Abstract
The phospholipid-lysophospholipid transacylase tafazzin is responsible for enrichment of the cardiolipin fraction of mitochondria with tetralinoleoyl-cardiolipin. The specificity for linoleoyl hydrocarbon chains is now explained by the specific action of tafazzin on negatively curved lipid monolayers.
The lipid composition of mitochondrial inner membranes has attracted attention because of its high content of phosphatidylethanolamines and cardiolipins, lipid species that are both known to form nonlamellar lipid phases with negatively curved lipid monolayers. Mitochondrial lipids of mammals are particularly rich in 1′,3′-bis[1,2-dilinoleoyl-sn-glycero-3-phospho]-sn-glycerol, a cardiolipin with four diunsaturated linoleic acid chains. The concentration of this lipid in the inner membranes of mitochondria is determined to a considerable extent by the phospholipid-lysophospholipid transacylase tafazzin. Disturbances in the cardiolipin content of the inner mitochondrial membrane, including shifts in the composition of cardiolipin fatty acids, have been related to Barth syndrome1, an X chromosome–linked genetic disorder that is caused by mutations to tafazzin2. However, previous studies have indicated that tafazzin has little selectivity among possible lipid substrates, raising the question as to why tafazzin mutations would cause such a specific outcome. In this issue, Schlame et al.3 shed light on this question, reporting model membrane studies suggesting that selective enrichment of linoleic acid in cardiolipins is related to a strong preference of tafazzin to transacylate phospholipids in negatively curved lipid monolayers as found in the cristae of mitochondrial inner membranes.
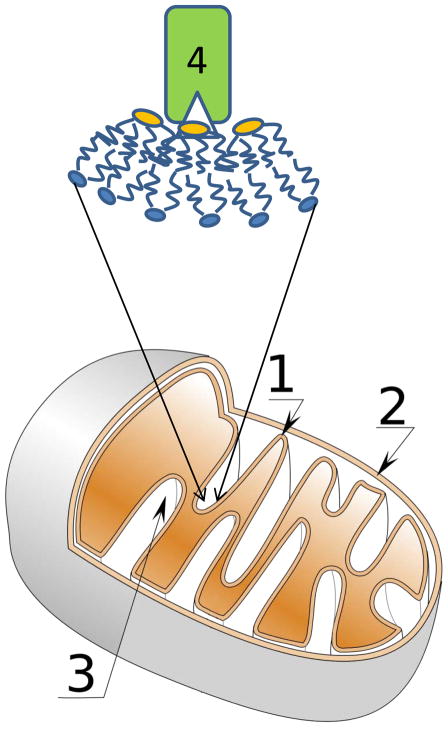
Tafazzin is expressed in high amounts in the mitochondria of cardiac and skeletal muscles. The enzyme is located in the intermembrane space between inner and outer membranes of mitochondria (see Fig. 1), where it associates peripherally with membrane leaflets4. The amino acid sequence of the protein predicted that tafazzin is a phospholipid acyltransferase5, with recombinant expression of tafazzin in Sf9 cells demonstrating unambiguously that it functions as a phospholipid-lysophospholipid transacylase6. In Barth syndrome patients, tafazzin is mutated and has impaired activity, resulting in a deficiency of tetralinoleoyl-cardiolipin in mitochondria7. The genetic disorder has been associated with weakening and enlargement of the heart (dilated cardiomyopathy); low counts of neutrophils, an abundant type of white blood cell (neutropenia); and weakness of skeletal muscles (skeletal myopathy), all of which are related to ultrastructural abnormalities in mitochondria of the corresponding tissues1,8.
Figure 1.
Schematic presentation of a mitochondrion. Shown are the inner membrane (1), outer membrane (2) and cristae (3). The transacylase tafazzin (4) prefers to interact with lipid monolayers that have negative curvature (shown in magnified membrane region). Phospholipids with diunsaturated linoleic acid chains selectively enrich in negatively curved monolayers. The selective action of tafazzin on those highly curved regions results in enrichment of the cardiolipin fraction with linoleic acid chains.
In the previous characterization of tafazzin, the reasons for specific enrichment of mitochondria with tetralinoleoyl-cardiolipin remained mysterious: contrary to expectations the extracted protein was shown to transacylate all phospholipids and lysolipids, regardless of their polar head groups or hydrophobic hydrocarbon chains3,6. Tafazzin even transfers acyl chains to the sn-1 and sn-2 positions of glycerol in phospholipids with equal probability9. What is then the mechanism responsible for the specific enrichment?
In elegant new experiments, Schlame et al.3 combine radiolabeling, MS and 31P-NMR of various lipid compositions before and after treatment with tafazzin to investigate a ‘thermodynamic remodeling’ hypothesis-the idea that not only chemical identity but also the physical properties of the lipid domain affect tafazzin function. In particular, phospholipids whose polar head group has a relatively small volume, such as phosphatidylethanolamine and cardiolipin, and unsaturated hydrocarbon chains, such as linoleic acid, have a shape asymmetry that leads to their accumulation in negatively curved lipid monolayers. Indeed, the formation of cardiolipin microdomains in negatively curved regions of Escherichia coli membranes was confirmed experimentally10.
This enrichment may even trigger a transition to an inverse hexagonal lipid phase (HII) consisting of inverted cylindrical micelles that have a water-filled, polar core. The micelles are packed into a hexagonal lattice, which gives the phase its name. For cardiolipin, HII phase formation is triggered by addition of divalent cations and can easily be detected by solid-state 31P-NMR. Schlame et al.3 observed that partial conversion of cardiolipin-containing lipid-water dispersions to an HII phase, as monitored by NMR, correlated with tafazzin-induced enrichment of cardiolipin with linoleoyl hydrocarbon chains, as detected by MS. Further work will be needed to determine whether the HII phase itself is the site of tafazzin action or whether the enzyme acts at the transition structures between lamellar and HII phases that also have negatively curved lipid monolayers and may be more easily accessible for the peripheral protein. The selective enrichment in tetralinoleoyl-cardiolipin by tafazzin suggests that lipids with the diunsaturated linoeoyl chains enrich specifically in those highly curved membrane regions.
The tafazzin story is an excellent example of how the search for a locus of a genetic disorder stimulated biochemical and biophysical investigations that linked the disease to the consequences of malfunction of a phospholipid-lysophospholipid transacylase, the lack of tetralinoleoyl-cardiolipin in mitochondrial inner membranes and the impairment of mitochondrial function. This remarkable depth of understanding of the molecular mechanisms of Barth syndrome pathogenesis has already resulted in easier diagnosis and earlier treatment of the disorder and also points at opportunities for basic research to develop new treatments. Tafazzin joins the ranks of proteins, such as protein kinase C, whose membrane association and function are influenced by lipids that prefer to be located in monolayers with negative curvature. Negative curvature packing stress was also identified as major cofactor in the formation of metarhodopsin-II, the photointermediate of the G protein–coupled membrane receptor rhodopsin, which activates the G protein transducin. Considering that phosphatidylethanolamines are very common phospholipids, it is almost certain that more proteins will be found whose function is regulated by negative curvature in lipid monolayers.
Footnotes
Competing financial interests
The author declares no competing financial interests.
References
- 1.Barth PG, et al. J Neurol Sci. 1983;62:327–355. doi: 10.1016/0022-510x(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 2.Bione S, et al. Nat Genet. 1996;12:385–389. doi: 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 3.Schlame M, et al. Nat Chem Biol. 2012;8:xxx. doi: 10.1038/nchembio.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claypool SM, McCaffery JM, Koehler CM. J Cell Biol. 2006;174:379–390. doi: 10.1083/jcb.200605043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuwald AF. Curr Biol. 1997;7:R465–R466. doi: 10.1016/s0960-9822(06)00237-5. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Malhotra A, Ren MD, Schlame M. J Biol Chem. 2006;281:39217–39224. doi: 10.1074/jbc.M606100200. [DOI] [PubMed] [Google Scholar]
- 7.Schlame M, et al. Ann Neurol. 2002;51:634–637. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- 8.Acehan D, et al. Mitochondrion. 2009;9:86–95. doi: 10.1016/j.mito.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malhotra A, Xu Y, Ren M, Schlame M. Biochim Biophys Acta. 2009;1791:314–320. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renner LD, Weibel DB. Proc Natl Acad Sci USA. 2011;108:6264–6269. doi: 10.1073/pnas.1015757108. [DOI] [PMC free article] [PubMed] [Google Scholar]