Abstract
Gα12 – RhoA signaling is a parathyroid hormone (PTH)-stimulated pathway that mediates effects in bone and may influence genetic susceptibility to osteoporosis. To further elucidate effects of the pathway in osteoblasts, UMR-106 osteoblastic cells were stably transfected with constitutively active (ca) Gα12 or caRhoA or dominant negative (dn) RhoA and co-cultured with RAW 264.7 cells to determine effects on hormone-stimulated osteoclastogenesis. Whereas PTH and calcitriol stimulated osteoclastogenesis in co-cultures with UMR-106 cells expressing pcDNA or dominant negative RhoA, the osteoclastogenic effects of PTH and calcitriol were significantly attenuated when the UMR-106 cells expressed either caRhoA or caGα12. These inhibitory effects were partially reversed by the Rho kinase inhibitor Y27632. None of the constructs affected osteoclastogenesis in untreated co-cultures, and the constructs did not inhibit the osteoclastogenic responses to receptor activator of NFκB ligand (RANKL). To investigate the mechanism of the inhibitory effects of caGα12 and caRhoA, expression of RANKL, osteoprotegerin (OPG), osteopontin (OPN) and intercellular adhesion molecule-1 (ICAM) in response to PTH or calcitriol was examined in the UMR-106 cells. In the cells expressing pcDNA or dnRhoA, PTH and calcitriol increased RANKL mRNA and decreased OPG mRNA, whereas these effects were absent in the cells expressing caGα12 or caRhoA. Basal expression of RANKL and OPG was unaffected by the constructs. The results suggest that Gα12 – RhoA signaling can inhibit hormone-stimulated osteoclastogenesis by effects on expression of RANKL and OPG. Since PTH can stimulate the Gα12 – RhoA pathway, the current findings could represent a homeostatic mechanism for regulating osteoclastogenic action.
Keywords: Osteoblast, Osteoclastogenesis, Gα12, RhoA, Parathyroid hormone, Calcitriol
INTRODUCTION
Gα12/13 proteins are a subfamily of heterotrimeric G proteins that act as molecular switches to exchange GDP for GTP and by this action transduce effects initiated through seven-transmembrane domain receptors [Kelly et al., 2007] and other effectors [Marty and Ye, 2010]. The Gα12/13 subfamily is expressed in most tissues [Spicher et al., 1994] and has been shown to be involved in multiple physiological and pathophysiological processes [Worzfeld et al., 2008]. The most extensively studied mediators of Gα12/13 signaling are the Rho family monomeric G proteins. These ~ 20 kDa proteins are activated by Gα12/13 through guanine nucleotide exchange factors, RhoGEFs [Siehler, 2009]. Downstream effectors of Rho family small G proteins include Rho kinase and MAP kinase, as well as other mediators [Bishop and Hall, 2000]. The pathway is best characterized for its effects on the actin cytoskeleton, but other cytoskeletal responses, cell polarity, vesicular trafficking, integrin signaling, membrane transport, cell survival, erythropoesis, lymphocyte development and cancer progression have been linked to the pathway [DeMali et al., 2003; Heasman and Ridley, 2008; Karlsson et al., 2009; Mulloy et al., 2010; Tybulewicz and Henderson, 2009].
Recent findings indicate that the Gα12/13 – RhoA – Rho kinase pathway has important roles in bone. In osteoclasts, RhoA is important for podosome assembly, osteoclast motility and bone resorption [Chellaiah et al., 2000; Ory et al., 2008]. In C3H10T1/2 murine mesenchymal stem cells, Rho A and Rho kinase regulate fluid-flow-induced osteogenic differentiation, and RhoA activation decreased adipogenic and chondrogenic differentiation [Arnsdorf et al., 2009].
Survival of MC3T3-E1 osteoblastic cells [Yoshida et al., 2009], and the integrity of the actin cytoskeleton [Kazmers et al., 2009] are dependent upon the RhoA and Rho kinase signaling. Activation of Rho family small G proteins and their translocation to membrane sites are dependent upon lipid modification [Roberts et al., 2008]. Hence, statins and bisphosphonates, which interfere with the synthesis of isoprenyl lipids, as well as geranylgeranyl transferase inhibitors, can prevent effects of RhoA [Fritz and Kaina, 2006; Liao and Laufs, 2005; Reszka and Rodan, 2004; Russell, 2007; Yuasa et al., 2007]. caRhoA is able to overcome the effects of the bisphosphonate alendronate to degrade the osteoblast cytoskeleton [Kazmers et al., 2009]. In UMR-106 osteoblastic cells, RhoA is crucial for PTH-stimulated protein kinase Cα (PKCα) translocation to the plasma membrane and is involved in the PTH-mediated stimulation of interleukin-6 expression [Radeff et al., 2004]. Particularly interesting are recent findings that genetic polymorphisms in RhoA and in the Rho GEF, ARHGEF3, show an association with bone mineral density Z score in postmenopausal women [Mullin et al., 2008; Mullin et al., 2009]. Gα12/13 signaling has been less extensively studied in bone, but has been found to stimulate phospholipase D activity through RhoA [Singh et al., 2005].
To further elucidate the roles of Gα12 and RhoA in osteoblasts, we investigated whether stable expression of mutant forms of the proteins in osteoblastic cells could affect hormone-stimulated osteoclastogenesis. The studies reported here show that expression of caGα12 or caRhoA in UMR-106 cells impairs their ability to induce osteoclastogenesis in response to PTH or calcitriol. The effect correlated with effects of caGα12 and caRhoA to prevent the stimulation of expression of RANKL mRNA in response to PTH or calcitriol..
METHODS
Materials
RAW 264.7 monocyte-macrophage lineage osteoclast precursor cells and UMR-106 osteoblastic cells were purchased from American Type Culture Collection (Manassa, VA). The G12 construct (G12Q231L) and the control pcDNA were kindly provided by Dr. Tatyana Voyno-Yasenetskaya, University of Illinois. The RhoA dominant negative (RhoA19N) and constitutively active (RhoA63L) constructs were kindly provided by Dr. Said Sebti, University of South Florida. PTH(1-34) was purchased from BaChem (Torrance, CA); human sRANKL was purchased from Peprotech (Rocky Hill, NJ); 1,25-(OH)2D3 (calcitriol) was a gift from Dr. Milan Uskokovic (Hoffmann-La Roche, Nutley, NJ).
Transfection
Rat osteoblastic UMR106 cells were transfected using a CalPhos mammalian transfection kit (Clontech, Mountain View, CA). Briefly, 2 µg of plasmid DNA was added to 12.4 µl 2M calcium solution, and diluted with deionized water to make 100 µl solution A. Solution A was then added dropwise into 100µl 2x HEPES-buffered saline (Solution B) with slow vortexing to make the transfection solution. After incubation at room temperature for 20 min, the transfection solution was added dropwise to cell culture plates. After 16 hr at 37°C, calcium phosphate-containing medium was removed and replaced with 2 ml fresh medium for 24 hr. Stable transfectants were selected and maintained with α-MEM + 10% fetal bovine serum containing 600 µM G418.
Cell co-culture
For osteoclastogenesis and MTT assays, cells were cultured in 0.2 ml αMEM + 10% fetal bovine serum in 96-well Corning plates (Corning, NY). UMR-106 cells were used between the 18th and 29th passage. RAW 264.7 cells were used between the 13th and 20th passage. UMR-106 and RAW 264.7 cells were were seeded at a density of 104/ml for UMR-106 cells and 2500/ml for RAW 264.7 cells, a ratio found to be optimal in preliminary experiments. Treatments (PTH, calcitriol, RANKL) were added the day after cell seeding. The medium was replaced with fresh treatment-containing medium after 48 hours. The total treatment time was 5 days. For RT-PCR, UMR-106 cells expressing the constructs were cultured in 6-well plates in 2 ml α-MEM + 10% fetal bovine serum. Treatments (PTH, calcitriol) were for 16 hours.
TRAP assay
At the end of the treatment period, medium was removed and cells were fixed by 10-minute treatment with 50 µl 10% formalin. After a wash with 100 µl 95% ethanol, wells were dried and incubated for 30 minutes with 100 µl TRAP substrate (phosphatase substrate, cat #P4744, Sigma-Aldrich, St. Louis, MO), 1.36 mg/ml in buffer consisting of 50 mM Na citrate/10 mM tartrate buffer, pH 4.6. This was then added to 100 µl 0.1 N NaOH, and the absorbance read at 410 nm on a microplate reader (Dynatech, Chantilly, VA)
Formation of multinucleated cells
Cells were stained with 200µl TRAP reaction solution, which was prepared by dissolving 5 mg napthol AS-MX phosphate (Sigma-Aldrich) dissolved in 0.5 ml N,N-dimethylformamide). This was mixed with 50 ml of buffer consisting of 100mM Na acetate, 50 mM Na tartrate, pH 5.0, and adding 30 mg Fast Violet LB salt (Sigma-Aldrich). The duration of staining was 2–4 hr. Cells with three or more nuclei were counted.
RT-PCR
cDNA was synthesized using 1 µg total RNA and a reverse transcription system (Promega, Madison, WI). PCR was performed in a 25-µl reaction solution containing Platinum Taq DNA polymerase (InVitrogen, Carlsbad, CA) and cDNA template. After denaturing the template and activating the polymerase at 94°C for 2 minutes, the PCR reaction was run for 30–40 cycles at: 94°C for 15 seconds, 55–60°C for 30 seconds, and 72°C for 60 seconds. The forward and reverse primers of RANKL were 5’-TCG GGT TCC CAT AAA GTC AG-3’ and 5’-CTT GGG ATT TTG ATG CTG GT-3’. For OPG, forward and reverse primers were 5’-TGG GAA TGA AGA TCC TCC AG-3’ and 5’-GAG GAA GGA AAG GGC CTA TG-3’. For osteopontin, forward and reverse primers were 5’-CTA AGC CTC AGC ATC CTT GG-3’ and 5’-TGT AAT GCG CCT TCT CCT CT-3’. For ICAM, forward and reverse primers were 5’-AGG TAT CCA TCC ATC CCA CA-3’ and 5’-GCC ACA GTT CTC AAA GCA CA-3’. The forward and reverse primers for housekeeping gene Rpl13a were 5’-CCC TCC ACC CTA TGA CAA GA-3’ and 5’-CCT TTT CTT TCC GTT TCT CC-3’
Statistics
The significance of changes was assessed by ANOVA and Bonferroni post-test. A value of p<0.05 was considered significant.
RESULTS
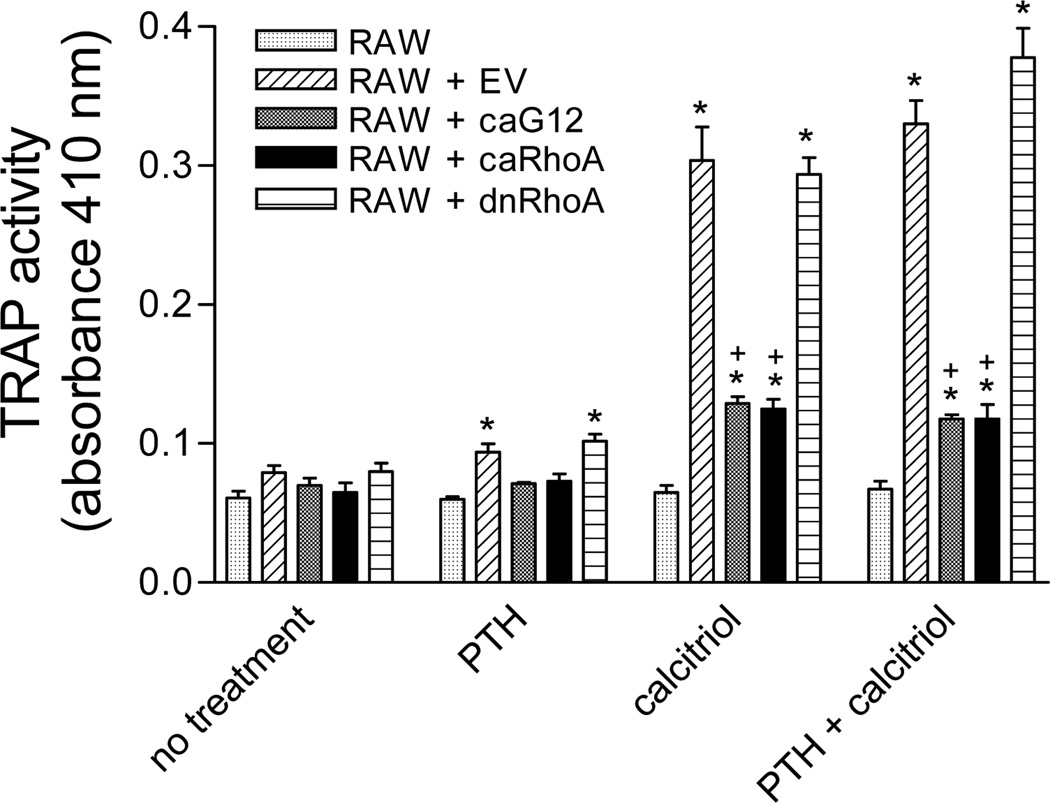
In co-cultures of UMR-106 osteoblastic cells and RAW 264.7 osteoclast precursor cells, treatment for 5 days with PTH (30 nM), calcitriol (100 nM) or the combination stimulated osteoclastogenesis as shown by increased TRAP activity (Figure 1). However, stable transfection of the UMR-106 cells with caGα12 or caRhoA prevented the osteoclastogenic effects of PTH and calcitriol. Cells expressing dnRhoA responded similarly to cells expressing the empty vector, with PTH, calcitriol or the combination eliciting a significant increase in TRAP activity. No stimulation of TRAP activity was detected when the RAW 264.7 cells were cultured in the absence of UMR-106 cells. The responses in co-cultures containing UMR-106 cells transfected with EV were not different from those in co-cultures containing non-transfected UMR-106 cells (data not shown). There was no TRAP activity in UMR-106 cells cultured in the absence of RAW 264.7 cells (data not shown).
Figure 1.
The osteoclastogenic effects of PTH (30 nM) and calcitriol (100 nM) in UMR-106/RAW 264.7 cell co-cultures as assessed by TRAP activity are markedly attenuated when the UMR-106 cells stably express caGα12 or caRhoA constructs. Cells were seeded at a density of 104/ml for UMR-106 cells and 2,500/ml for RAW 264.7 cells in 96 well plates and maintained in α-MEM supplemented with 10% fetal bovine serum. Five-day treatments began on the day after seeding. * p<0.05 vs. co-cultures without PTH or calcitriol, + p<0.05 vs. co-cultures containing EV-transfected UMR-106 cells. N = 3.
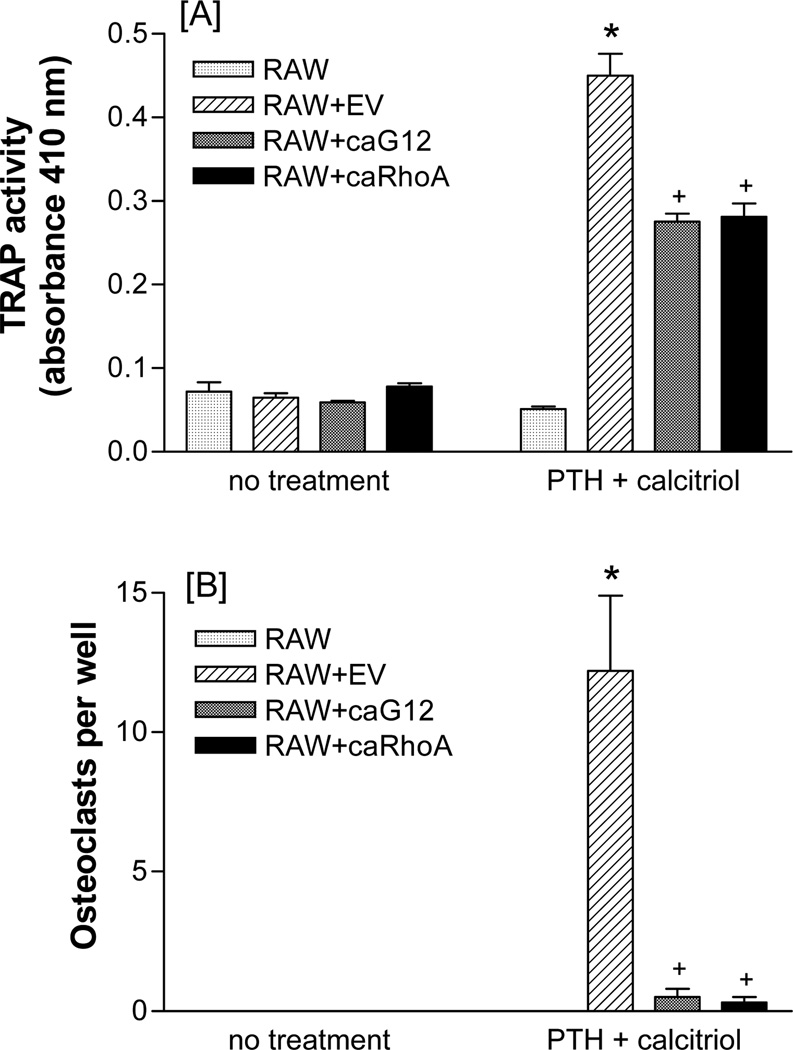
Although the 5-day treatment with calcitriol or the combination did not elicit large numbers of osteoclasts, the effects on TRAP were reflected in the numbers of multinucleated osteoclasts formed. Expression of caGα12 or caRhoA in the UMR-106 cells largely prevented the formation of multinucleated cells in the co-cultures (Figure 2).
Figure 2.
CaGα12 or caRhoA stably expressed in UMR-106 cells prevent the effect of PTH (30 nM) + calcitriol (100 nM) on [A] TRAP activity and [B] multinucleation in UMR-106/RAW 264.7 cell co-cultures. Cells were seeded at a density of 104/ml for UMR-106 cells and 2,500/ml for RAW 264.7 cells in 96 well plates and maintained in α-MEM supplemented with 10% fetal bovine serum. Five-day treatments began on the day after seeding. * p<0.05 vs. co-cultures without PTH + calcitriol, + p<0.05 vs. co-cultures containing EV-transfected UMR-106 cells. N = 6.
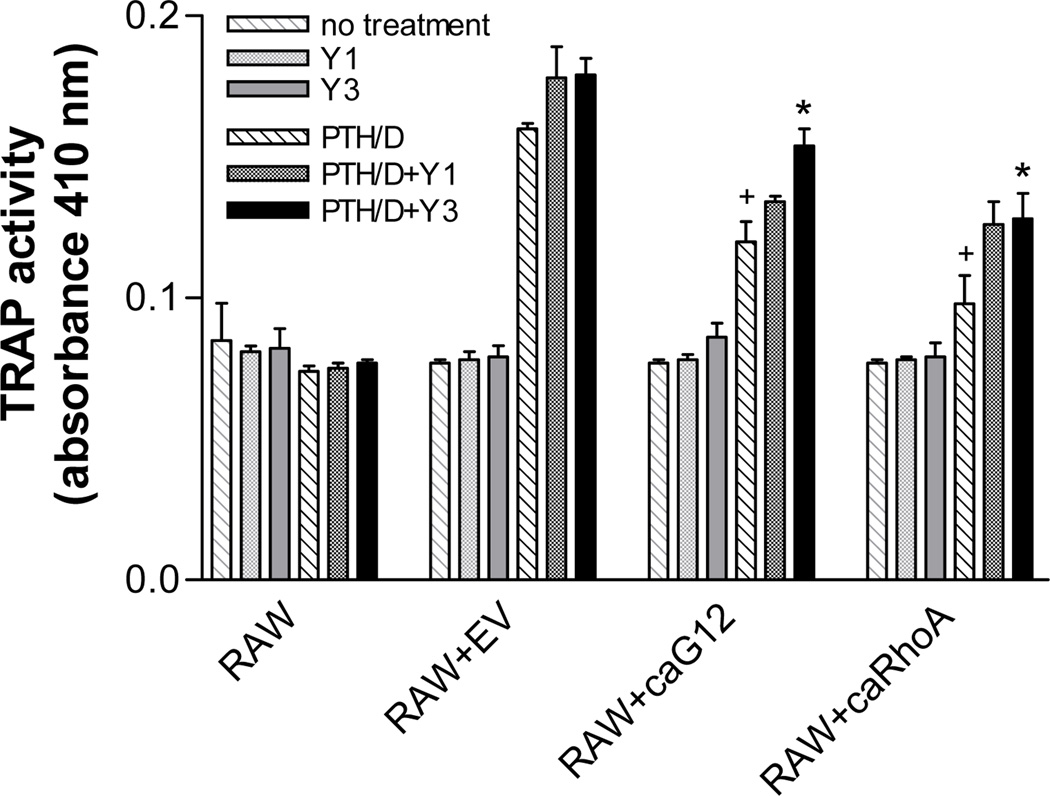
Since Gα12 signaling is often transduced through RhoA, and RhoA effects can be mediated through the downstream kinase, Rho kinase, the effects of the Rho kinase inhibitor Y27632 on TRAP activity in the co-cultures was determined. The inhibitor, at a concentration of 3 µM, significantly reversed the inhibitory effects of caGα12 and caRhoA on TRAP activity in co-cultures treated with PTH + calcitriol (Figure 3). There were no direct effects of Y27632 on TRAP activity in the RAW 264.7 cells under the conditions used.
Figure 3.
The Rho kinase inhibitor, Y27632, reverses the inhibitory effects of stable expression of caGα12 or caRhoA in UMR-106 cells on PTH (30 nM) + calcitriol (100 nM)-stimulated osteoclastogenesis in co-cultures with RAW 264.7 cells. Cells were seeded at a density of 104/ml for UMR-106 cells and 2,500/ml for RAW 264.7 cells in 96 well plates and maintained in α-MEM supplemented with 10% fetal bovine serum. Five-day treatments began on the day after seeding. * p<0.05 vs. no Y27632, + p<0.05 vs. response to PTH + calcitriol in co-cultures containing EV-transfected UMR-106 cells. N = 3.
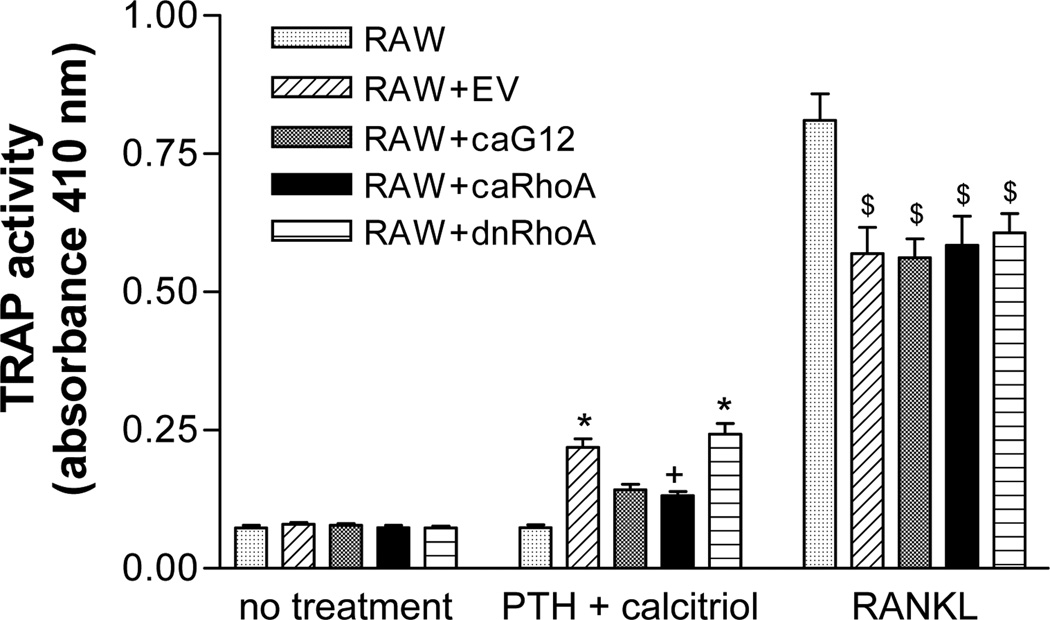
To determine whether the constructs could influence the response to RANKL, the co-cultures were treated with 30 ng/ml RANKL. As expected, RANKL markedly stimulated TRAP activity in the RAW 264.7 cells in the absence of UMR-106 cells (Figure 4). Interestingly, the addition of any osteoblastic cells decreased the response of the UMR-106 cells to RANKL, an effect that was observed in most experiments. However, expression of the caGα12 or caRhoA constructs failed to alter the response to RANKL, although in the same experiment it produced the usual effect to decrease the response to PTH + calcitriol.
Figure 4.
Stable expression of caGα12 or caRhoA in UMR-106 cells fails to differentially attenuate responses to RANKL (30 ng/ml) in UMR-106/RAW 264.7 cell co-cultures. In the same experiment, caGα12 and caRhoA attenuated the response to PTH (30 nM) + calcitriol (100 nM). Cells were seeded at a density of 104/ml for UMR-106 cells and 2,500/ml for RAW 264.7 cells in 96 well plates and maintained in α-MEM supplemented with 10% fetal bovine serum. Five-day treatments began on the day after seeding. * p<0.05 vs. co-cultures without PTH + calcitriol, + p<0.05 vs. co-cultures containing UMR-106 cells stably expressing dnRhoA or EV, $ p<0.05 vs. effect of RANKL in RAW 264.7 cells alone. N = 6.
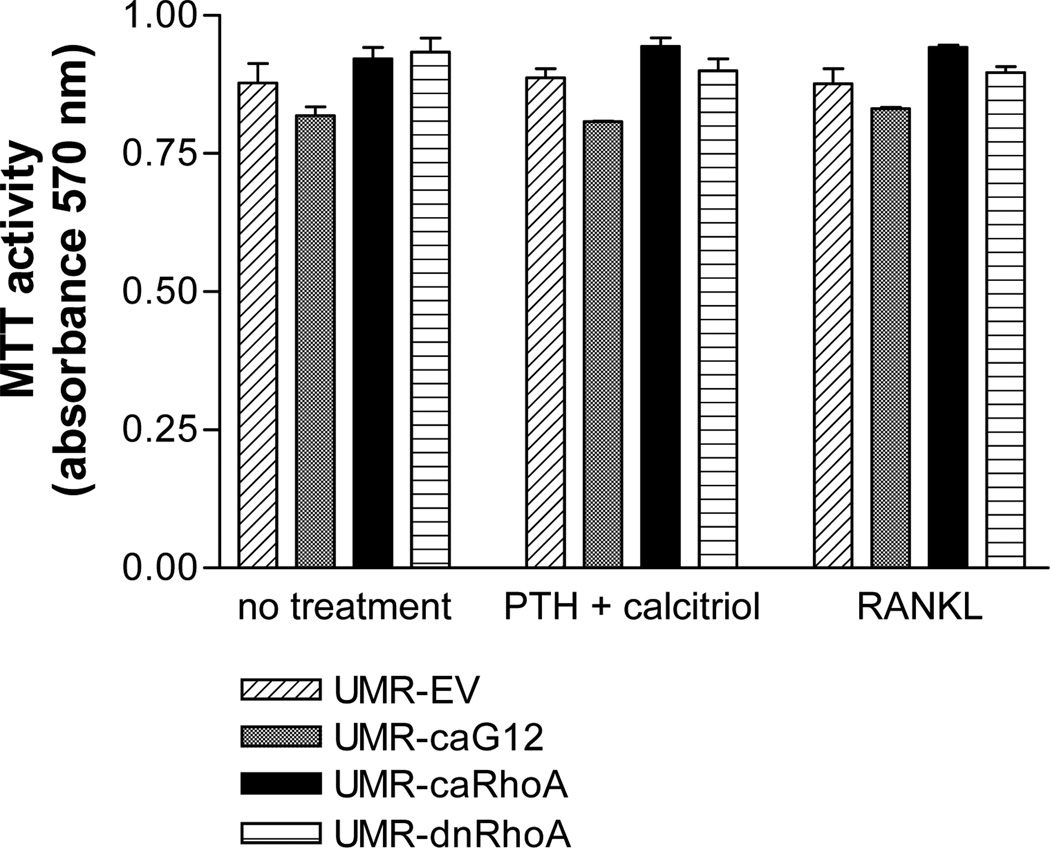
No significant differences in metabolic or proliferative activity, assessed by MTT assay, were observed in UMR-106 cells transfected with the different constructs (Figure 5).
Figure 5.
Stable expression of caGα12, caRhoA or dnRho A in UMR-106 cells do not affect cell viability, as assessed by MTT assay. UMR-106 cells were seeded at a density of 104/ml in 96 well plates and maintained in α-MEM supplemented with 10% fetal bovine serum. Five-day treatments began on the day after seeding. N = 3.
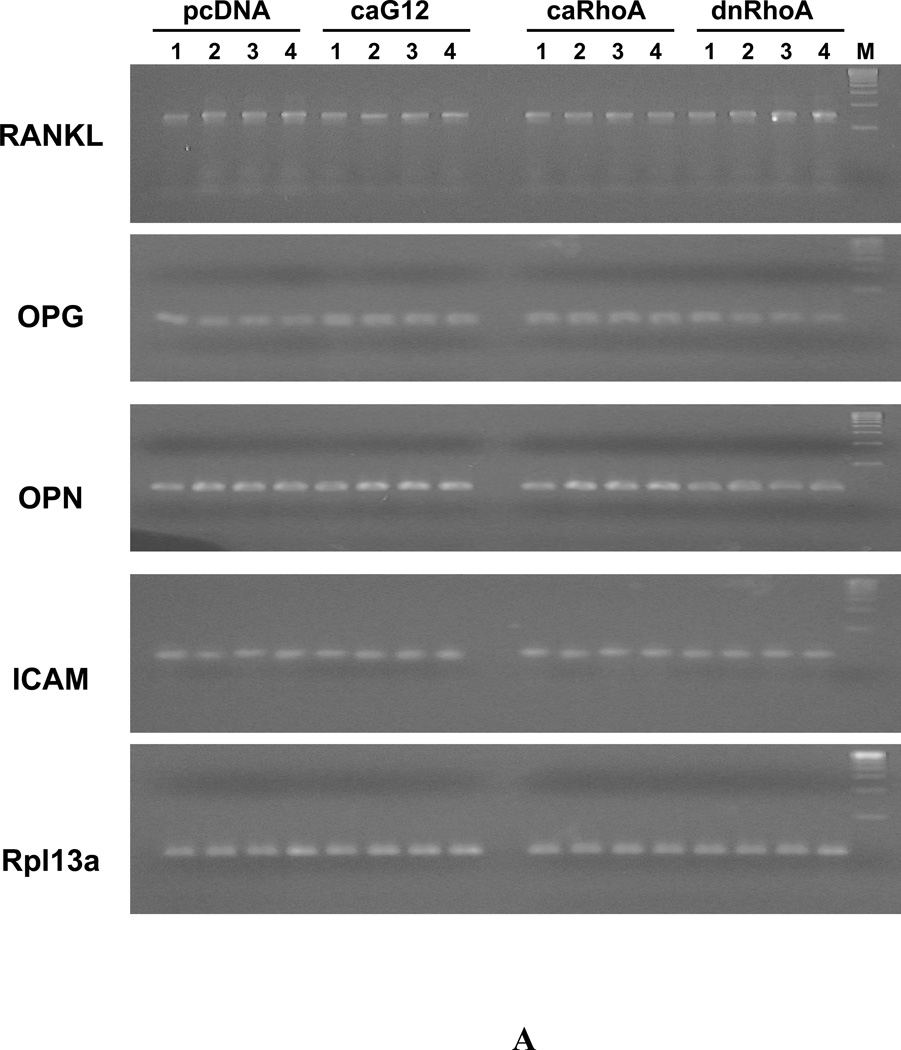
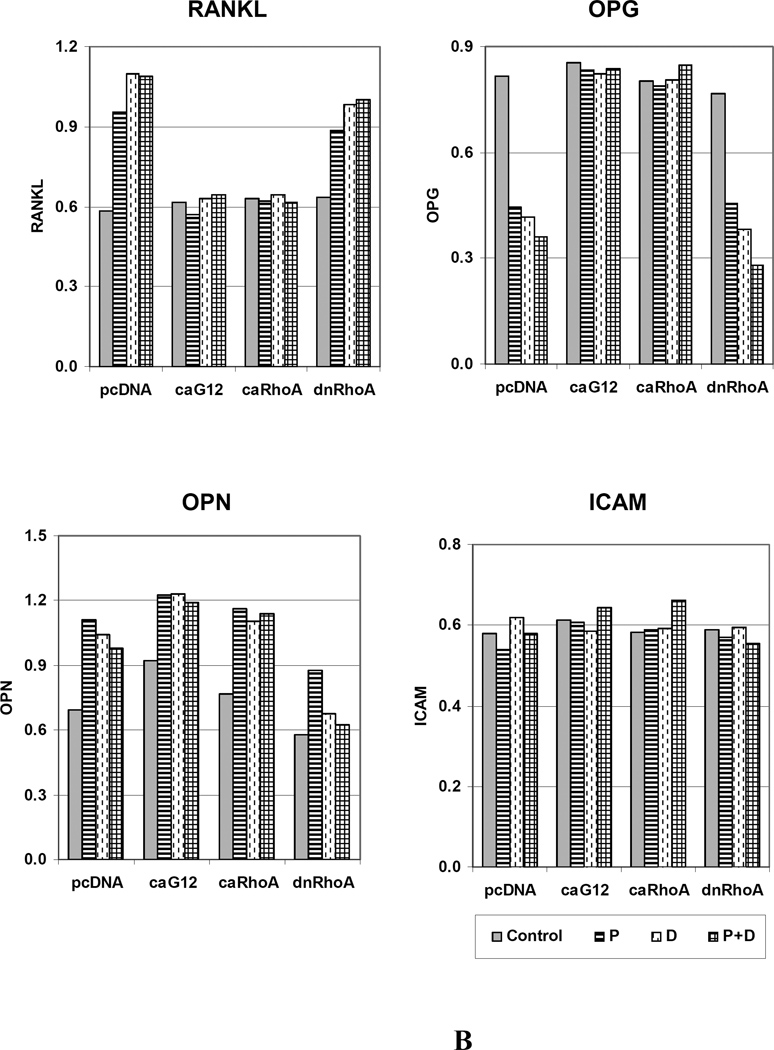
Since the findings indicate that expression of caGα12 or caRhoA in osteoblastic cells impairs their ability to activate osteoclast precursor cells in response to the osteoclastogenic ligands PTH and calcitriol, experiments were carried out to determine the effects of the ligands on the expression of the osteoblast cytokines RANKL and OPG, the former being a principal stimulator of osteoclastogenesis, and the latter a decoy receptor for RANKL that prevents its effects on the receptor RANK on the preosteoclast target cell. PTH, calcitriol, or the combination increased RANKL mRNA and decreased OPG mRNA in UMR-106 cells expressing pcDNA or dominant negative RhoA (Figure 6). However, in cells expressing caGα12 or caRhoA, these responses were prevented or even slightly reversed. In addition to RANKL and OPG mRNA, osteopontin (OPN) and intercellular adhesion molecule-1 (ICAM) mRNA expression were examined. OPN has been found to antagonize some effects of PTH [Ono et al., 2008] and ICAM is regulated by RhoA and Rho kinase [Anwar et al., 2004] and is involved in cell-cell interactions mediating PTH-stimulated osteoclast differentiation and bone resorption [Okada et al., 2002]. If these genes were altered by the constructs, they could potentially mediate the observed effects. OPN expression was slightly increased by caGα12, an observation that we had noted previously in microarray studies [Wang, 2008]. However, OPN expression in response to PTH, calcitriol and the combination were not markedly different in cells expressing the constitutively active constructs and in cells expressing the empty vector, although they were decreased in cells expressing dnRhoA. Also, there were no differences in the expression of ICAM among the treatments or with the different constructs.
Figure 6.
Constitutively active G12 and RhoA constructs attenuate the effects of PTH, calcitriol and the combination to increase RANKL and decrease OPG expression in UMR-106 cells, but do not influence PTH, calcitriol or PTH + calcitriol responses to OPN or ICAM. (A) RT-PCR of effects of treatments on RANKL, OPG, OPN and ICAM. 1. Control; 2. PTH (P); 3. Calcitriol (D); 4. PTH+Calcitriol (P+D); M. 100bp molecular ruler. (B) The relative expression levels of genes were analyzed and normalized against the housekeeping gene Rpl13a. The bands shown in Figure 6A were scanned and quantified for the analysis.
DISCUSSION
Osteoblast-stimulated osteoclastogenesis is an important component of bone remodeling as well as a potential limitation in the therapeutic use of parathyroid hormone (PTH) to build bone. Excessive calcitriol can also stimulate osteoclastogenesis. Factors that modulate this osteoclastogenic effect are important for understanding bone physiology and could lead to new therapeutic agents that lack this limitation. The current studies reveal that in co-cultures of UMR-106 osteoblastic cells and RAW 264.7 osteoclast precursor cells, expression of caGα12 or caRhoA in the UMR-106 cells impairs the ability of PTH or calcitriol to the differentiation of the RAW 264.7 cells into cells producing TRAP (Figures 1 to 4) and having the ability to form multinucleated osteoclasts (Figure 2). The cells expressing caGα12 or caRhoA also had impaired ability to express RANKL in response to PTH or calcitriol (Figure 6). The small decrease in expression of OPG in response to PTH or calcitriol was not seen in the cells transfected with caGα12 or caRhoA. However, it seemed unlikely that this was an important factor in the response, since the cells expressing the constitutively active constructs were not different from empty vector or dnRhoA-expressing cells in their response to RANKL. Thus the effect appears to be mediated at the level of RANKL production rather than by interference with the response to RANKL. The results demonstrate that the Gα12 – RhoA signaling pathway in osteoblastic cells is able to modulate hormone-stimulated osteoclastogenesis.
Several years ago, Wang et al. [Wang et al., 2002] described experiments on the effects of geranylgeranoic acid on bone, which are pertinent to the current findings, since geranylgeranoic acid could conceivably serve as a geranylgeranyl group donor and activate RhoA. They had investigated the effects of geranylgeranoic acid as a retinoid, a class of compounds that they had found to induce osteoblast differentiation and to inhibit osteoclast formation [Ishimi, 1999; Park et al., 1997]. Wang et al. found that geranylgeranoic acid inhibited osteoclast formation induced by calcitriol in co-cultures of mouse bone marrow cells and primary osteoblasts, an effect similar to what we observed in the co-cultures of RAW 264.7 cells with UMR-106 osteoblatic cells treated with caGα12 or caRhoA. They also observed that when ST2 stromal cells were treated with geranylgeranoic acid, the effects of calcitriol or prostaglandin E2 to decrease OPG mRNA were antagonized, similar to what we observed in the UMR-106 cells expressing caGα12 or caRhoA. They observed that geranylgeranoic acid inhibited the effect of RANKL in cultures of bone marrow macrophages. This is not necessarily inconsistent with our results showing that the constructs did not affect the response to RANKL, since the studies in macrophages would represent an effect of geranylgeranoic acid directly on the osteoclast precursor, which our experiments were designed to circumvent. Even Y-27632, added directly to the cultures, failed to have significant effects unless osteoblastic cells were present in addition to the RAW cells. Wang et al [Wang et al., 2002] also found that geranylgeranoic acid increased bone mineral density when administered in vivo to SAMP6 senescence-accelerated mice. It is possible that a similar effect might be seen with activation of Gα12 or RhoA in osteoblasts.
The processes downstream of RhoA that lead to the observed effects on RANKL and on osteoclastogenesis remains to be determined. However, the effects could have physiological implications. Although both PTH and calcitriol can have effects leading to bone formation, prolonged continuous treatment with PTH or exposure to high concentrations of calcitriol can result in accelerated resorption and bone loss, likely through stimulation of RANKL expression. Since PTH can activate RhoA [Radeff et al., 2004] possibly through Gα12 [Singh et al., 2005], although conceivably through other pathways, the current studies suggest the presence of a homeostatic mechanism by which stimuli that could lead to excess resorption could be modulated, resulting in the protection and preservation of bone.
Acknowledgments
Contract grant sponsor: NIH/NIAMS; Contract grant number: AR11262
REFERENCES
- Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol. 2004;173:6965–6972. doi: 10.4049/jimmunol.173.11.6965. [DOI] [PubMed] [Google Scholar]
- Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348(Pt 2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. Journal of Biological Chemistry. 2000;275:11993–12002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Wennerberg K, Burridge K. Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol. 2003;15:572–582. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Fritz G, Kaina B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr Cancer Drug Targets. 2006;6:1–14. [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Ishimi Y, Ohmura M, Wang XX, Yamaguchi M, Ikegami S. Inhibition by carotenoids and retinoic acid of osteoclast-like cell formation induced by bone-resorbing agents in vitro. J Clin Biochem Nutr. 1999;27:113–122. [Google Scholar]
- Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796:91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Kazmers NH, Ma SA, Yoshida T, Stern PH. Rho GTPase signaling and PTH 3-34, but not PTH 1-34, maintain the actin cytoskeleton and antagonize bisphosphonate effects in mouse osteoblastic MC3T3-E1 cells. Bone. 2009;45:52–60. doi: 10.1016/j.bone.2009.03.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Casey PJ, Meigs TE. Biologic functions of the G12 subfamily of heterotrimeric g proteins: growth, migration, and metastasis. Biochemistry. 2007;46:6677–6687. doi: 10.1021/bi700235f. [DOI] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C, Ye RD. Heterotrimeric G protein signaling outside the realm of seven transmembrane domain receptors. Mol Pharmacol. 2010;78:12–18. doi: 10.1124/mol.110.063453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BH, Prince RL, Dick IM, Hart DJ, Spector TD, Dudbridge F, Wilson SG. Identification of a role for the ARHGEF3 gene in postmenopausal osteoporosis. Am J Hum Genet. 2008;82:1262–1269. doi: 10.1016/j.ajhg.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin BH, Prince RL, Mamotte C, Spector TD, Hart DJ, Dudbridge F, Wilson SG. Further genetic evidence suggesting a role for the RhoGTPase-RhoGEF pathway in osteoporosis. Bone. 2009;45:387–391. doi: 10.1016/j.bone.2009.04.254. [DOI] [PubMed] [Google Scholar]
- Mulloy JC, Cancelas JA, Filippi MD, Kalfa TA, Guo F, Zheng Y. Rho GTPases in hematopoiesis and hemopathies. Blood. 2010;115:936–947. doi: 10.1182/blood-2009-09-198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Morimoto I, Ura K, Watanabe K, Eto S, Kumegawa M, Raisz L, Pilbeam C, Tanaka Y. Cell-to-Cell adhesion via intercellular adhesion molecule-1 and leukocyte function-associated antigen-1 pathway is involved in 1alpha,25(OH)2D3, PTH and IL-1alpha-induced osteoclast differentiation and bone resorption. Endocr J. 2002;49:483–495. doi: 10.1507/endocrj.49.483. [DOI] [PubMed] [Google Scholar]
- Ono N, Nakashima K, Rittling SR, Schipani E, Hayata T, Soma K, Denhardt DT, Kronenberg HM, Ezura Y, Noda M. Osteopontin negatively regulates parathyroid hormone receptor signaling in osteoblasts. J Biol Chem. 2008;283:19400–19409. doi: 10.1074/jbc.M800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory S, Brazier H, Pawlak G, Blangy A. Rho GTPases in osteoclasts: orchestrators of podosome arrangement. Eur J Cell Biol. 2008;87:469–477. doi: 10.1016/j.ejcb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Park CK, Ishimi Y, Ohmura M, Yamaguchi M, Ikegami S. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J Nutr Sci Vitaminol (Tokyo) 1997;43:281–296. doi: 10.3177/jnsv.43.281. [DOI] [PubMed] [Google Scholar]
- Radeff JM, Nagy Z, Stern PH. Rho and Rho kinase are involved in parathyroid hormone-stimulated protein kinase C alpha translocation and IL-6 promoter activity in osteoblastic cells. J Bone Miner Res. 2004;19:1882–1891. doi: 10.1359/JBMR.040806. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem. 2004;4:711–719. [PubMed] [Google Scholar]
- Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, Cox AD, Wilson O, Kirschmeier P, Der CJ. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(Suppl 2):S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- Siehler S. Regulation of RhoGEF proteins by G12/13-coupled receptors. Br J Pharmacol. 2009;158:41–49. doi: 10.1111/j.1476-5381.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PH. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology. 2005;146:2171–2175. doi: 10.1210/en.2004-1283. [DOI] [PubMed] [Google Scholar]
- Spicher K, Kalkbrenner F, Zobel A, Harhammer R, Nurnberg B, Soling A, Schultz G. G12 and G13 alpha-subunits are immunochemically detectable in most membranes of various mammalian cells and tissues. Biochem Biophys Res Commun. 1994;198:906–914. doi: 10.1006/bbrc.1994.1129. [DOI] [PubMed] [Google Scholar]
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9:630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yoshida T, Stern PH. Stable expression of constitutively active Galpha 12 in osteoblastic cells promotes matrix protein expression and alkaline phosphatase activity. J Bone Miner Res. 2008;23:S290. [Google Scholar]
- Wang X, Wu J, Shidoji Y, Muto Y, Ohishi N, Yagi K, Ikegami S, Shinki T, Udagawa N, Suda T, Ishimi Y. Effects of geranylgeranoic acid in bone: induction of osteoblast differentiation and inhibition of osteoclast formation. J Bone Miner Res. 2002;17:91–100. doi: 10.1359/jbmr.2002.17.1.91. [DOI] [PubMed] [Google Scholar]
- Worzfeld T, Wettschureck N, Offermanns S. G(12)/G(13)-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci. 2008;29:582–589. doi: 10.1016/j.tips.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Clark MF, Stern PH. The small GTPase RhoA is crucial for MC3T3-E1 osteoblastic cell survival. J Cell Biochem. 2009;106:896–902. doi: 10.1002/jcb.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T, Kimura S, Ashihara E, Habuchi T, Maekawa T. Zoledronic acid - a multiplicity of anti-cancer action. Curr Med Chem. 2007;14:2126–2135. doi: 10.2174/092986707781389600. [DOI] [PubMed] [Google Scholar]