEGF+/+ or VEGF−/− fibrosarcoma cells (5 × 106), MT/ret cells (5 × 105), or CT-26 tumor cells (5 × 105) suspended in 100 µl Matrigel (BD)/PBS (1:1) were injected s.c. in the left flanks of 6–8-wk-old mice. For the adoptive transfer experiment, 106 sorted CD4+CD25+ T reg cells from WT mice in a total volume of 100 µl PBS or equal volume of PBS alone (as control) were injected i.v. directly before s.c. tumor cell transplantation. For CD4+Foxp3+ T reg cell depletion, DEREG mice were injected i.p. with 30 ng/g body weight of DT (Merck) 1 d before tumor transplantation and at days 2, 5, and 8 p.t. Tumor volume was quantified by caliper measurement at days 3, 7, and 11 (length × width × height). Mice were sacrificed at the indicated time points or when the first tumor within an experimental group had grown to 2 cm3.
RNA isolation and quantitative real-time PCR.
RNA isolation from cancer cell lines and tumor biopsies was performed by using Qiazol Lysis Reagent (QIAGEN) according to the manufacturer’s instructions including DNase I treatment (Fermentas). Afterward, 1 µg of each sample was reverse transcribed using RevertAid H Minus First strand c-DNA Synthesis kit in a final volume of 20 µl (Fermentas). Real-time PCR was performed in an ABI PRISM cycler (Life Technologies) with a SYBR Green PCR kit from Applied Biosystems (Life Technologies) and specific primers for VEGF (5′-ATCCGCATGATCTGCATGG-3′ and 5′-AGTCCCATGAAGTGATCAAGTTCA-3′), TGF-β (5′-ACCTGGGTTGGAAGTGGAT-3′ and 5′-GAAGCGCCCGGGTTGTGTTGGTT-3′), Sema3A (5′-CAAAGGCAAGCTGAATGGAT-3′ and 5′-AGAGCTAGAAAGAAATGGGAGAGT-3′), and RPS9 (5′-CTGGACGAGGGCAAGATGAAGC-3′ and 5′-TGACGTTGGCGGATGAGCACA-3′). Relative mRNA levels were determined by using included standard curves for each individual gene and further normalization to the housekeeping gene RPS9, as described previously (Hansen et al., 2007).
Flow cytometry.
Flow cytometric expression analyses were performed using anti–mouse CD4 (L3T4), CD8 (Ly-2), CD25 (PC61), CD69 (H1.2F3), CD44 (IM7), CD11c (HL3), CD11b (M1/79), CD19 (1D3), NK1.1 (PH136; all BD), and anti–Nrp-1 (R&D Systems) as Pacific blue, FITC, allophycocyanin, or Phycoerythrin conjugates. Alternatively, unconjugated goat anti–Nrp-1 antibody (R&D Systems) was used, followed by incubation with Alexa Fluor 647–conjugated anti–goat antibody (Invitrogen). Intracellular staining was performed using the Foxp3 staining kit from eBioscience (NatuTec) with anti-Foxp3 (FJK-16s) or anti–granzyme B (GB11) according to the manufacturer’s recommendations. IFN-γ expression was analyzed by stimulating cells for 4 h with 10 ng/ml PMA (Sigma-Aldrich) and 100 µg/ml ionomycin (Sigma-Aldrich) in the presence of 5 µg/ml Brefeldin A (Sigma-Aldrich), treated with 2% paraformaldehyde and 0.1% NP-40 and stained with anti–mouse IFN-γ (XMG1.2, BD). FACS analyses were performed with LSRII and DIVA software (BD).
Cell separation.
CD4+ T cells, enriched from splenocytes by using the CD4+ T cell isolation kit (Miltenyi Biotec) according to the manufacturer’s recommendations, or whole erythrocyte-depleted splenocytes were labeled with anti-CD4 (L3T4; BD) and anti-CD25 antibodies (PC61; BD). CD4+CD25+ and CD4+CD25− were separated using a MoFlow (Cytomation) or FACSAria II (BD) and purity was >97%. Tumors were removed from animals, cut in small pieces, crushed through a 100-µm nylon mash, and depleted of erythrocytes before staining with different antibodies for flow cytometric analysis.
Proliferation.
105 sorted CD4+CD25+ T cells and CD4+CD25− T cells were either cultured alone or co-cultured with CD4+CD25− responder T cells at different ratios in the presence of 1 µg/ml anti-CD3 and irradiated splenocytes as APCs (4 × 105) for 72 h. Proliferation and suppression assays were performed in triplicate in a final volume of 200 µl. Cells were pulsed with 1 µCi/well of [3H]-thymidine for the final 8–18 h of the experiment and [3H]-thymidine incorporation was measured by scintillation counting. Alternatively, 105 sorted CD45.1+CD8+ T cells were labeled with CFSE and cultured alone or co-cultured with 105 CD4+CD25+ T cells from CD45.2+ Nrp-1KO or Nrp-1WT mice in the presence of 1 µg/ml anti-CD3 and irradiated splenocytes as APCs (4 × 105) for 72 h. Proliferation of CD45.1+CD8 + responder cells was assessed as loss of CFSE dye by flow cytometry.
Immunohistochemical-based analyses.
Consecutive cryosections (7 µm) were stained using rat anti–Foxp3-FITC (NatuTec), rat anti-CD4 (BD), rabbit anti-CD8 (Abcam), goat anti–Nrp-1 (R&D Systems), and corresponding IgG as control (Dianov) as previously described (Helfrich et al., 2010). Nuclei were stained using propidium iodide (Merck). Quantification of individual cell populations (CD8+, CD4+, Foxp3+, and Nrp-1+) per tumor area (mm2) was calculated using the mean of three tumor sections per tumor (top, middle, and base) of concordant distance at indicated time points. A total of five regions of interest (ROI) per tumor section were evaluated. Means were calculated for all ROI. All analyses were performed on microscopes (Olympus) and corresponding Cell P Software. Slides fluorescence was examined by confocal laser-scanning microscopy (TCS SP2; Leica).
Migration.
16.2.11 hybridoma cells (Weber et al., 1992) were infected with a retroviral vector encoding Nrp-1 cDNA (provided by H. Fujisawa, Nagoya University, Nagoya, Japan) as described previously (Reinwald et al., 2008). Migration assays were performed using transwell chambers with 5 µm polycarbonate filters (Costar) as previously described (Heuer et al., 2006). In brief, 100 ng/ml recombinant VEGF or SDF-1β (Tebu) diluted in migration assay media or as negative control assay media alone was added to the lower chamber of the transwells. The fibronectin-coated transwell inserts were placed on top, and 106 16.2.11 cells, retroviral transduced 16.2.11 hybridoma cells, or 106 CD4+CD25+ T reg cells from either Nrp-1WT or Nrp-1KO mice were added to the upper chamber. After 2.5–3.5 h of incubation at 37°C, cells that had migrated through the filter were collected and counted.
X-ray computed tomography.
Mice were mask narcotized using Isoflurane (Abbott). A thin-slice x-ray computed tomography (CT) of mice was performed using Dual Source CT (SOMATOM Definition; Siemens). Ultrathin 0.4 mm slices covering the whole body were acquired and calculated in window center 50 and window width 350. A three-dimensional reconstruction was made in volume rendering technique to visualize the surface of each animal in 19 standardized planes.
Statistical analysis.
Student’s t test was used for the determination of statistical significance between experimental groups. Results were expressed as mean ± SEM. P values <0.05 were considered statistically significant.
Acknowledgments
We thank Werner Müller (University of Manchester, Manchester, UK) for providing CD4-cre mice, David D. Ginty (John Hopkins University, Baltimore, USA) for providing Nrp-1flox/flox mice, Masahi Kato (Chubu University, Aichi, Japan) for providing of MT/ret mice, and H. Fujisawa (Nagoya University, Nagoya, Japan) for providing Nrp-1 cDNA. We are very grateful to Nadine Hochhard, Carolin Wevers, Tanja Toepfer, Katharina Grabovacki, and Mechthild Hemmler-Rohloff for excellent technical assistance. We thank Annika Frede, Withold Bartosik, and Patrick Juszczak for cell sortings and staff of the animal facility at the University Hospital Essen for mouse colony management.
This work was supported by Deutsche Forschungsgemeinschaft to W. Hansen, Deutsche Krebshilfe to W. Hansen and J. Buer, Melanoma International Foundation to I. Helfrich, and Bio-NRW to D. Schadendorf.
The authors have no conflicting financial interest to declare.
Footnotes
Abbreviations used:
- DEREG
- depletion of T reg cell
- dLN
- draining LN
- DT
- diphtheria toxin
- EAE
- experimental autoimmune encephalomyelitis
- FIC
- Foxp3–IRES–cre recombinase
- mLN
- mesenteric LN
- MS
- multiple sclerosis
- MT
- mouse metallothionein I promoter-enhancer
- Nrp-1
- Neuropilin 1
- p.t.
- post transplantation
- Ret
- rearranged during transfection
- SDF
- stromal cell–derived factor
- Sema 3A
- Semaphorin 3A
- TIL
- tumor-infiltrating lymphocyte
- VEGF
- vascular endothelial growth factor
References
- Andreu P., Johansson M., Affara N.I., Pucci F., Tan T., Junankar S., Korets L., Lam J., Tawfik D., DeNardo D.G., et al. 2010. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 17:121–134 10.1016/j.ccr.2009.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony P.A., Piccirillo C.A., Akpinarli A., Finkelstein S.E., Speiss P.J., Surman D.R., Palmer D.C., Chan C.C., Klebanoff C.A., Overwijk W.W., et al. 2005. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 174:2591–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F., Coussens L.M. 2004. Cancer: an inflammatory link. Nature. 431:405–406 10.1038/431405a [DOI] [PubMed] [Google Scholar]
- Battaglia A., Buzzonetti A., Monego G., Peri L., Ferrandina G., Fanfani F., Scambia G., Fattorossi A. 2008. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology. 123:129–138 10.1111/j.1365-2567.2007.02737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielenberg D.R., Pettaway C.A., Takashima S., Klagsbrun M. 2006. Neuropilins in neoplasms: expression, regulation, and function. Exp. Cell Res. 312:584–593 10.1016/j.yexcr.2005.11.024 [DOI] [PubMed] [Google Scholar]
- Bruder D., Probst-Kepper M., Westendorf A.M., Geffers R., Beissert S., Loser K., von Boehmer H., Buer J., Hansen W. 2004. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 34:623–630 10.1002/eji.200324799 [DOI] [PubMed] [Google Scholar]
- Busse A., Keilholz U. 2011. Role of TGF-β in melanoma. Curr. Pharm. Biotechnol. 12:2165–2175 10.2174/138920111798808437 [DOI] [PubMed] [Google Scholar]
- Catalano A., Caprari P., Moretti S., Faronato M., Tamagnone L., Procopio A. 2006. Semaphorin-3A is expressed by tumor cells and alters T-cell signal transduction and function. Blood. 107:3321–3329 10.1182/blood-2005-06-2445 [DOI] [PubMed] [Google Scholar]
- Chen C., Li M., Chai H., Yang H., Fisher W.E., Yao Q. 2005. Roles of neuropilins in neuronal development, angiogenesis, and cancers. World J. Surg. 29:271–275 10.1007/s00268-004-7818-1 [DOI] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave S.S., Wright G., Tan B., Rosenwald A., Gascoyne R.D., Chan W.C., Fisher R.I., Braziel R.M., Rimsza L.M., Grogan T.M., et al. 2004. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N. Engl. J. Med. 351:2159–2169 10.1056/NEJMoa041869 [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Takagi S., Hirata T. 1995. Growth-associated expression of a membrane protein, neuropilin, in Xenopus optic nerve fibers. Dev. Neurosci. 17:343–349 10.1159/000111304 [DOI] [PubMed] [Google Scholar]
- Galon J., Costes A., Sanchez-Cabo F., Kirilovsky A., Mlecnik B., Lagorce-Pagès C., Tosolini M., Camus M., Berger A., Wind P., et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 313:1960–1964 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- Glinka Y., Prud’homme G.J. 2008. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory T cell activity. J. Leukoc. Biol. 84:302–310 10.1189/jlb.0208090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graells J., Vinyals A., Figueras A., Llorens A., Moreno A., Marcoval J., Gonzalez F.J., Fabra A. 2004. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J. Invest. Dermatol. 123:1151–1161 10.1111/j.0022-202X.2004.23460.x [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E.R., Reimert D.V., Shu T., Fritzsch B., Richards L.J., Kolodkin A.L., Ginty D.D. 2003. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell. 5:45–57 10.1016/S1534-5807(03)00169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Joshi K., Wig J.D., Arora S.K. 2007. Intratumoral FOXP3 expression in infiltrating breast carcinoma: Its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 46:792–797 10.1080/02841860701233443 [DOI] [PubMed] [Google Scholar]
- Hansen W., Westendorf A.M., Reinwald S., Bruder D., Deppenmeier S., Groebe L., Probst-Kepper M., Gruber A.D., Geffers R., Buer J. 2007. Chronic antigen stimulation in vivo induces a distinct population of antigen-specific Foxp3 CD25 regulatory T cells. J. Immunol. 179:8059–8068 [DOI] [PubMed] [Google Scholar]
- Helfrich I., Scheffrahn I., Bartling S., Weis J., von Felbert V., Middleton M., Kato M., Ergün S., Schadendorf D. 2010. Resistance to antiangiogenic therapy is directed by vascular phenotype, vessel stabilization, and maturation in malignant melanoma. J. Exp. Med. 207:491–503 10.1084/jem.20091846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer K., Sylvester M., Kliche S., Pusch R., Thiemke K., Schraven B., Freund C. 2006. Lipid-binding hSH3 domains in immune cell adapter proteins. J. Mol. Biol. 361:94–104 10.1016/j.jmb.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Jones E.A., Yuan L., Breant C., Watts R.J., Eichmann A. 2008. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 135:2479–2488 10.1242/dev.014902 [DOI] [PubMed] [Google Scholar]
- Kato M., Takahashi M., Akhand A.A., Liu W., Dai Y., Shimizu S., Iwamoto T., Suzuki H., Nakashima I. 1998. Transgenic mouse model for skin malignant melanoma. Oncogene. 17:1885–1888 10.1038/sj.onc.1202077 [DOI] [PubMed] [Google Scholar]
- Kawakami T., Tokunaga T., Hatanaka H., Kijima H., Yamazaki H., Abe Y., Osamura Y., Inoue H., Ueyama Y., Nakamura M. 2002. Neuropilin 1 and neuropilin 2 co-expression is significantly correlated with increased vascularity and poor prognosis in nonsmall cell lung carcinoma. Cancer. 95:2196–2201 10.1002/cncr.10936 [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. 1999. A requirement for neuropilin-1 in embryonic vessel formation. Development. 126:4895–4902 [DOI] [PubMed] [Google Scholar]
- Kitsukawa T., Shimizu M., Sanbo M., Hirata T., Taniguchi M., Bekku Y., Yagi T., Fujisawa H. 1997. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 19:995–1005 10.1016/S0896-6273(00)80392-X [DOI] [PubMed] [Google Scholar]
- Klages K., Mayer C.T., Lahl K., Loddenkemper C., Teng M.W., Ngiow S.F., Smyth M.J., Hamann A., Huehn J., Sparwasser T. 2010. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 70:7788–7799 10.1158/0008-5472.CAN-10-1736 [DOI] [PubMed] [Google Scholar]
- Lahl K., Loddenkemper C., Drouin C., Freyer J., Arnason J., Eberl G., Hamann A., Wagner H., Huehn J., Sparwasser T. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204:57–63 10.1084/jem.20061852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chao Q.G., Ping L.Z., Xue C., Xia Z.Y., Qian D., Shi-ang H. 2009. The prevalence of FOXP3+ regulatory T-cells in peripheral blood of patients with NSCLC. Cancer Biother. Radiopharm. 24:357–367 10.1089/cbr.2008.0612 [DOI] [PubMed] [Google Scholar]
- Loser K., Hansen W., Apelt J., Balkow S., Buer J., Beissert S. 2005. In vitro-generated regulatory T cells induced by Foxp3-retrovirus infection control murine contact allergy and systemic autoimmunity. Gene Ther. 12:1294–1304 10.1038/sj.gt.3302567 [DOI] [PubMed] [Google Scholar]
- Mizui M., Kikutani H. 2008. Neuropilin-1: the glue between regulatory T cells and dendritic cells? Immunity. 28:302–303 10.1016/j.immuni.2008.02.012 [DOI] [PubMed] [Google Scholar]
- Murga M., Fernandez-Capetillo O., Tosato G. 2005. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood. 105:1992–1999 10.1182/blood-2004-07-2598 [DOI] [PubMed] [Google Scholar]
- Nasarre P., Thomas M., Kruse K., Helfrich I., Wolter V., Deppermann C., Schadendorf D., Thurston G., Fiedler U., Augustin H.G. 2009. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 69:1324–1333 10.1158/0008-5472.CAN-08-3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H., Sakaguchi S. 2010. Regulatory T cells in tumor immunity. Int. J. Cancer. 127:759–767 [DOI] [PubMed] [Google Scholar]
- Ohara M., Yamaguchi Y., Matsuura K., Murakami S., Arihiro K., Okada M. 2009. Possible involvement of regulatory T cells in tumor onset and progression in primary breast cancer. Cancer Immunol. Immunother. 58:441–447 10.1007/s00262-008-0570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka S., Tawara I., Shimizu J., Sakaguchi S., Fujita T., Nakayama E. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 59:3128–3133 [PubMed] [Google Scholar]
- Pan Q., Chanthery Y., Liang W.C., Stawicki S., Mak J., Rathore N., Tong R.K., Kowalski J., Yee S.F., Pacheco G., et al. 2007. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67 10.1016/j.ccr.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Price D.J., Miralem T., Jiang S., Steinberg R., Avraham H. 2001. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 12:129–135 [PubMed] [Google Scholar]
- Proescholdt M.A., Jacobson S., Tresser N., Oldfield E.H., Merrill M.J. 2002. Vascular endothelial growth factor is expressed in multiple sclerosis plaques and can induce inflammatory lesions in experimental allergic encephalomyelitis rats. J. Neuropathol. Exp. Neurol. 61:914–925 [DOI] [PubMed] [Google Scholar]
- Reinwald S., Wiethe C., Westendorf A.M., Breloer M., Probst-Kepper M., Fleischer B., Steinkasserer A., Buer J., Hansen W. 2008. CD83 expression in CD4+ T cells modulates inflammation and autoimmunity. J. Immunol. 180:5890–5897 [DOI] [PubMed] [Google Scholar]
- Roland C.L., Lynn K.D., Toombs J.E., Dineen S.P., Udugamasooriya D.G., Brekken R.A. 2009. Cytokine levels correlate with immune cell infiltration after anti-VEGF therapy in preclinical mouse models of breast cancer. PLoS ONE. 4:e7669 10.1371/journal.pone.0007669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe W.A., Welsh M.E., Carter D.E., Karlik S.J. 2009. VEGF and angiogenesis in acute and chronic MOG((35-55)) peptide induced EAE. J. Neuroimmunol. 209:6–15 10.1016/j.jneuroim.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Sarris M., Andersen K.G., Randow F., Mayr L., Betz A.G. 2008. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 28:402–413 10.1016/j.immuni.2008.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q., Gu C., Fujisawa H., Sabelko K., Gertsenstein M., Nagy A., Taniguchi M., Kolodkin A.L., Ginty D.D., Shima D.T., Ruhrberg C. 2004. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 18:2822–2834 10.1101/gad.322904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Sakaguchi S. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163:5211–5218 [PubMed] [Google Scholar]
- Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. 1998. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 92:735–745 10.1016/S0092-8674(00)81402-6 [DOI] [PubMed] [Google Scholar]
- Solomon B.D., Mueller C., Chae W.J., Alabanza L.M., Bynoe M.S. 2011. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 108:2040–2045 10.1073/pnas.1008721108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton C.A., Kumar I., Reed M.W., Brown N.J. 2007. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 212:237–248 10.1002/path.2182 [DOI] [PubMed] [Google Scholar]
- Stockmann C., Doedens A., Weidemann A., Zhang N., Takeda N., Greenberg J.I., Cheresh D.A., Johnson R.S. 2008. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 456:814–818 10.1038/nature07445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straume O., Akslen L.A. 2003. Increased expression of VEGF-receptors (FLT-1, KDR, NRP-1) and thrombospondin-1 is associated with glomeruloid microvascular proliferation, an aggressive angiogenic phenotype, in malignant melanoma. Angiogenesis. 6:295–301 10.1023/B:AGEN.0000029408.08638.aa [DOI] [PubMed] [Google Scholar]
- Strauss L., Bergmann C., Szczepanski M., Gooding W., Johnson J.T., Whiteside T.L. 2007. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 13:4345–4354 10.1158/1078-0432.CCR-07-0472 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Nakamura F., Jin Z., Kalb R.G., Strittmatter S.M. 1998. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat. Neurosci. 1:487–493 10.1038/2203 [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C.D., Cleary K.R., Ellis L.M. 1995. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 55:3964–3968 [PubMed] [Google Scholar]
- Tordjman R., Lepelletier Y., Lemarchandel V., Cambot M., Gaulard P., Hermine O., Roméo P.H. 2002. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3:477–482 [DOI] [PubMed] [Google Scholar]
- Turk M.J., Guevara-Patiño J.A., Rizzuto G.A., Engelhorn M.E., Sakaguchi S., Houghton A.N. 2004. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J. Exp. Med. 200:771–782 10.1084/jem.20041130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338–344 10.1038/ni1180 [DOI] [PubMed] [Google Scholar]
- Wada J., Suzuki H., Fuchino R., Yamasaki A., Nagai S., Yanai K., Koga K., Nakamura M., Tanaka M., Morisaki T., Katano M. 2009. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 29:881–888 [PubMed] [Google Scholar]
- Wang L., Zeng H., Wang P., Soker S., Mukhopadhyay D. 2003. Neuropilin-1-mediated vascular permeability factor/vascular endothelial growth factor-dependent endothelial cell migration. J. Biol. Chem. 278:48848–48860 10.1074/jbc.M310047200 [DOI] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. 1992. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 356:793–796 10.1038/356793a0 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]
- Zou W. 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6:295–307 10.1038/nri1806 [DOI] [PubMed] [Google Scholar]