Abstract
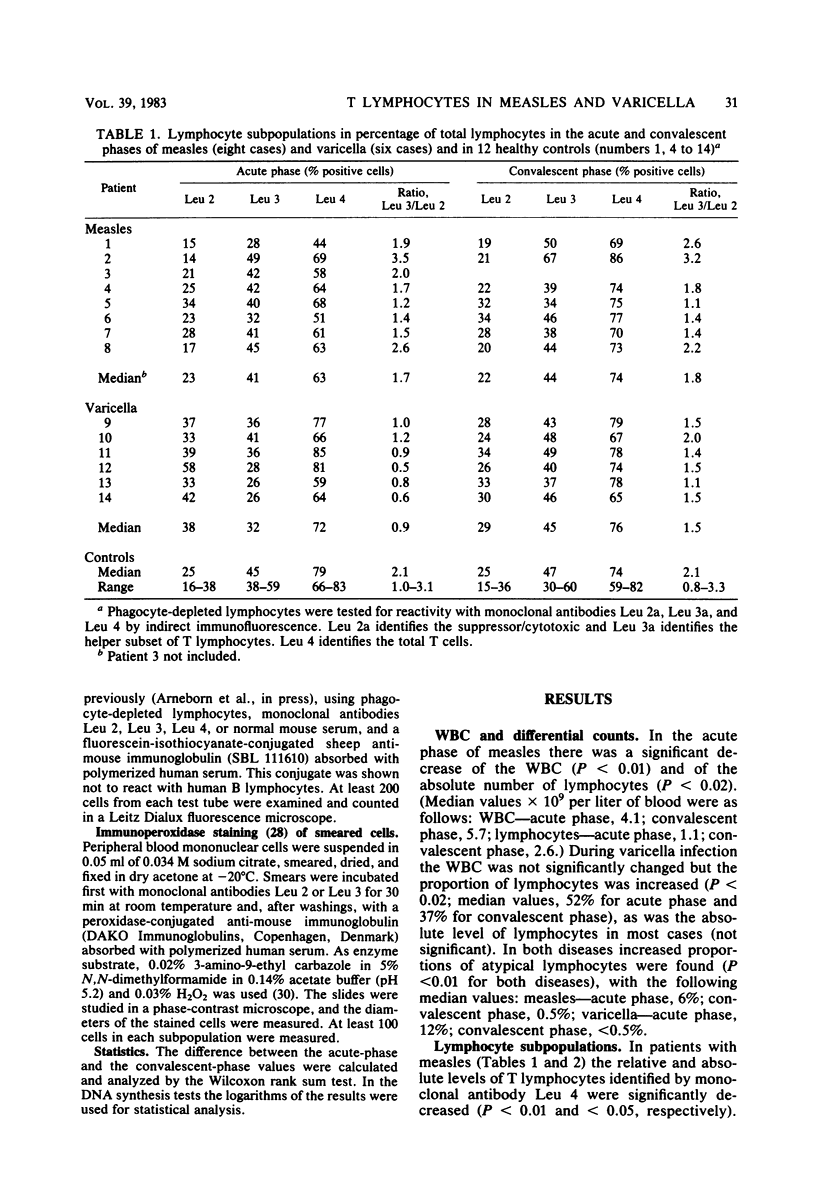
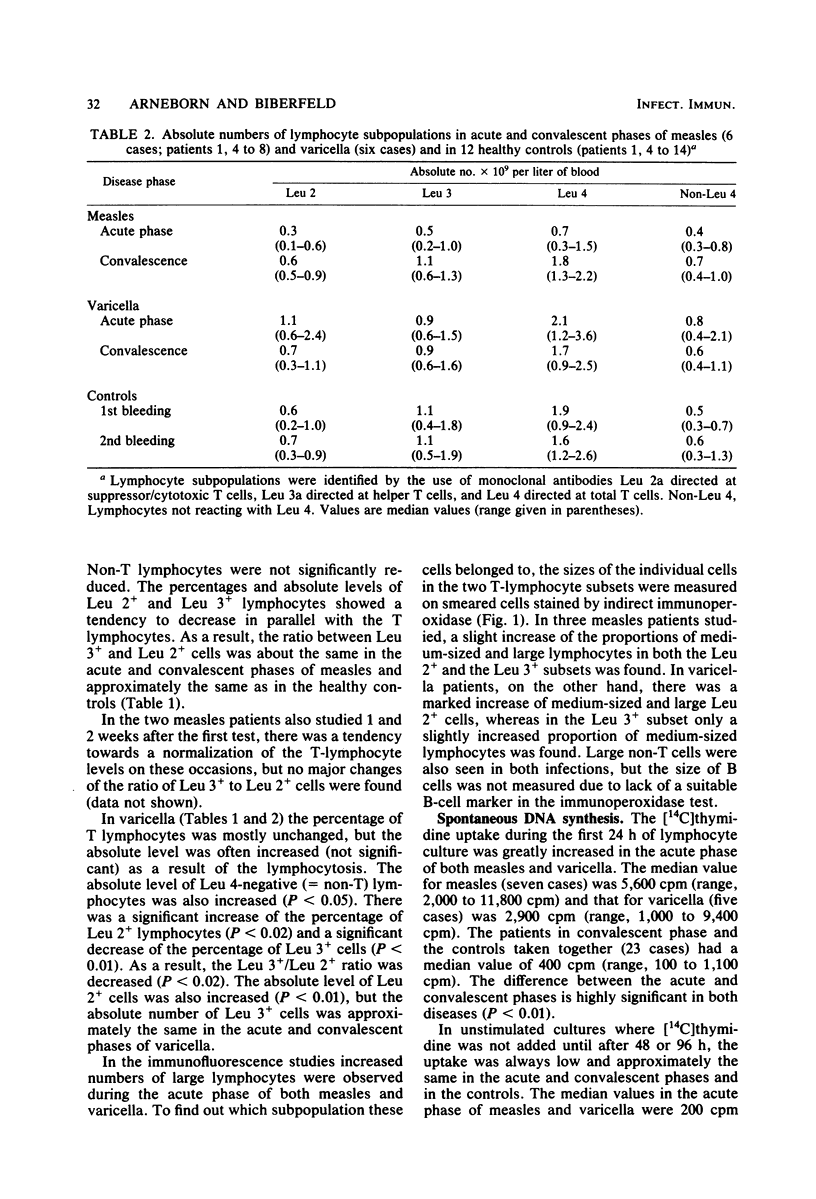
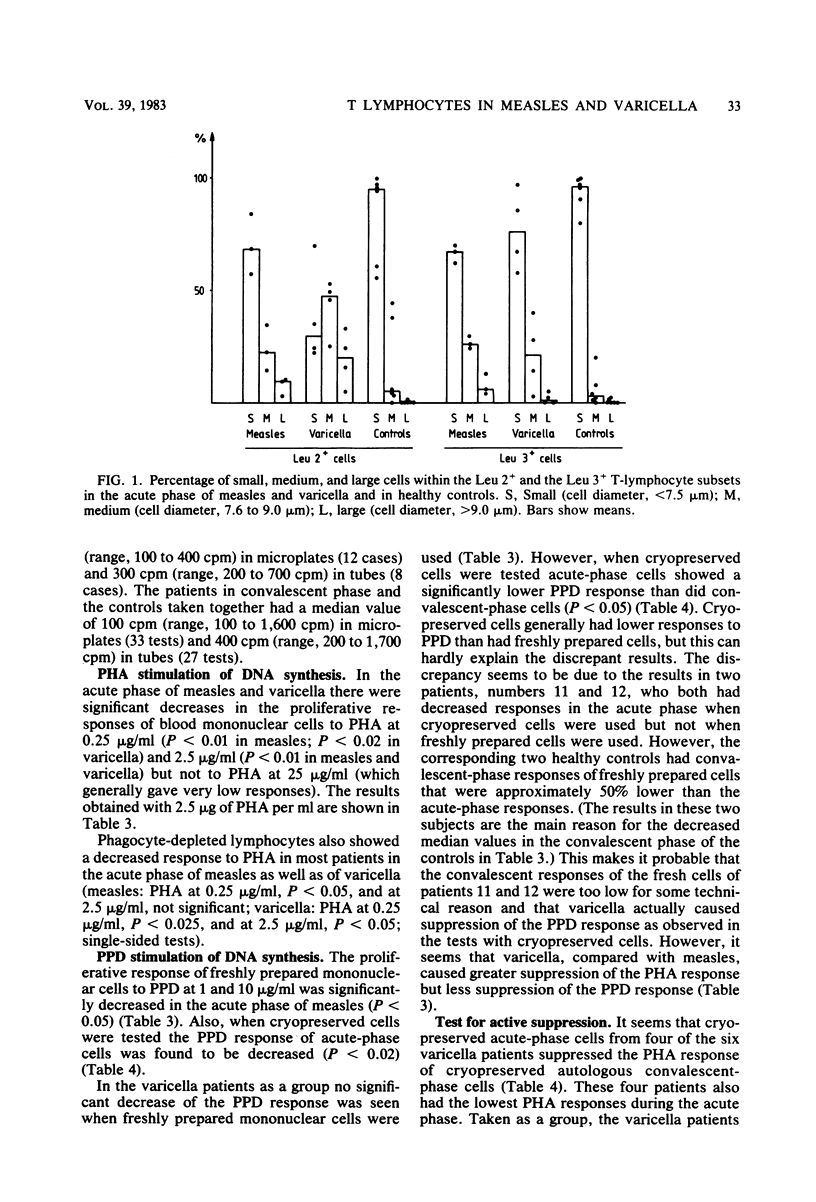
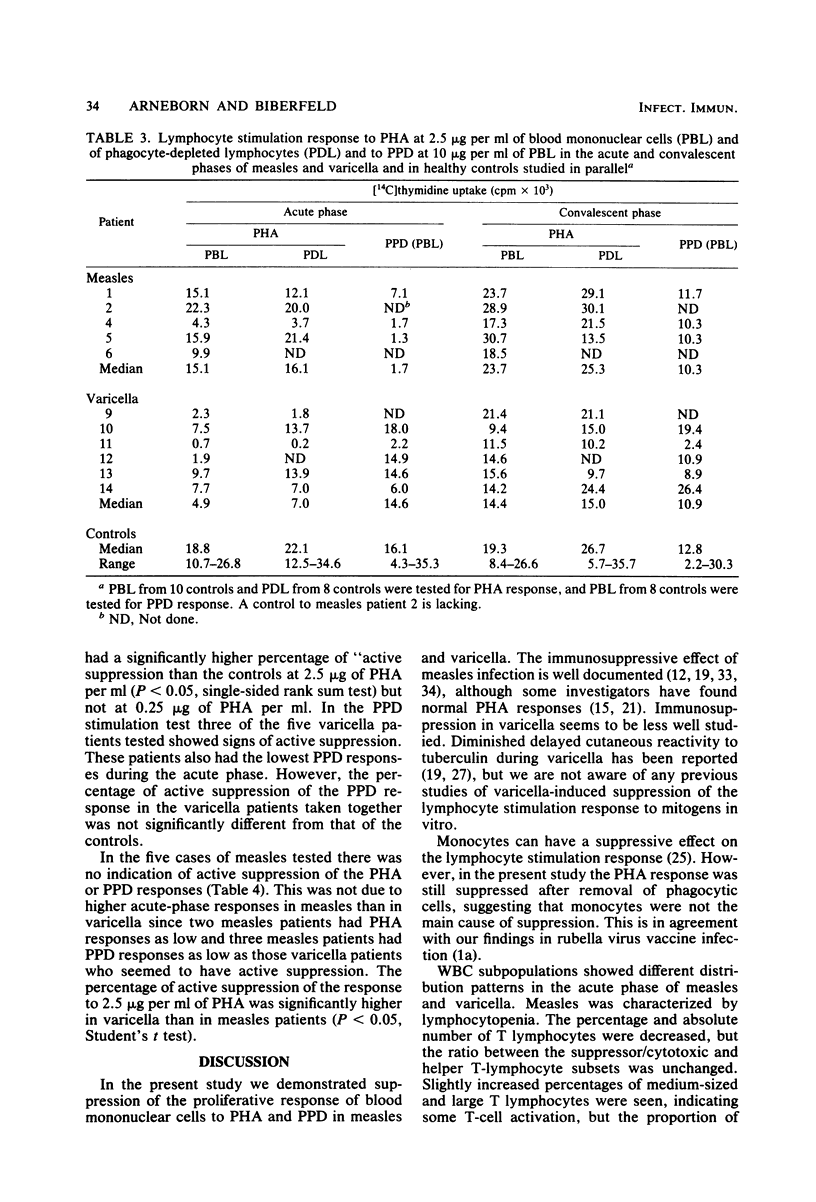
Patients with measles or varicella were studied during the acute phase (first week) of illness and, after recovery, by lymphocyte stimulation tests and determination of T-lymphocyte subpopulations, using the monoclonal antibodies Leu 2a and Leu 3a directed at the suppressor/cytotoxic and the helper T-cell subsets, respectively. Low proliferative responses to phytohemagglutinin were found during the acute phase of both diseases. The response to purified protein derivate of tuberculin was also low in all measles patients tested and in some of the varicella patients. In both infections increased spontaneous DNA synthesis was demonstrated. In the acute phase of measles there was T lymphocytopenia but no change of the ratio between T lymphocytes of helper and suppressor/cytotoxic cell phenotypes. In the acute phase of varicella the percentage and the absolute number of Leu 2-positive (suppressor/cytotoxic) T cells were increased. Measurement of the size of the lymphocytes indicated activation of this subset. Cryopreserved blood mononuclear cells from the acute phase of varicella could suppress the phytohemagglutinin response of autologous convalescent-phase cells. This was not seen when cells from measles patients were tested. The suppression of the lymphocyte stimulation response in varicella is probably in part caused by activation of suppressor cells, whereas the suppression of the stimulation response in measles seems to be due mainly to other mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Häyry P. Clonal isolation of alloantigen-reactive T-cells and characterization of their memory functions. Transplant Rev. 1975;25:121–162. doi: 10.1111/j.1600-065x.1975.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Arneborn P., Biberfeld G., Wasserman J. Immunosuppression and alterations of T-lymphocyte subpopulations after rubella vaccination. Infect Immun. 1980 Jul;29(1):36–41. doi: 10.1128/iai.29.1.36-41.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arneborn P., Biberfeld G., von Stedingk L. V. T lymphocyte subpopulations defined by monoclonal antibodies and FC receptor binding in relation to immunosuppression in vaccine-induced rubella infection. Acta Pathol Microbiol Immunol Scand C. 1982 Jun;90(3):163–170. doi: 10.1111/j.1699-0463.1982.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Bach M. A., Bach J. F. The use of monoclonal anti-T cell antibodies to study T cell imbalances in human diseases. Clin Exp Immunol. 1981 Sep;45(3):449–456. [PMC free article] [PubMed] [Google Scholar]
- Biberfeld G., Forsgren M., Von Stedingk L. V., Arneborn P. PPD-induced viral antibody production in human blood lymphocytes. Clin Exp Immunol. 1980 Nov;42(2):364–369. [PMC free article] [PubMed] [Google Scholar]
- Brody J. A., Harlem M. M., Plank C. R., White L. R. Freezing human peripheral lymphocytes and a technique for culture in monolayers. Proc Soc Exp Biol Med. 1968 Dec;129(3):968–972. doi: 10.3181/00379727-129-33471. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Cooper L. Z. Immunosuppression and isolation of rubella virus from human lymphocytes after vaccination with two rubella vaccines. Infect Immun. 1979 Jul;25(1):352–356. doi: 10.1128/iai.25.1.352-356.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Carter R. L. The mitotic activity of circulating atypical mononuclear cells in infectious mononucleosis. Blood. 1965 Nov;26(5):579–586. [PubMed] [Google Scholar]
- Crawford D. H., Brickell P., Tidman N., McConnell I., Hoffbrand A. V., Janossy G. Increased numbers of cells with suppressor T cell phenotype in the peripheral blood of patients with infectious mononucleosis. Clin Exp Immunol. 1981 Feb;43(2):291–297. [PMC free article] [PubMed] [Google Scholar]
- De Waele M., Thielemans C., Van Camp B. K. Characterization of immunoregulatory T cells in EBV-induced infectious mononucleosis by monoclonal antibodies. N Engl J Med. 1981 Feb 19;304(8):460–462. doi: 10.1056/NEJM198102193040804. [DOI] [PubMed] [Google Scholar]
- Finkel A., Dent P. B. Abnormalities in lymphocyte proliferation in classical and atypical measles infection. Cell Immunol. 1973 Jan;6(1):41–48. doi: 10.1016/0008-8749(73)90004-x. [DOI] [PubMed] [Google Scholar]
- Gump D. W., Fekety F. R., Jr The relationship of infection and DNA-synthesizing cells in human blood. J Lab Clin Med. 1967 Mar;69(3):428–437. [PubMed] [Google Scholar]
- Hirsch R. L., Mokhtarian F., Griffin D. E., Brooks B. R., Hess J., Johnson R. T. Measles virus vaccination of measles seropositive individuals suppresses lymphocyte proliferation and chemotactic factor production. Clin Immunol Immunopathol. 1981 Dec;21(3):341–350. doi: 10.1016/0090-1229(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Defective helper factor (LMF) production in patients with acute measles infection. Clin Immunol Immunopathol. 1981 Aug;20(2):215–223. doi: 10.1016/0090-1229(81)90179-3. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Dissociation of lymphokine production and blastogenesis in children with measles infections. Clin Immunol Immunopathol. 1978 Jul;10(3):335–343. doi: 10.1016/0090-1229(78)90190-3. [DOI] [PubMed] [Google Scholar]
- MACKINNEY A. A., Jr TISSUE CULTURE OF CELLS ALREADY IN DNA SYNTHESIS FROM PATIENTS WITH INFECTIOUS MONONUCLEOSIS. Blood. 1965 Jul;26:36–48. [PubMed] [Google Scholar]
- McFarland H. F. The effect of measles virus infection on T and B lymphocytes in the mouse. I. Suppression of helper cell activity. J Immunol. 1974 Dec;113(6):1978–1983. [PubMed] [Google Scholar]
- O'Shea S., Parsons G., Best J. M., Banatvala J. E., Balfour H. H., Jr How well do low levels of rubella antibody protect? Lancet. 1981 Dec 5;2(8258):1284–1284. doi: 10.1016/s0140-6736(81)91517-8. [DOI] [PubMed] [Google Scholar]
- Osunkoya B. O., Cooke A. R., Ayeni O., Adejumo T. A. Studies on leukocyte cultures in measles. I. Lymphocyte transformation and giant cell formation in leukocyte cultures from clinical cases of measles. Arch Gesamte Virusforsch. 1974;44(4):313–322. [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Perlin E. Atypical lymphocytes in acute infectious mononucleosis. Identification by multiple T and B lymphocyte markers. N Engl J Med. 1974 Nov 28;291(22):1145–1148. doi: 10.1056/NEJM197411282912201. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Rice L., Laughter A. H., Twomey J. J. Three suppressor systems in human blood that modulate lymphoproliferation. J Immunol. 1979 Mar;122(3):991–996. [PubMed] [Google Scholar]
- SCHMID J. R., OECHSLIN R. J., MOESCHLIN S. INFECTIOUS MONONUCLEOSIS, AN AUTORADIOGRAPHIC STUDY OF DNA- AND RNA-SYNTHESIS. Scand J Haematol. 1965;2:18–30. doi: 10.1111/j.1600-0609.1965.tb01275.x. [DOI] [PubMed] [Google Scholar]
- STARR S., BERKOVICH S. THE DEPRESSION OF TUBERCULIN REACTIVITY DURING CHICKENPOX. Pediatrics. 1964 May;33:769–772. [PubMed] [Google Scholar]
- Thomas H. C. T cell subsets in patients with acute and chronic HBV infection, primary biliary cirrhosis and alcohol induced liver disease. Int J Immunopharmacol. 1981;3(3):301–305. doi: 10.1016/0192-0561(81)90023-0. [DOI] [PubMed] [Google Scholar]
- Tubbs R. R., Velasco M. E., Benjamin S. P. Immunocytochemical identification of human chorionic gonadotropin. Comparative study of diaminobenzidine and 3-amino, 9-ethylcarbazole, a nonhazardous chromogen. Arch Pathol Lab Med. 1979 Sep;103(10):534–536. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Buimovici-Klein E. Lymphocyte responses to rubella antigen and phytohemagglutinin after administration of the RA 27/3 strain of live attenuated rubella vaccine. Infect Immun. 1975 Apr;11(4):748–753. doi: 10.1128/iai.11.4.748-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood T. A., Frenkel E. P. The atypical lymphocyte. Am J Med. 1967 Jun;42(6):923–936. doi: 10.1016/0002-9343(67)90073-3. [DOI] [PubMed] [Google Scholar]
- Zweiman B., Lisak R. P., Waters D., Koprowski H. Effects of purified measles virus components on proliferating human lymphocytes. Cell Immunol. 1979 Oct;47(2):241–247. doi: 10.1016/0008-8749(79)90334-4. [DOI] [PubMed] [Google Scholar]



