Abstract
MicroRNAs are known to contribute significantly to stem cell phenotype by post-transcriptionally regulating gene expression. Most of our knowledge of microRNAs comes from the study of canonical microRNAs that require two sequential cleavages by the Drosha/Dgcr8 heterodimer and Dicer to generate mature products. In contrast, non-canonical microRNAs bypass the cleavage by the Drosha/Dgcr8 heterodimer within the nucleus but still require cytoplasmic cleavage by Dicer. The function of non-canonical microRNAs in embryonic stem cells (ESCs) remains obscure. It has been hypothesized that non-canonical microRNAs have important roles in ESCs based upon the phenotypes of ESC lines that lack these specific classes of microRNAs; Dicer-deficient ESCs lacking both canonical and non-canonical microRNAs have much more severe proliferation defect than Dgcr8-deficient ESCs lacking only canonical microRNAs. Using these cell lines, we identified two non-canonical microRNAs, miR-320 and miR-702, that promote proliferation of Dgcr8-deficient ESCs by releasing them from G1 arrest. This is accomplished by targeting the 3′-untranslated regions of the cell cycle inhibitors p57 and p21 and thereby inhibiting their expression. This is the first report of the crucial role of non-canonical microRNAs in ESCs.
Keywords: miRNA, Dicer, Dgcr8, proliferation, miR-320, miR-702
INTRODUCTION
Initially, all microRNAs (miRNAs) were thought to require processing by two different RNase III-containing enzymes, Drosha and Dicer. These “canonical” miRNAs first undergo a cleavage step within the nucleus by the Microprocessor complex that contains the enzyme Drosha and the indispensable double stranded RNA binding protein Dgcr8 to convert the primary miRNA (pri-miRNA) transcript into the precursor miRNA (pre-miRNA) [1–3]. The second cleavage step occurs in the cytoplasm by Dicer to release from the pre-miRNA the functional, final miRNA product that is usually 22 nucleotides in length [4, 5]. More recently, however, less abundant “non-canonical” miRNAs that do not require the initial cleavage step by the Microprocessor complex have been discovered [6]. Although non-canonical miRNAs do not need processing by the Drosha/Dgcr8 heterodimer, they still require Dicer cleavage in the cytoplasm. Compared to canonical miRNAs, the function of non-canonical miRNAs is much less clear, especially in ESCs.
The critical roles that miRNAs play in early development and ESCs are well established. Both Dicer-deficient and Dgcr8-deficient mouse embryos start to arrest prior to embryonic day (E) 7.5 [7–9]. Furthermore, miRNAs are essential for dedifferentiation reprogramming [10]. Remarkably, Dicer-deficient ESCs have been isolated in multiple labs [11, 12]. Although these cells which lack both canonical and non-canonical miRNAs are able to indefinitely expand and to express ESC-specific markers, they have profound proliferation and differentiation defects. However, Dgcr8-deficient ESCs which lack only canonical miRNAs exhibit a less severe phenotype with respect to proliferation and differentiation when compared to Dicer-deficient ESCs [9] (Fig 1A). We hypothesized that this difference may be due to non-canonical miRNAs that are present in Dgcr8-deficient ESCs but absent in Dicer-deficient ESCs. We focused on the proliferation phenotype of Dicer-deficient ESCs and searched for uncharacterized non-canonical miRNAs that confer a proliferative advantage in Dgcr8-deficient ESCs over Dicer-deficient ESCs.
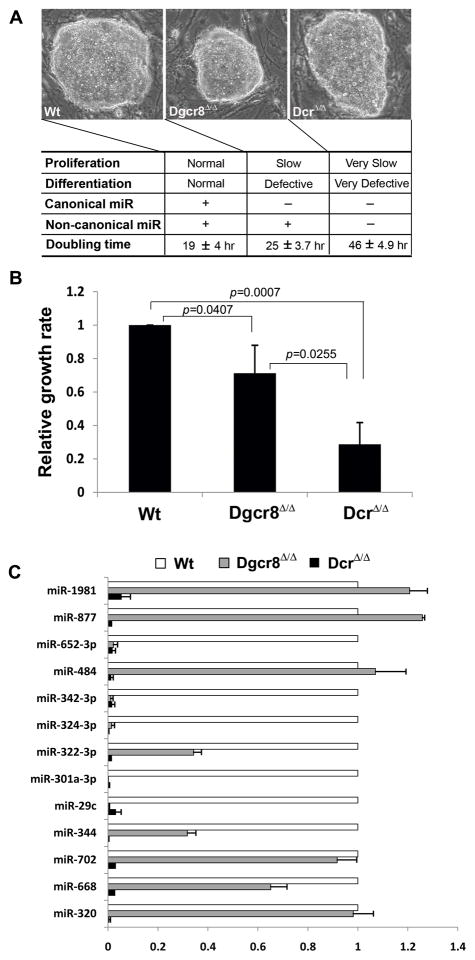
Figure 1. Dicer-deficient ESCs proliferate slower than Dgcr8-deficient ESCs.
(A) Dgcr8- deficient (Dgcr8Δ/Δ) ESCs which lack canonical miRNAs have a differentiation defect and slower proliferation compared to wild-type (Wt) ESCs. Dicer-deficient (DcrΔ/Δ) ESCs which lack both canonical and non-canonical miRNAs have even more extreme differentiation and proliferation defects. (B) Proliferation rate of three ESC lines was measured with the MTS assay which shows different proliferation phenotypes. Each value is represented relative to an assigned wild-type value of 1.0. Data are presented as mean +/− SD with N=3. (C) Non-canonical miRNA expression levels of the mature form in wild-type, Dgcr8-deficient, and Dicer-deficient ESC lines measured with qRT-PCR. Dgcr8-deficient ESCs expressed significant levels of most non-canonical miRNAs tested, while Dicer-deficient ESCs expressed none in any significant amount. Each value is represented relative to an assigned wild-type value of 1.0 for that miRNA. Data are presented as mean +/− SD with N=3.
Certain canonical miRNAs such ESC-cell cycle regulating (ESCC) miRNAs which include the miR-290 and miR-302 clusters have already been implicated in promoting ESC proliferation [13]. ESCC miRNAs are found to enhance proliferation by targeting Cyclin E/Cdk2 complex inhibitors such as p21 [13]. Yet uncharacterized non-canonical miRNAs may have a similar proliferative function in ESCs. Indeed, we identified two non-canonical miRNAs, miR-320 and miR-702, that function as promoters of proliferation in Dgcr8-deficient ESCs by releasing them from G1 arrest and promote proliferation by targeting the cell cycle inhibitors p57 and p21, respectively. The function of these two miRNAs has not been described in ESCs, and this is the first time that non-canonical miRNAs have been implicated in the regulation of proliferation in ESCs.
MATERIALS AND METHODS
Animal and Cell Culture
MEFs were prepared from E13.5 Dicerf/f embryos and TTFs from Dicerf/f adult mice [14] and cultured in DMEM containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1× nonessential amino acids, and 0.1 mM 2-mercaptoethanol (Invitrogen). Three mouse ESC lines, a germline-competent wild-type (W4), Dgcr8-deficient (Dgcr8Δ/Δ) [9], and Dicer-deficient (DicerΔ/Δ) [12], were cultured on irradiated MEFs in serum-containing ESC medium, DMEM with 15% FBS, 2 mM L-glutamine, 1× nonessential amino acids, 0.1 mM 2-mercaptoethanol, and 1,000 U/ml leukemia inhibitory factor (Chemicon).
Transfection and Cell Growth Rate
Wild-type, Dgcr8Δ/Δ and DicerΔ/Δ ESCs were plated in gelatinized 12-well plates on day 0 in leukemia inhibitory factor media. On day 1, miRIDIAN miRNA mimics or hairpin inhibitors (Dharmacon) were transfected at a concentration of 50–100 nM using Dharmafect1 (Dharmacon) following the manufacturer’s protocol. Media was changed daily. Cell growth rate was performed using cell proliferation assay kit (Promega) according to the manufacturer’s instructions. To remove functional Dicer, MEFs or TTFs were treated with Adeno-Cre virus at a multiplicity of infection (MOI) of ~100, (University of Iowa) before miRNA mimic transfection. Medium was replaced with MTS media and incubated at 37 °C for 3 hours. Absorbance was recorded at 490 nm.
Quantitative RT-PCR and Western Blot Analysis
Total RNA was extracted by using TriZol reagent (Invitrogen) and treated with RNase-free DNase (Ambion). For the miRNA qPCR, RNA was polyadenylated using ATP and poly (A) polymerase (Ambion) and reverse-transcribed using oligo-dT adapter by SuperScript III (Invitrogen) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using primers listed in Supplemental Table S1 on the CFX96 Real-Time System (Bio-Rad). Expression of individual transcripts was normalized to U6 snRNA and 18S RNA for miRNA and Gapdh expression for cell cycle inhibitors. Protein blots were analyzed using antibodies to p21 (1:500, BD), p27 (1:1000, BD), p57 (1:1000, Abcam), and alpha-tubulin (1:2000, Abcam).
Flow Cytometry
Single-cell suspension was prepared by filtering through a 30-um strainer and fixing in ethanol. After treating with 10 mg/ml of RNase A, cells were labeled with 10 ug/ml of propidium iodine (Sigma), followed by washing in PBS, and analyzed by flow cytometry. Flow cytometry was performed on a Becton Dickinson FACScan and the data were analyzed using FlowJo software (Tree Star).
Luciferase reporter assay
We cloned wild-type and mutated p21 and p57 3′-untranslated regions (UTRs) by PCR from the cDNA of reverse-transcribed wild-type ESC mRNA. PCR products were subcloned to pmirGLO vector (Promega) and sequenced. Sequences of primers are listed in the supporting information (Supplemental Table S1). 293 cells were plated and grown for at least 16 hours before transfection. For transfection experiments, we transfected 100 nM of miRNA mimics or inhibitors using Dharmafect1 (Dharmacon) and luciferase reporters using Lipofectamin 2000 (Invitrogen). Cells were lysed 12–16 hours later and processed for luciferase assay using Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was measured by VICTOR X5 (Perkin Elmer). Data are presented as normalized ratios of firefly:Renilla luciferase.
RESULTS
Dicer-deficient ESCs proliferate slower than Dgcr8-deficient ESCs
The nuclear Drosha/Dgcr8 heterodimer processes canonical miRNAs but not non- canonical miRNAs. The Dicer RNase enzyme, in contrast, processes both canonical and non-canonical miRNAs since the Dicer-mediated cleavage of pre-miRNAs to generate mature miRNAs is the final processing step that is required by almost all miRNAs [6]. Dgcr8-deficient (Dgcr8Δ/Δ) ESCs lack only canonical miRNAs and have a phenotype of slow proliferation and defective differentiation [9]. However, Dicer-deficient (DicerΔ/Δ) ESCs lack both canonical and non-canonical miRNAs, and their phenotype is more severe than that of Dgcr8-null ESCs [11, 12], suggesting that non-canonical miRNAs also play a role in ESC proliferation and differentiation (Fig 1A). One measure of proliferation, the doubling times for wild-type, Dgcr8-deficient, and Dicer-deficient ESCs were 19 hours (standard deviation (SD) 4.0 hours), 25 hours (SD 3.7 hours), and 46 hours (SD 4.9 hours), respectively (Fig 1A). Based on the MTS assay, Dgcr8-deficient ESCs proliferated 29% slower than wild-type ESCs, while Dicer-deficient ESCs proliferated 59% slower than Dgcr8-deficient ESCs (Fig 1B). This difference in proliferation rate between Dgcr8- and Dicer-deficient ESCs is likely due to non-canonical miRNAs present only in Dgcr8-deficient ESCs.
Most miRNAs are canonical, and non-canonical miRNAs constitute only a small fraction of total miRNAs [15]. To identify non-canonical miRNAs that endow a proliferative advantage in Dgcr8-deficient ESCs over Dicer-deficient ESCs, we compiled a list of non-canonical miRNAs already published in the literature [6, 16]. We performed reverse transcription-quantitative polymerase chain reaction (qRT-PCR) to measure the expression levels of mature, non-canonical miRNAs in wild-type, Dgcr8-deficient, and Dicer-deficient ESC lines (Fig 1C). As expected, Dgcr8-deficient ESCs expressed significant levels of most non-canonical miRNAs tested, while Dicer-deficient ESCs expressed none in any significant amount, supporting the model that these non-canonical miRNAs require processing by Dicer but not the Microprocessor. We reasoned that only those non-canonical miRNAs that are significantly expressed in Dgcr8-deficient ESCs are viable candidates that endow these cells with proliferative advantage over Dicer-deficient ESCs.
Forced expression of non-canonical microRNAs miR-320 and miR-702 in Dicer-deficient ESCs promotes proliferation
To identify individual non-canonical miRNAs that have pro-proliferative function in ESCs, we introduced mimics of those non-canonical miRNAs that are significantly expressed in Dgcr8-deficient ESCs into Dicer-deficient ESCs, and assessed for growth promoting effects. miRNA mimics are analogous to endogenous, mature miRNAs, and can be used in Dicer-deficient ESCs because they do not require any further processing by Dicer. Once transfected individually into Dicer-deficient ESCs, two candidates, miR-320 and miR-702, increased their proliferation rate to that of Dgcr8-deficient ESCs, rescuing the severe proliferation defect (Fig 2A). Hence, we focused our investigation on these two miRNAs.
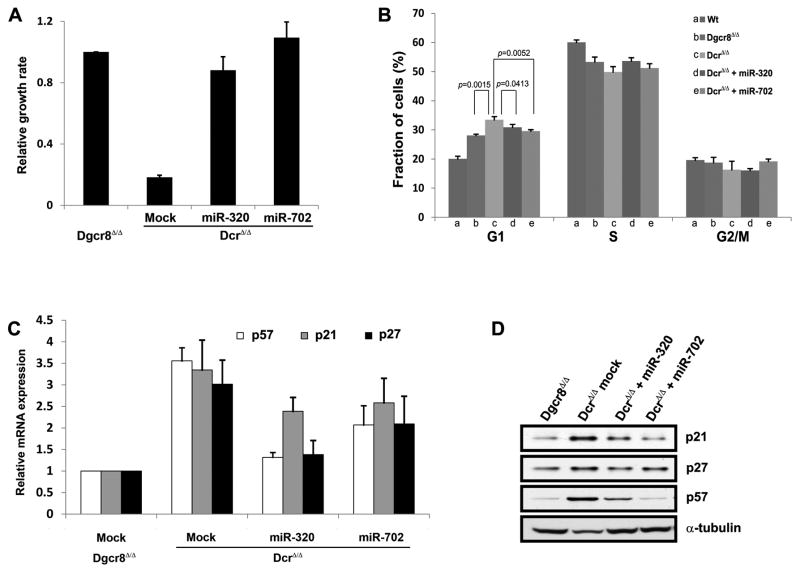
Figure 2. Forced expression of non-canonical microRNAs miR-320 and miR-702 in Dicer- deficient ESCs promotes proliferation.
(A) miR-320 and miR-702 increased the proliferation rate of Dicer-deficient (DcrΔ/Δ) ESCs to that of Dgcr8-deficient (Dgcr8Δ/Δ) ESCs, rescuing the severe proliferation defect. Each value is represented relative to an assigned Dgcr8Δ/Δ value of 1.0. Data are presented as mean +/− SD with N=3. (B) Dgcr8-deficient ESCs accumulated in the G1 phase of the cell cycle compared to wild-type (Wt) ESCs. Dicer-deficient ESCs accumulated in the G1 phase even more than Dgcr8-deficient ESCs. Transducing miR-320 or miR-702 mimic into Dicer-deficient ESCs decreased the proportion of cells in G1. Data are presented as mean +/− SD with N=5. (C, D) Expression levels determined by qRT-PCR (C) and immunoblot analysis (D) of cyclin-dependent kinase inhibitors p21, p27, and p57 demonstrate upregulation in Dicer-deficient ESCs compared to Dgcr8-deficient ESCs. With the introduction of miR-320 or miR-702 into Dicer-deficient ESCs, mRNA levels of all three cyclin-dependent kinase inhibitors showed decreasing trend (C), while only p21 and p57 protein levels were clearly downregulated (D). α-tubulin expression level was used as loading control for the protein blot. For (C), each value is represented relative to an assigned Dgcr8Δ/Δ values of 1.0, and data are presented as mean +/− SD with N=3.
Unlike other cell types, wild-type ESCs can proliferate more rapidly because they have a shortened G1 phase and absence of the G1/S checkpoint [17]. However, in accordance with published results, Dgcr8-deficient ESCs accumulated in the G1 phase of the cell cycle compared to wild-type ESCs (Fig 2B). This phenotype is at least partially due to lack of ESCC miRNAs comprised of the miR-290 cluster that promote proliferation and cell cycle progression [9]. Consistent with their extreme proliferation defect, Dicer-deficient ESCs accumulated in the G1 phase even more than Dgcr8-deficient ESCs (33% vs 28%, respectively) (Fig 2B). To test whether miR-320 and miR-702 rescue proliferation by reducing the fraction of Dicer-deficient ESCs in G1, we transduced each mimic into Dicer-deficient ESCs. Both miRNAs decreased the proportion of cells in G1 and increased the proportion in the S phase (Fig 2B). This result suggests that miR-320 and miR-702 promote ESC proliferation by facilitating the G1 to S phase transition of these cells.
Since miRNAs are repressors of gene expression, we hypothesized that miR-320 and miR-702 promote the G1-S transition by targeting its key inhibitors. To test this hypothesis, we measured expression levels of well known inhibitors of the G1-S transition after miR-320 or miR-702 mimic was transduced into Dicer-deficient ESCs. The cyclin D-Cdk4,6 and cyclin E- Cdk2 complexes are the two main regulators of the G1-S transition, but only the cyclin E-Cdk2 complex functions in mouse ESCs [17, 18]. Mouse ESCs have an unrestricted G1 to S phase transition mediated by the constitutively active cyclin E-Cdk2 complex. Three cyclin-dependent kinase inhibitors of the Cip/Kip family, Cdkn1a (p21), Cdkn1b (p27), and Cdkn1c (p57), respectively, modulate the activity of the cyclin E-Cdk2 complex [19]. As expected, p21, p27, and p57 mRNA and protein levels were upregulated in Dicer-deficient ESCs compared to Dgcr8-deficient ESCs (Fig 2C, D), consistent with a slower growth rate in Dicer-deficient ESCs. We then introduced miR-320 or miR-702 into Dicer-deficient ESCs and measured its effect on the expression levels of p21, p27, and p57. p21 and p57 protein levels were clearly downregulated with the transduction of miR-320 or miR-702 into Dicer-deficient ESCs, though a trend towards lower levels of p27 mRNA was also seen (Fig 2D).
miR-320 and miR-702 directly target p57 and p21, respectively
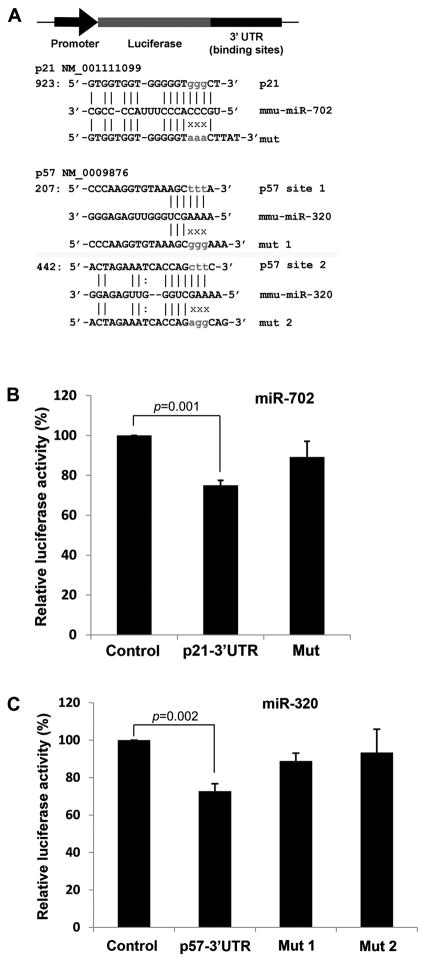
miR-320 and miR-702 reduce G1 accumulation and promote proliferation by downregulating p21 and p57 in ESCs, and we sought to determine whether they directly target p21 and p57. The computational programs Miranda and Targetscan identified p21 and p57 as predicted targets for miR-702 and miR-320, respectively [20, 21]. (Fig 3A). However, p27 was not a predicted target for either miR-702 or miR-320. To experimentally ascertain whether miR-702 and miR-320 target p21 and p57, we constructed luciferase reporters that test direct binding of miRNA to predicted target sequences within the 3′-UTR. We cloned the 3′-UTR of p21 or p57 downstream of a constitutively expressed firefly luciferase (Fig 3A). When the miR-702 mimic was co-transfected with the p21 reporter construct, luciferase activity was inhibited by 25% compared to the reporter construct alone, suggesting that miR-702 directly binds to the predicted target sequence within the 3′-UTR of p21 (Fig 3B). When we performed this experiment using a reporter construct with a mutation in the predicted target sequence, luciferase activity decreased only 11% (Fig 3A, B). Similar luciferase reporter experiments were performed with miR-320 and the 3′-UTR of p57. Again, co-transfecting miR-320 with the p57 reporter construct suppressed luciferase activity by 30% (Fig 3C). The 3′-UTR of p57 contains two predicted target sequences of miR-320, and mutating either sequence significantly increased luciferase activity, further supporting direct binding of miR-320 to either of these predicted sequences (Fig 3A, C). Of note, mutating both sequences did not further enhance luciferase activity, indicating that these target sequences have redundant function and miRNA-320 binding to either one has a similar but not additive effect.
Figure 3. miR-320 and miR-702 directly target p57 and p21, respectively.
(A) The computational programs identified p21 and p57 as predicted targets for miR-702 and miR-320, respectively. Luciferase reporters that test direct binding of miRNA to predicted target sequences within the 3′-UTR were constructed by cloning the 3′-UTR of p21 or p57 downstream of a constitutively expressed firefly luciferase. Reporters with a mutation (mut) in the predicted target sequence were also constructed. (B) When the miR-702 mimic was co-transfected with the p21 reporter construct, luciferase activity was inhibited by 25% compared to the reporter construct alone (Control). When this experiment was performed using the reporter construct with a mutation in the predicted target sequence, luciferase activity decreased by only 11%. Each value is represented relative to an assigned control value of 100%. Data are presented as mean +/− SD with N=5. (C) Similar luciferase reporter experiments were performed with miR-320 and the 3′-UTR of p57. Co-transfecting miR-320 with the p57 reporter construct suppressed luciferase activity by 30%. The 3′ UTR of p57 contains two predicted target sequences of miR- 320, and mutating either sequence significantly increased luciferase activity, further supporting direct binding of miR-320 to either of these predicted sequences. Each value is represented relative to an assigned control value of 100%. Data are presented as mean +/− SD with N=5.
Inhibitors of miR-320 and miR-702 suppress proliferation of Dgcr8-deficient ESCs
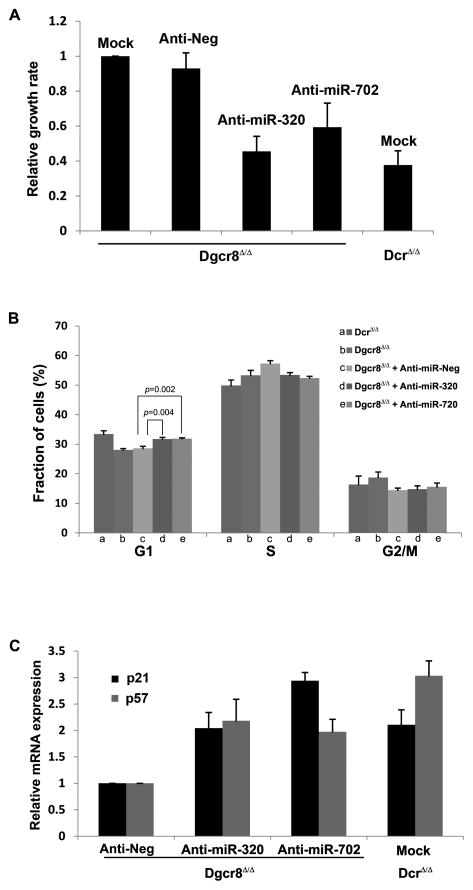
To further corroborate the function of miR-320 and miR-702 in ESCs, we performed a set of “reverse” experiments using inhibitors of miR-320 and miR-702 that suppress miRNA activity to study the loss-of-function effects. When we transfected anti-miR-320 or anti-miR-702 into Dgcr8-deficient ESCs, their proliferation rate declined significantly whereas negative control anti-miR did not decrease the growth rate (Fig 4A).
Figure 4. Inhibitors of miR-320 and miR-702 suppress proliferation of Dgcr8-deficient ESCs.
(A) When we transfected anti-miR-320 or anti-miR-702 into Dgcr8-deficient ESCs, their proliferation rate declined significantly whereas transfecting negative control anti-miR did not decrease the growth rate compared to mock-transfection. Each value is represented relative to an assigned Dgcr8Δ/Δ mock transfection value of 1.0. Data are presented as mean +/− SD with N=3. (B) Cell cycle analysis of Dgcr8-deficient ESCs transfected with either anti-miR-320 or anti-miR-702 showed increased cellular fraction in the G1 phase, implicating miR-320 and miR-702 as promoters of ESC proliferation that facilitate the G1 to S phase transition. Data are presented as mean +/− SD with N=5. (C) mRNA expression levels of p57 and p21 increased when anti-miR-320 or anti-miR-702 was transduced into Dgcr8-deficient ESCs. Although miR-320 has been shown to directly target only p57 and miR-702 only p21, p57 expression increased with the introduction of anti-miR-702, and p21 expression increased with anti-miR-320. This effect likely stems from either indirect regulation of cyclin-dependent kinase inhibitors by miRNAs or direct regulation through unrecognized target sequences within the cyclin-dependent kinase inhibitor mRNA. Each value is represented relative to an assigned Dgcr8Δ/Δ anti-negative control value of 1.0. Data are presented as mean +/− SD with N=3.
We next performed cell cycle analysis of Dgcr8-deficient ESCs transfected with anti-miR-320 or anti-miR-702. Supporting our earlier data using miRNA mimics, introducing either inhibitor into Dgcr8-deficient ESCs increased the cellular fraction in the G1 phase (Fig 4B), again indicating that miR-320 and miR-702 facilitate the G1 to S phase transition. Although the magnitude of increase was relatively small (3%), this change was consistent and statistically significant.
Finally, we measured the expression levels of p57 and p21 when anti-miR-320 or anti-miR-702 was transduced into Dgcr8-deficient ESCs. Consistent with previous results, p57 and p21 expression levels increased with the introduction of miRNA inhibitors (Fig 4C). Although miR-320 has been shown to directly target only p57 and miR-702 only p21, the expression data from both miRNA mimic and inhibitor studies demonstrate changes in the expression levels of non-targeted cyclin-dependent kinase inhibitors (Fig 2C, D; 4C). For example, the introduction of anti-miR-702 into Dgcr8-deficient ESCs increased the expression level of not only p21, but also p57 which does not contain any known miR-702 target sequence in the 3′-UTR (Fig 4C). This phenomenon may represent either an indirect regulation of cyclin-dependent kinase inhibitors by proliferation promoting miRNAs or a direct regulation through unrecognized target sequences within the cyclin-dependent kinase inhibitor mRNA.
miR-320 and miR-702 do not have pro-proliferative effect in mouse embryonic fibroblasts or adult tail-tip fibroblasts
We have demonstrated that miR-320 and miR-702 promote proliferation and relieve G1 accumulation of ESCs by directly inhibiting the expression of p57 and p21, respectively. However, it is not clear whether their role is ESC-specific or is generalizable to other cell types. Thus, we tested the effects of miR-320 and miR-702 in Dicer-deficient mouse embryonic fibroblasts (MEFs) and adult tail-tip fibroblasts (TTFs). These cells were derived by applying Cre recombinase to appropriate tissues harvested from conditional Dicer knockout mice that have key Dicer exons flanked with lox P sites [8]. Unlike ESCs, the growth rate of Dicer-deficient MEFs and TTFs did not change much with the introduction of miR-320 (Supplemental Fig S1A). Similarly, miR-702 did not increase the proliferation rate of Dicer-deficient TTFs although it did slightly enhance proliferation of Dicer-deficient MEFs by 13%. Consistent with the lack of significant growth promoting effect on Dicer-deficient MEFs and TTFs, miR-320 and miR-702 did not repress expression levels of p57 and p21 in these cell types (Supplemental Fig S1B, C).
DISCUSSION
Unlike canonical miRNAs that require two sequential cleavages by RNase enzymes Drosha and Dicer for proper processing, more recently identified non-canonical miRNAs only need the Dicer cleavage. Despite our understanding of their biogenesis, non-canonical miRNAs’ function in ESCs is almost completely unknown. One of the strongest pieces of evidence that non-canonical miRNAs play a crucial role in ESCs is that Dicer-null ESCs lacking both canonical and non-canonical miRNAs have more severe phenotypes than Dgcr8-null ESCs lacking only canonical miRNAs [9]. Our study for the first time identifies specific non-canonical miRNAs that function in ESC proliferation and the G1 to S phase transition. Until now, the role of miR-320 and miR-702 in ESCs has been unknown. Our study suggest that miR-320 and miR-702 promote Dgcr8-deficient ESC proliferation by inhibiting the expression of p57 and p21, respectively, and releasing the cells from the G1 phase. However, it is likely that other miRNAs, including ESCC miRNAs, also target cell cycle inhibitors to promote proliferation [13]. Most mRNAs have conserved target sites for multiple miRNAs which would enable combinatorial control of these messages [22]. To maintain cell cycle kinetics with a shortened G1 phase and an unrestricted G1 to S phase transition, ESCs appear to utilize redundancy with multiple miRNAs, both canonical and non-canonical, to repress key cell cycle inhibitors.
Finally, the proliferation promoting function of miR-320 and miR-702 seems to be specific to ESCs and does not extend to MEFs or TTFs. In fact, a recent report describes a very different role of miR-320 in fibroblasts; it functions as a critical component of the Pten tumor suppressor axis that acts in stromal fibroblasts [23]. Different functions in different cell types are not surprising given that miRNAs are capable of targeting multiple genes, and their role is likely context-dependent.
Supplementary Material
Highlights.
Embryonic stem cells (ESCs) lacking non-canonical miRNAs proliferate slower.
miR-320 and miR-702 are two non-canonical miRNAs expressed in ESCs.
miR-320 and miR-702 promote proliferation of Dgcr8-deficient ESCs.
miR-320 targets p57 and helps to release Dgcr8-deficient ESCs from G1 arrest.
miR-702 targets p21 and helps to release Dgcr8-deficient ESCs from G1 arrest.
Acknowledgments
We thank Drs. Sangnam Oh and Joo-Hye Song for FACS analysis. The research was supported by NIH grant to M.Y.C (K08 DK078641).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 2.Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 3.Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 4.Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 6.Babiarz JE, Ruby JG, Wang Y, Bartel DP, Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other Microprocessor-independent, Dicer-dependent small RNAs. Genes Dev. 2008;22:2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 8.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci U S A. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BM, Thier MC, Oh S, Sherwood R, Kanellopoulou C, Edenhofer F, Choi MY. MicroRNAs Are Indispensable for Reprogramming Mouse Embryonic Fibroblasts into Induced Stem Cell-Like Cells. PLoS One. 2012;7:e39239. doi: 10.1371/journal.pone.0039239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Baskerville S, Shenoy A, Babiarz JE, Baehner L, Blelloch R. Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet. 2008;40:1478–1483. doi: 10.1038/ng.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15:410–415. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892–903. doi: 10.1016/j.molcel.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babiarz JE, Hsu R, Melton C, Thomas M, Ullian EM, Blelloch R. A role for noncanonical microRNAs in the mammalian brain revealed by phenotypic differences in Dgcr8 versus Dicer1 knockouts and small RNA sequencing. Rna. 2011;17:1489–1501. doi: 10.1261/rna.2442211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 18.Savatier P, Lapillonne H, van Grunsven LA, Rudkin BB, Samarut J. Withdrawal of differentiation inhibitory activity/leukemia inhibitory factor up-regulates D-type cyclins and cyclin-dependent kinase inhibitors in mouse embryonic stem cells. Oncogene. 1996;12:309–322. [PubMed] [Google Scholar]
- 19.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 20.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 22.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ, Martin CK, Li F, Yu L, Fernandez SA, Pecot T, Rosol TJ, Cory S, Hallett M, Park M, Piper MG, Marsh CB, Yee LD, Jimenez RE, Nuovo G, Lawler SE, Chiocca EA, Leone G, Ostrowski MC. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.