Abstract
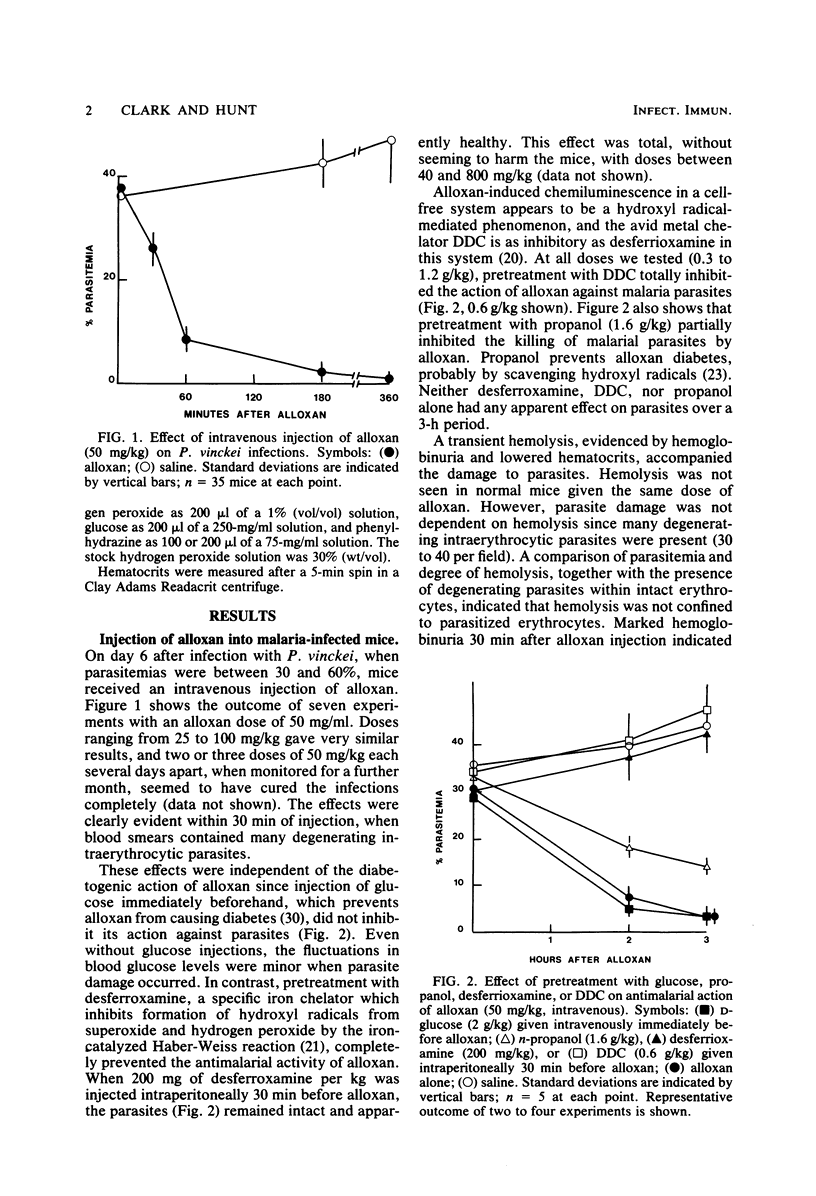
A rapid reduction in parasitemia associated with damage to intraerythrocytic parasites was observed in Plasmodium vinckei-infected mice after they had received a single intravenous injection of alloxan. This was not prevented by prior injection of glucose, but was prevented by desferrioxamine or diethyldithiocarbamate. Prior injection of propanol partially blocked the phenomenon. A transient hemolysis was observed in malaria-infected mice, but not in controls, after injection of alloxan. This was also blocked by desferrioxamine, but not by glucose. Both the fall in parasitemia and hemolysis occurred, but less dramatically, when phenylhydrazine or hydrogen peroxide was injected into parasitized mice. Again, the hemolysis was blocked by desferrioxamine. These observations are consistent with the parasite death and hemolysis being mediated by reactive oxygen species, possibly hydroxyl radicals, and have implications for our understanding of hemolysis, endothelial damage, and parasite suppression in acute malaria. Our evidence that malaria parasites are susceptible to free oxygen radicals supports the view that high intraerythrocytic oxidative stress may contribute to the high frequencies in malarial areas of genes for certain erythrocyte-related traits and suggests that some antimalarial drugs may suppress parasites partly through oxidative damage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVING A. S., CARSON P. E., FLANAGAN C. L., ICKES C. E. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science. 1956 Sep 14;124(3220):484–485. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L. J. Qinghaosu: a new antimalarial. Br Med J (Clin Res Ed) 1982 Mar 13;284(6318):767–768. doi: 10.1136/bmj.284.6318.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Does endotoxin cause both the disease and parasite death in acute malaria and babesiosis? Lancet. 1978 Jul 8;2(8080):75–77. doi: 10.1016/s0140-6736(78)91386-7. [DOI] [PubMed] [Google Scholar]
- Clark I. A. Thromboxane may be important in the organ damage and hypotension of malaria. Med Hypotheses. 1981 May;7(5):625–631. doi: 10.1016/0306-9877(81)90007-4. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- Das S. K., Nair R. C. Superoxide dismutase, glutathione peroxidase, catalase and lipid peroxidation of normal and sickled erythrocytes. Br J Haematol. 1980 Jan;44(1):87–92. doi: 10.1111/j.1365-2141.1980.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Degowin R. L., Eppes R. B., Powell R. D., Carson P. E. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bull World Health Organ. 1966;35(2):165–179. [PMC free article] [PubMed] [Google Scholar]
- Fee J. A., Teitelbaum H. D. Evidence that superoxide dismutase plays a role in protecting red blood cells against peroxidative hemolysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):150–158. doi: 10.1016/0006-291x(72)90022-8. [DOI] [PubMed] [Google Scholar]
- Fein I. A., Rackow E. C., Shapiro L. Acute pulmonary edema in Plasmodium falciparum malaria. Am Rev Respir Dis. 1978 Aug;118(2):425–429. doi: 10.1164/arrd.1978.118.2.425. [DOI] [PubMed] [Google Scholar]
- Fischer L. J., Hamburger S. A. Inhibition of alloxan action in isolated pancreatic islets by superoxide dismutase, catalase, and a metal chelator. Diabetes. 1980 Mar;29(3):213–216. doi: 10.2337/diab.29.3.213. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Oxidant damage mediates variant red cell resistance to malaria. Nature. 1979 Jul 19;280(5719):245–247. doi: 10.1038/280245a0. [DOI] [PubMed] [Google Scholar]
- Frischer H., Bowman J. Hemoglobin E, an oxidatively unstable mutation. J Lab Clin Med. 1975 Apr;85(4):531–539. [PubMed] [Google Scholar]
- Goldberg B., Stern A., Peisach J. The mechanism of superoxide anion generation by the interaction of phenylhydrazine with hemoglobin. J Biol Chem. 1976 May 25;251(10):3045–3051. [PubMed] [Google Scholar]
- Goldstein B. D., McDonagh E. M. Spectrofluorescent detection of in vivo red cell lipid peroxidation in patients treated with diaminodiphenylsulfone. J Clin Invest. 1976 May;57(5):1302–1307. doi: 10.1172/JCI108398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K. Alloxan-induced luminol luminescence as a tool for investigating mechanisms of radical-mediated diabetogenicity. Biochem J. 1981 Dec 15;200(3):685–690. doi: 10.1042/bj2000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Sehlin J., Täljedal I. B. Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic action of alloxan on pancreatic islet cells in vitro. Biochem J. 1979 Jul 15;182(1):17–25. doi: 10.1042/bj1820017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S. The prevention of alloxan-induced diabetes in mice by the iron-chelator detapac: suggestion of a role for iron in the cytotoxic process. Experientia. 1982 Mar 15;38(3):378–379. doi: 10.1007/BF01949404. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Winston B., Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976 May 1;25(9):1085–1092. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- Kistler G. S., Caldwell P. R., Weibel E. R. Development of fine structural damage to alveolar and capillary lining cells in oxygen-poisoned rat lungs. J Cell Biol. 1967 Mar;32(3):605–628. doi: 10.1083/jcb.32.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Effects of superoxide on the erythrocyte membrane. J Biol Chem. 1978 Mar 25;253(6):1838–1845. [PubMed] [Google Scholar]
- Lynch R. E., Fridovich I. Permeation of the erythrocyte stroma by superoxide radical. J Biol Chem. 1978 Jul 10;253(13):4697–4699. [PubMed] [Google Scholar]
- MOTULSKY A. G. Metabolic polymorphisms and the role of infectious diseases in human evolution. Hum Biol. 1960 Feb;32:28–62. [PubMed] [Google Scholar]
- McDaniel M. L., Roth C. E., Fink C. J., Lacy P. E. Effect of anomers of D-glucose on alloxan inhibition of insulin release in isolated perifused pancreatic islets. Endocrinology. 1976 Aug;99(2):535–540. doi: 10.1210/endo-99-2-535. [DOI] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972 Nov 10;247(21):6960–6962. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med. 1981 May 1;153(5):1302–1315. doi: 10.1084/jem.153.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R. L., Raventos-Suarez C., Fabry M. E., Tanowitz H., Sicard D., Labie D. Impairment of the growth of Plasmodium falciparum in HbEE erythrocytes. J Clin Invest. 1981 Jul;68(1):303–305. doi: 10.1172/JCI110248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasvol G., Weatherall D. J., Wilson R. J. Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977 Nov 10;270(5633):171–173. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]
- RIGDON R. H., MICKS D. W., BRESLIN D. Effect of phenylhydrazine hydrochloride of plasmodium knowlesi infection in the monkey. Am J Hyg. 1950 Nov;52(3):308–322. doi: 10.1093/oxfordjournals.aje.a119426. [DOI] [PubMed] [Google Scholar]
- ROSE C. S., GYORGY P. Hemolysis with alloxan and alloxan-like compounds, and the protective action of tocopherol. Blood. 1950 Nov;5(11):1062–1074. [PubMed] [Google Scholar]
- ROSE C. S., GYORGY P. Specificity of hemolytic reaction in vitamin E-deficient erythrocytes. Am J Physiol. 1952 Feb;168(2):414–420. doi: 10.1152/ajplegacy.1952.168.2.414. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz E. A., Shohet S. B., Lubin B. H. Lipid membrane peroxidation in beta-thalassemia major. Blood. 1976 Mar;47(3):495–505. [PubMed] [Google Scholar]
- Rest J. R., Wright D. H. Electron microscopy of cerebral malaria in golden hamsters (Mesocricetus auratus) infected with Plasmodium berghei. J Pathol. 1979 Mar;127(3):115–120. doi: 10.1002/path.1711270303. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Gunther R., Gee M., Flynn J., Demling R. H. Leukocytes, platelets, and thromboxane A2 in endotoxin-induced lung injury. Surgery. 1981 Jul;90(1):102–107. [PubMed] [Google Scholar]
- Stocks J., Kemp M., Dormandy T. L. Increased susceptibility of red-blood-cell lipids to autooxidation in haemolytic states. Lancet. 1971 Feb 6;1(7693):266–269. doi: 10.1016/s0140-6736(71)91004-x. [DOI] [PubMed] [Google Scholar]
- Stocks J., Offerman E. L., Modell C. B., Dormandy T. L. The susceptibility to autoxidation of human red cell lipids in health and disease. Br J Haematol. 1972 Dec;23(6):713–724. doi: 10.1111/j.1365-2141.1972.tb03486.x. [DOI] [PubMed] [Google Scholar]
- TARLOV A. R., BREWER G. J., CARSON P. E., ALVING A. S. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med. 1962 Feb;109:209–234. doi: 10.1001/archinte.1962.03620140081013. [DOI] [PubMed] [Google Scholar]
- TARLOV A. R., KELLERMEYER R. W. The hemolytic effect of primaquine. XI. Decreased catalase activity in primaquine-sensitive erythrocytes. J Lab Clin Med. 1961 Aug;58:204–216. [PubMed] [Google Scholar]
- Wei E. P., Kontos H. A., Dietrich W. D., Povlishock J. T., Ellis E. F. Inhibition by free radical scavengers and by cyclooxygenase inhibitors of pial arteriolar abnormalities from concussive brain injury in cats. Circ Res. 1981 Jan;48(1):95–103. doi: 10.1161/01.res.48.1.95. [DOI] [PubMed] [Google Scholar]
- Wind M., Stern A. Comparison of human adult and fetal hemoglobin: aminophenol-induced methemoglobin formation. Experientia. 1977 Nov 15;33(11):1500–1501. doi: 10.1007/BF01918834. [DOI] [PubMed] [Google Scholar]



