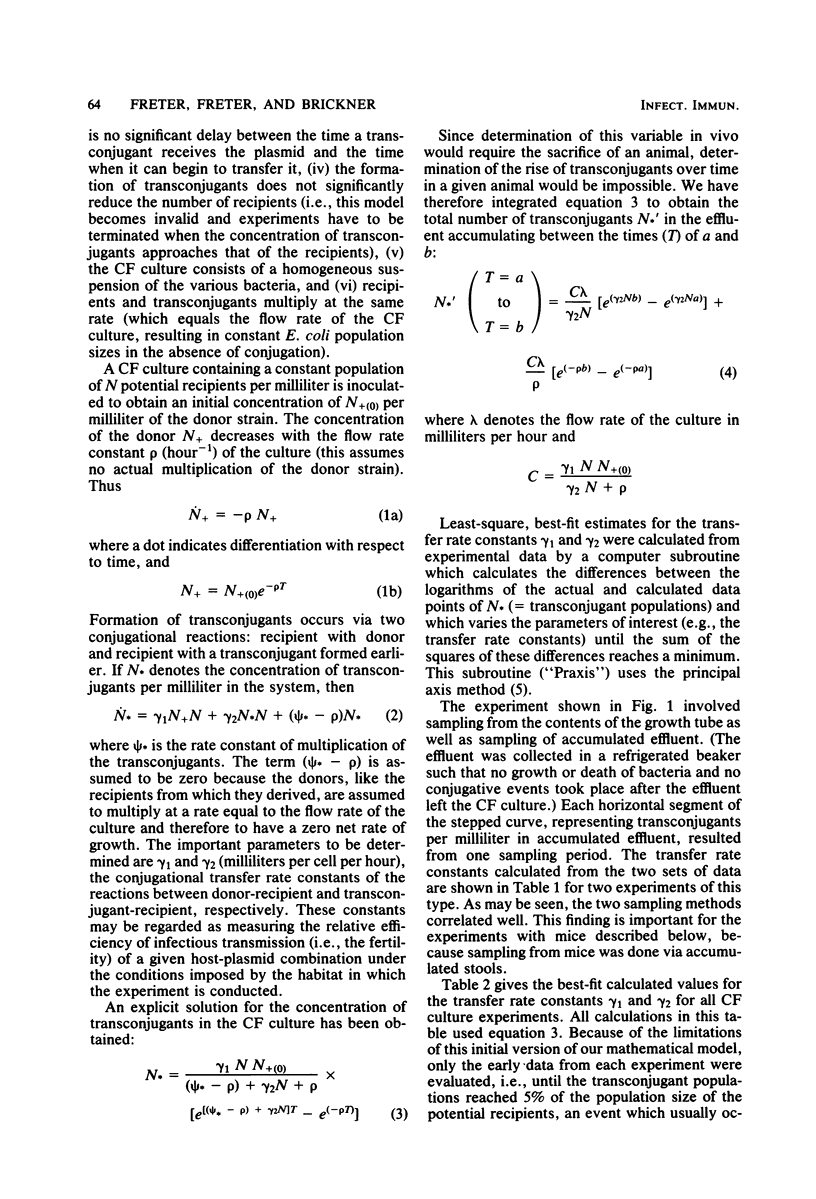
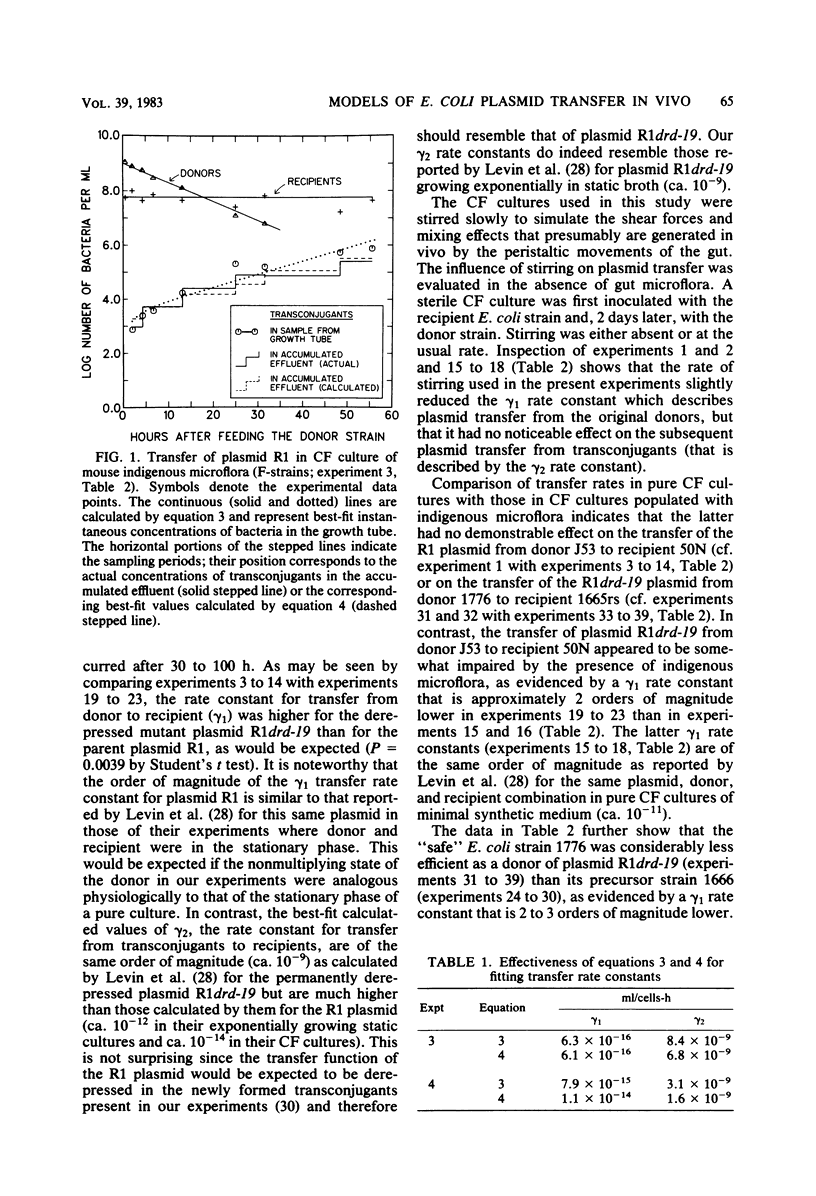
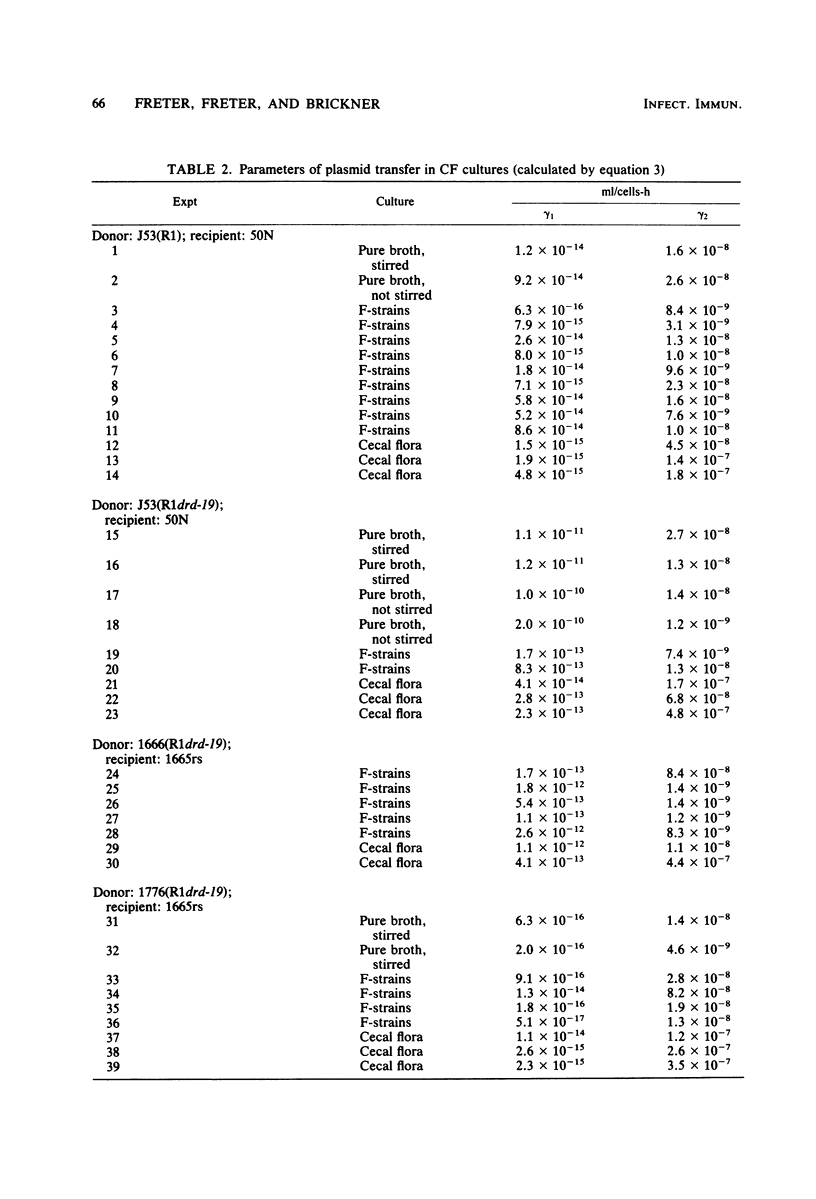
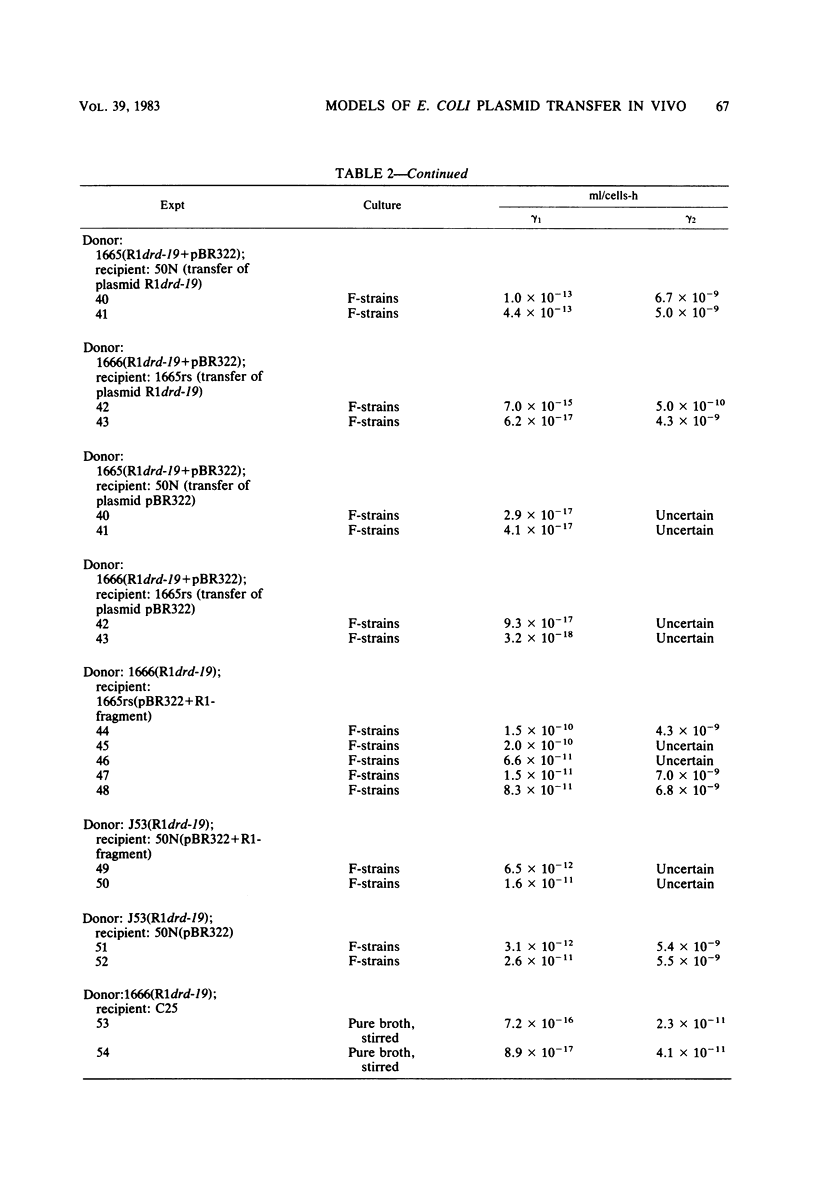
Abstract
Little is known about the factors that govern plasmid transfers in natural ecosystems such as the gut. The consistent finding by earlier workers that plasmid transfer in the normal gut can be detected only at very low rates, if at all, has given rise to numerous speculations concerning the presence in vivo of various inhibitors of plasmid transfer. Plasmids R1, R1drd-19, and pBR322 were studied in Escherichia coli K-12 and wild-type E. coli hosts in two experimental systems: (i) gnotobiotic mice carrying a synthetic indigenous microflora (F-strains) which resemble in their function the normal indigenous microflora of the mouse large intestine, and (ii) anaerobic continuous-flow cultures of indigenous large intestinal microflora of the mouse, which can simulate bacterial interactions observed in the mouse gut. Mathematical models were developed to estimate plasmid transfer rates as a measure of the “fertility,” i.e., of the intrinsic ability to transfer the plasmid under the environmental conditions of the gut. The models also evaluate the effects of plasmid segregation, reduction of the growth rates of plasmid-bearing bacterial hosts, repression of transfer functions, competition for nutrients, and bacterial attachment to the wall of the gut or culture vessel. Some confidence in the validity of these mathematical models was gained because they were able to reproduce a number of known phenomena such as the repression of fertility of the R1 plasmid, as well as known differences in the transmission and mobilization of the plasmids studied. Interpretation of the data obtained permitted a number of conclusions, some of which were rather unexpected. (i) Fertility of plasmid-bearing E. coli in the normal intestine was not impaired. The observed low rates of plasmid transfer in the normal gut can be explained on quantitative grounds alone and do not require hypothetical inhibitory mechanisms. (ii) Conditions for long-term spread and maintenance throughout human or animal populations of a diversity of conjugative and nonconjugative plasmids may be optimal among E. coli strains of low fertility, as are found among wild-type strains. (iii) E. coli strains carrying plasmid pBR322 plus R1drd-19 were impaired in their ability to transfer R1drd-19, but strains carrying pBR322 were significantly better recipients of R1drd-19 than a plasmid-free recipient E. coli. (iv) Long-term coexistence of plasmid-bearing and plasmid-free E. coli, in spite of undiminished fertility, appeared to be due to a detrimental effect of the plasmid on the growth rate of its host bacterium, rather than due to high rates of plasmid segregation. (v) Mathematical analysis of experimental data published by earlier investigators is consistent with the conclusion that plasmid transfer occurs consistently in the human gut, but that the resulting transconjugant E. coli populations are too small to be detected regularly with the culture methods used by earlier investigators. It is concluded that the long-term interactions observed were often the consequences of minor differences in parameters such as growth rates, fertility, rates of segregation, etc., which were too small to be detected except by precise mathematical analysis of long-term experiments, but which were nevertheless decisive determinants of the ultimate fates of the plasmids and their hosts.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. Plasmid transfer in Escherichia coli. J Infect Dis. 1978 May;137(5):686–687. doi: 10.1093/infdis/137.5.686. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. Viability of, and transfer of a plasmid from, E. coli K12 in human intestine. Nature. 1975 Jun 5;255(5508):502–504. doi: 10.1038/255502a0. [DOI] [PubMed] [Google Scholar]
- Anderson J. D. Factors that may prevent transfer of anti-biotic resistance between gram-negative bacteria in the gut. J Med Microbiol. 1975 Feb;8(1):83–88. doi: 10.1099/00222615-8-1-83. [DOI] [PubMed] [Google Scholar]
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Burman L. G. Expression of R-plasmid functions during anaerobic growth of an Escherichia coli K-12 host. J Bacteriol. 1977 Jul;131(1):69–75. doi: 10.1128/jb.131.1.69-75.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Crosa J. H., Falkow S. Mobilization of the Escherichia coli plasmid ColE1 (colicin E1) and ColE1 vectors used in recombinant DNA experiments. J Infect Dis. 1978 May;137(5):676–680. doi: 10.1093/infdis/137.5.676. [DOI] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Rousseau M. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun. 1981 Dec;34(3):957–969. doi: 10.1128/iai.34.3.957-969.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Tancrede C., Rousseau M. R-plasmic transfer from Serratia liquefaciens to Escherichia coli in vitro and in vivo in the digestive tract of gnotobiotic mice associated with human fecal flora. Infect Immun. 1980 Jun;28(3):981–990. doi: 10.1128/iai.28.3.981-990.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- FISHER K. W. The role of the Krebs cycle in conjugation in Escherichia coli K-12. J Gen Microbiol. 1957 Feb;16(1):120–135. doi: 10.1099/00221287-16-1-120. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A. Transfer of multiple drug resistance from Escherichia coli to Salmonella typhi murium in the mouse intestine. Antonie Van Leeuwenhoek. 1965;31(3):314–322. doi: 10.1007/BF02045911. [DOI] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Jarolmen H., Kemp G. R factor transmission in vivo. J Bacteriol. 1969 Aug;99(2):487–490. doi: 10.1128/jb.99.2.487-490.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T., Curtiss R., 3rd Genetic exchange between Escherichia coli strains in the mouse intestine. J Bacteriol. 1970 Jul;103(1):71–80. doi: 10.1128/jb.103.1.71-80.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUYA M. TRANSFER OF DRUG RESISTANCE BETWEEN ENTERIC BACTERIA INDUCED IN THE MOUSE INTESTINE. J Bacteriol. 1964 Aug;88:322–328. doi: 10.1128/jb.88.2.322-328.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. R., Rice V. A. The kinetics of transfer of nonconjugative plasmids by mobilizing conjugative factors. Genet Res. 1980 Jun;35(3):241–259. doi: 10.1017/s0016672300014117. [DOI] [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M., Rice V. A. The kinetics of conjugative plasmid transmission: fit of a simple mass action model. Plasmid. 1979 Apr;2(2):247–260. doi: 10.1016/0147-619x(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M. The population biology of bacterial plasmids: a priori conditions for the existence of mobilizable nonconjugative factors. Genetics. 1980 Feb;94(2):425–443. doi: 10.1093/genetics/94.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Marshall B., Rowse-Eagle D., Onderdonk A. Survival of Escherichia coli host-vector systems in the mammalian intestine. Science. 1980 Jul 18;209(4454):391–394. doi: 10.1126/science.6992276. [DOI] [PubMed] [Google Scholar]
- Moodie H. L., Woods D. R. Anaerobic R factor transfer in Escherichia coli. J Gen Microbiol. 1973 Jun;76(2):437–440. doi: 10.1099/00221287-76-2-437. [DOI] [PubMed] [Google Scholar]
- Olsen R. H., DeBusscher G., McCombie W. R. Development of broad-host-range vectors and gene banks: self-cloning of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1982 Apr;150(1):60–69. doi: 10.1128/jb.150.1.60-69.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL E. O. Criteria for the growth of contaminants and mutants in continuous culture. J Gen Microbiol. 1958 Feb;18(1):259–268. doi: 10.1099/00221287-18-1-259. [DOI] [PubMed] [Google Scholar]
- Reed N. D., Sieckmann D. G., Georgi C. E. Transfer of infectious drug resistance in microbially defined mice. J Bacteriol. 1969 Oct;100(1):22–26. doi: 10.1128/jb.100.1.22-26.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER H., FORMAL S. B., BARON L. S. Experimental genetic recombination in vivo between Escherichia coli and Salmonella typhimurium. J Exp Med. 1961 Jul 1;114:141–148. doi: 10.1084/jem.114.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman T. C., Klemm L. Transfer of antibiotic resistance (R factor) in the mouse intestine. Proc Soc Exp Biol Med. 1968 Jun;128(2):392–394. doi: 10.3181/00379727-128-33020. [DOI] [PubMed] [Google Scholar]
- Sansonetti P., Lafont J. P., Jaffé-Brachet A., Guillot J. F., Chaslus-Dancla E. Parameters controlling interbacterial plasmid spreading in a gnotoxenic chicken gut system: influence of plasmid and bacterial mutations. Antimicrob Agents Chemother. 1980 Mar;17(3):327–333. doi: 10.1128/aac.17.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W. Is it safe to use Escherichia coli K12 in recombinant DNA experiments? J Infect Dis. 1978 May;137(5):655–660. doi: 10.1093/infdis/137.5.655. [DOI] [PubMed] [Google Scholar]
- Smith H. W. The transfer of antibiotic resistance between strains of enterobacteria in chicken, calves and pigs. J Med Microbiol. 1970 Feb;3(1):165–180. doi: 10.1099/00222615-3-1-165. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Smith M. G. Letter: R factor transfer in vivo in sheep with E. coli K12. Nature. 1976 May 27;261(5558):348–348. doi: 10.1038/261348a0. [DOI] [PubMed] [Google Scholar]
- Smith M. G. Transfer of R factors from Escherichia coli to salmonellas in the rumen of sheep. J Med Microbiol. 1977 Feb;10(1):29–35. doi: 10.1099/00222615-10-1-29. [DOI] [PubMed] [Google Scholar]
- Stallions D. R., Curtiss R., 3rd Bacterial conjugation under anaerobic conditions. J Bacteriol. 1972 Jul;111(1):294–295. doi: 10.1128/jb.111.1.294-295.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart F. M., Levin B. R. The Population Biology of Bacterial Plasmids: A PRIORI Conditions for the Existence of Conjugationally Transmitted Factors. Genetics. 1977 Oct;87(2):209–228. doi: 10.1093/genetics/87.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton J. In vivo transfer of infectious drug resistance. Nature. 1966 Jul 16;211(5046):312–313. doi: 10.1038/211312a0. [DOI] [PubMed] [Google Scholar]
- Wiedemann B. Die Ubertragung extrachromosomaler Resistenzfaktoren in der Darmflora und ihre Hemmung. Zentralbl Bakteriol Orig A. 1972 May;220(1):106–123. [PubMed] [Google Scholar]
- Wiedemann B., Knothe H., Doll E. Ubertragung von R-Faktoren in der Darmflora des Menschen. Zentralbl Bakteriol Orig. 1970;213(2):183–193. [PubMed] [Google Scholar]



