Abstract
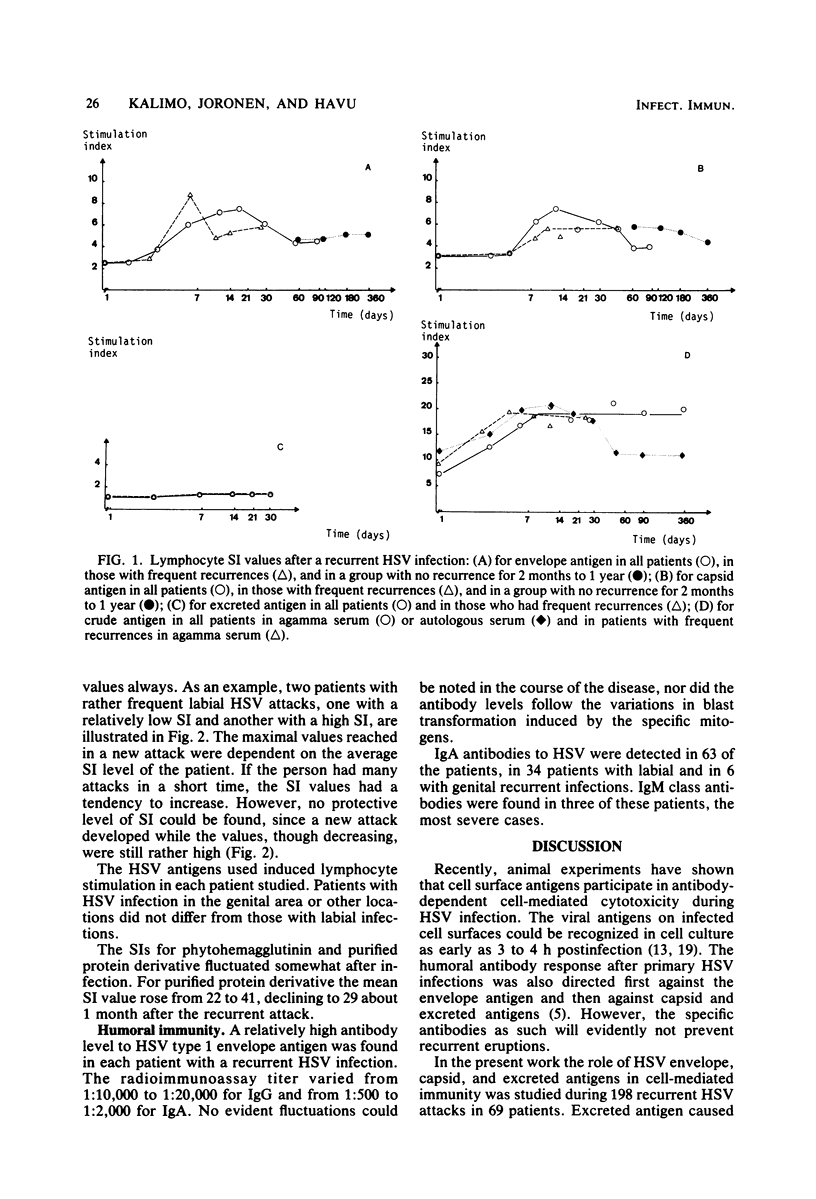
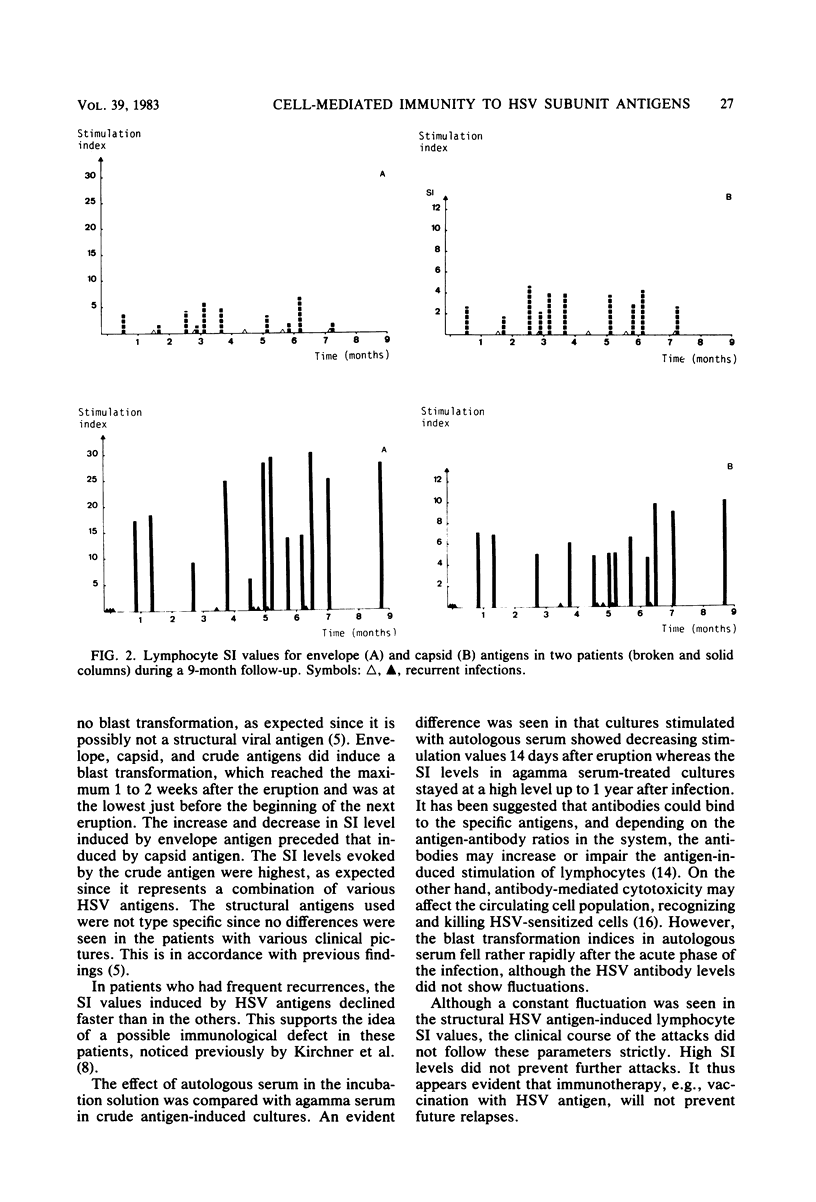
Cell-mediated immunity to herpes simplex virus envelope, capsid, excreted, and crude antigens was studied by in vitro lymphocyte stimulation tests during 198 recurrent attacks in 69 patients. Excreted antigen caused no blast transformation. Envelope and capsid antigen-induced lymphocyte stimulation was at the maximum 7 to 14 days postinfection, declining thereafter to a rather constant level in 1 to 2 months. The lowest levels were measured just a few days before a new attack. In persons with frequent relapses, the fluctuation was more rapid and stimulation index levels stayed higher, although no protective level seemed to exist. Cultures stimulated with the crude antigen in autologous serum showed rapid increases and declines in the stimulation index values, contrary to those grown in agamma serum, in which the stimulation level stayed rather constant up to 1 year postinfection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Czarnetzki B. M., Macher E. In vitro studies on the human cellular immune response to commercially available herpes simplex antigens. Arch Dermatol Res. 1980;268(3):247–255. doi: 10.1007/BF00404285. [DOI] [PubMed] [Google Scholar]
- Douglas R. G., Jr, Couch R. B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970 Feb;104(2):289–295. [PubMed] [Google Scholar]
- Gange R. W., de Bats A., Park J. R., Bradstreet C. M., Rhodes E. L. Cellular immunity and circulating antibody to herpes simplex virus in subjects with recurrent herpex simplex lesions and controls as measured by the mixed leukocyte migration inhibition test and complement fixation. Br J Dermatol. 1975 Nov;93(5):539–544. doi: 10.1111/j.1365-2133.1975.tb02246.x. [DOI] [PubMed] [Google Scholar]
- Kalimo K. O., Marttila R. J., Granfors K., Viljanen M. K. Solid-phase radioimmunoassay of human immunoglobulin M and immunoglobulin G antibodies against herpes simplex virus type 1 capsid, envelope, and excreted antigens. Infect Immun. 1977 Mar;15(3):883–889. doi: 10.1128/iai.15.3.883-889.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo K. O., Ziola B. R., Viljanen M. K., Granfors K., Toivanen P. Solid-phase radioimmunoassay of herpes simplex virus IgG and IgM antibodies. J Immunol Methods. 1977;14(2):183–195. doi: 10.1016/0022-1759(77)90008-4. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Erickson J. S., Ben-Porat T. Synthesis of proteins in cells infected with herpesvirus. X. Proteins excreted by cells infected with herpes simplex virus, types 1 and 2. Virology. 1975 Mar;64(1):132–143. doi: 10.1016/0042-6822(75)90085-9. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Schwenteck M., Northoff H., Schöpf E. Defective in vitro lymphoproliferative responses to herpes simplex virus in patients with frequently recurring herpes infections during the disease-free interval. Clin Immunol Immunopathol. 1978 Nov;11(3):267–274. doi: 10.1016/0090-1229(78)90050-8. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Courtney R. J., Eberle R., Schaffer P. A., O'Hara M. K., Rouse B. T. Cell-mediated immunity to herpes simplex virus: specificity of cytotoxic T cells. Infect Immun. 1980 Nov;30(2):451–461. doi: 10.1128/iai.30.2.451-461.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. L., Palmer E. L., Kissling R. E. Complement-fixing antigens of herpes simplex virus types 1 and 2: reactivity of capsid, envelope, and soluble antigens. Infect Immun. 1972 Feb;5(2):248–254. doi: 10.1128/iai.5.2.248-254.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller-Larsen A., Haahr S., Black F. T. Cellular and humoral immune responses to herpes simplex virus during and after primary gingivostomatitis. Infect Immun. 1978 Nov;22(2):445–451. doi: 10.1128/iai.22.2.445-451.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff J. M., Enders J. F. Poliovirus replication and cytopathogenicity in monolayer hamster cell cultures fused with beta propiolactone-inactivated Sendai virus. Proc Soc Exp Biol Med. 1968 Jan;127(1):260–267. doi: 10.3181/00379727-127-32668. [DOI] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Cromeans T. L., Nahmias A. J. Participation of three major glycoprotein antigens of herpes simplex virus type 1 early in the infectious cycle as determined by antibody-dependent cell-mediated cytotoxicity. Infect Immun. 1980 Apr;28(1):38–44. doi: 10.1128/iai.28.1.38-44.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Chibbaro A., Anger E., Lopez C. Cell-mediated immune responses in patients with recurrent Herpes Simplex infections. II. Infection-associated deficiency of lymphokine production in patients with recurrent herpes labialis or herpes progenitalis. J Immunol. 1977 Mar;118(3):1095–1102. [PubMed] [Google Scholar]
- Oppenheim J. J. Modulation of in vitro lymphocyte transformation by antibodies: enhancement by antigen-antibody complexes and inhibition by antibody excess. Cell Immunol. 1972 Mar;3(3):341–360. doi: 10.1016/0008-8749(72)90243-2. [DOI] [PubMed] [Google Scholar]
- Pelton B. K., Duncan I. B., Denman A. M. Herpes simplex virus depresses antibody production by affecting T-cell function. Nature. 1980 Mar 13;284(5752):176–177. doi: 10.1038/284176a0. [DOI] [PubMed] [Google Scholar]
- Pettersson D., Mellstedt H., Holm G. IgG on human blood lymphocytes studied by immunofluorescence. Scand J Immunol. 1978;8(6):535–542. doi: 10.1111/j.1365-3083.1978.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Norrild B. Early damage of herpes-infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specified cell-surface antigens and input virus. J Immunol. 1979 Nov;123(5):2239–2244. [PubMed] [Google Scholar]
- Steele R. W., Vincent M. M., Hensen S. A., Fuccillo D. A., Chapa I. A., Canales L. Cellular immune responses to Herpes simplex virus type 1 in recurrent herpes labialis: in vitro blastogenesis and cytotoxicity to infected cell line. J Infect Dis. 1975 May;131(5):528–534. doi: 10.1093/infdis/131.5.528. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Vincent M. M., Hensen S. A., Fuccillo D. A., Rola-Pleszczynski M., Bellanti J. A. Depressed specific cell-mediated immunity to Herpes simplex virus type 1 in patients with recurrent herpes labialis. Infect Immun. 1975 Jul;12(1):76–80. doi: 10.1128/iai.12.1.76-80.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. Effect of serum on human rosett-forming cells in fetuses and adult blood. Clin Immunol Immunopathol. 1973 Apr;1(3):408–413. doi: 10.1016/0090-1229(73)90057-3. [DOI] [PubMed] [Google Scholar]



