Abstract
A male presented at age 2.2 years with a 6-week history of intermittent vomiting and hyperpigmentation. Investigations showed salt wasting with hyperkalaemia, a grossly impaired cortisol response to ACTH stimulation, elevated renin and ACTH. Family history revealed that two maternal uncles had died soon after birth. A third uncle failed to thrive during infancy but improved with a course of cortisone, then being untreated until further investigation revealed adrenal insufficiency. A fourth uncle died aged 10 days, with urinary salt loss and hypoplastic adrenal glands at postmortem. Molecular studies on the proband, his mother, maternal grandmother, and surviving uncle showed a novel C to G substitution at nucleotide position 794 (missense mutation T265R) in the DAX1 (NR0B1) gene. The proband has responded well to steroid replacement but has proved sensitive to 9α-fludrocortisone treatment, developing hypertension on a dose of 133 μg/m2/day. At 8.8 years he was noted to have testicular volumes of 4 ml, despite no other evidence of secondary sexual development and prepubertal gonadotrophin levels. Novel features of this family include a novel DAX1 mutation, marked variability in age of presentation, hypertension on ‘standard’ doses of 9α-fludrocortisone and mild testicular enlargement.
Keywords: DAX-1, NR0B1, Adrenal insufficiency, Adrenal hypoplasia congenita, X-linked, Mineralocorticoid
Introduction
Adrenal failure in childhood is a life-threatening but treatable condition. One of the most common causes of primary adrenal failure is X-linked adrenal hypoplasia congenita (AHC) which was initially described by Sikl [1] in 1948 and which in 1994 was shown to be due to mutations in the nuclear receptor gene DAX1 (dosage-sensitive sex reversal, AHC, on the X chromosome, gene 1) that is now designated NR0B1 [2, 3]. The DAX-1 protein plays a key role in the development of the adrenal gland and the hypothalamic-pituitary-gonadal axis [4, 5].
Boys with this condition typically present with a salt-losing crisis in the first 2 months of life or more insidiously during childhood with adrenal failure [6, 7]. Classically, AHC is associated with arrested or absent puberty due to disordered gonadotrophin release in adolescence, and infertility due to an intrinsic defect in spermatogenesis [4, 8–10]. Making the diagnosis has important implications therefore for the patient and their family.
The phenotype associated with DAX1 mutations is highly variable and may include features such as presentation with isolated mineralocorticoid insufficiency [11], prolonged testosterone secretion in infancy [12, 13], sexual precocity or testicular enlargement [14–16], and a late-onset form of the condition presenting in adulthood [9, 17, 18].
Here we report a novel missense mutation in DAX-1 with severe loss of function in a boy from a family with a history of multiple deaths in the maternal uncles. We highlight our index patient’s sensitivity to mineralocorticoid treatment and the unexpected finding of testicular enlargement during late childhood.
Case Report
History
The proband, a Caucasian male, was born at 41 weeks’ gestation by Ventouse delivery weighing 3.72 kg and was well during the newborn period. He subsequently presented to hospital aged 2.2 years with a 6-week history of intermittent vomiting and hyperpigmentation, following an episode of croup. His mother commented that he had appeared to stop growing some months ago and that he suffered from repeated intercurrent illnesses. Past medical history included an intermittent wheeze from 6 weeks of age treated by bronchodilators but not inhaled corticosteroids, eczema, and otitis media with effusion treated with insertion of ventilation tubes. There was a family history of adrenal disease (see below).
Examination
On examination the child was unwell and dehydrated with hyperpigmentation. The height was −1.1 SD and weight −2.2 SD, according to current UK growth standards [19]. Biochemistry showed hyponatraemia (123 mmol/l), hyperkalaemia (6.0 mmol/l) and uraemia (9.4 mmol/l). He improved with the administration of intravenous fluids and was then further investigated. A standard synacthen test in the dose of 250 μg intravenously showed extremely low basal/peak cortisol values of 35/70 nmol/l, grossly elevated basal ACTH (2,333 ng/l; reference range 0–80) and elevated plasma renin activity (22 ng/ml/h; reference range < 9.00). Adrenal antibodies were negative.
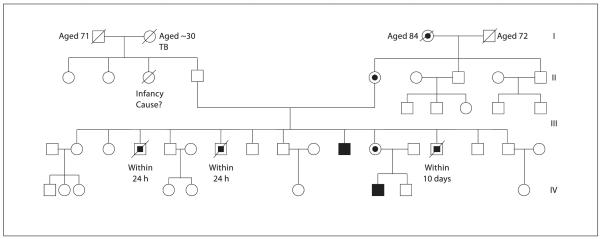
Family History (fig. 1)
Fig. 1.
Kindred with X-linked AHC. Partially filled circles denote carrier (heterozygous) females, partially filled squares denote putatively affected males, and filled squares denote affected (hemizygous) males.
Two maternal uncles had died very shortly after birth at term. Both were of normal birth weight (approximately 3 kg), but unfortunately few other details are known. A third maternal uncle failed to thrive during infancy but improved with a short course of cortisone treatment. He was then untreated until the age of 5 years when, following the early neonatal death of a further male sibling (see below), he was further investigated and found to have adrenal insufficiency. He went on to require pubertal induction, and is currently fit and well, receiving replacement therapy with testosterone, hydrocortisone 16.6 mg/m2/day, and fludrocortisone 56 μg/m2/day.
A fourth maternal uncle was born in good condition weighing approximately 3.5 kg. However, he became unwell and died after 10 days. He was reported to have high urinary sodium losses and small adrenal glands at postmortem.
Treatment and Progress
The child was started on steroid replacement with hydrocortisone (5 mg mane and 2.5 mg nocte; 12.5 mg/m2/day) and 9α-fludrocortisone (50 μg mane; 83.3 μg/m2/day).
He responded well to treatment with an improvement in energy, appetite and weight gain. At 2.4 years, blood pressure was 90/50 mm Hg and the testes were noted to be modestly enlarged at 3 ml (expected range 1–2 ml). The dose of 9α-fludrocortisone was increased to 100 μg mane (169.5 μg/m2/day).
Aged 3.6 years the child was admitted with vomiting and drowsiness in association with an intercurrent illness. At this time the daily hydrocortisone and fludrocortisone doses were 11.9 mg/m2 and 158.7 μg/m2, respectively. During the admission he was found to be hypertensive with systolic values and diastolic values of 137–171 and 82–123 mm Hg, respectively, and pulse rate was 106. Plasma renin activity was completely suppressed (<0.3 ng/ml/h) and electrolytes were normal: potassium 3.7 mmol/l and sodium 139 mmol/l. He was given intravenous hydrocortisone in view of the acute episode and improved rapidly. His blood pressure fell to 120/80 following temporary treatment with nifedipine.
Following this episode of frank hypertension the blood pressure remained elevated, systolic and diastolic values ranging between 100–134 and 60–87 mm Hg, respectively. At 7.2 years, a decision was taken to decrease the daily 9α-fludrocortisone dose from 100 μg (113.6 μg/m2) to 50 μg (56.8 μg/m2/day). On this regimen, serum renin was <0.3 ng/ml/h, ACTH 20 ng/l, cortisol <28.0 nmol/l and urea and electrolytes all within the normal range. The blood pressure fell slightly but not to normal levels, with median systolic and diastolic values of 120 and 80 mm, which represent 91st and 98.6th centiles, respectively, according to the blood pressure centile charts constructed using UK normative data [20].
Now aged 9.9 years the patient continues to show catch-up growth with height 135.4 cm (−0.45 SD), consistent with the midparental height (−0.49 SD), and weight 32 kg (0.22 SD). At chronological age 6.2 years, the bone age according to the TW2 system of Tanner and Whitehouse [21] was delayed by just over 1 year. Examination shows a normal-sized penis with no pubic or axillary hair. His testes show persistent enlargement (left 3.8 ml, right 3.9 ml), with firm texture but no discernible masses. Peak LH and FSH levels following stimulation with 100 μg gonadotrophin-releasing hormone (GnRH) are 1.2 and 0.6 U/l, respectively, with a basal testosterone of <0.5 nmol/l. Thyroid function is normal (free T4 18.0 pmol/l; reference range 9.0–26.0; TSH 1.58 mU/l; reference range 0.37–6.00).
Genetic and Molecular Studies
Mutational Analysis
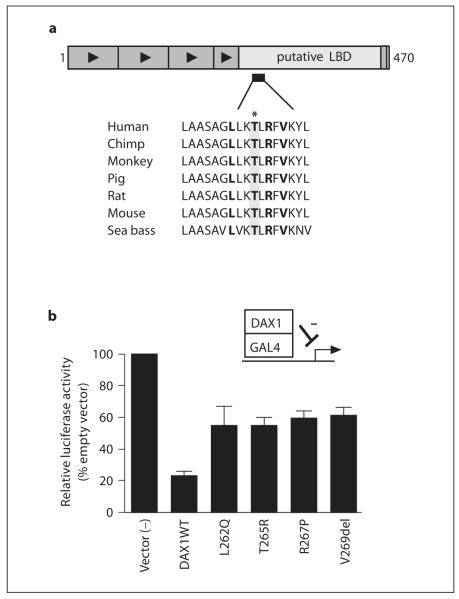
Sequence analysis of the DAX1 gene (NR0B1) showed the proband to have a C → G transversion at nucleotide 794 within exon 1. This mutation changes amino acid 265 from threonine to arginine (T265R). This is a previously unpublished nonconservative missense mutation that causes the substitution of an uncharged polar amino acid for a charged amino acid within a hydrophobic core of the putative DAX-1 ligand binding domain [22] (fig. 2a). The mutation is also present in the proband’s maternal grandmother, mother and affected surviving maternal uncle, but not in his brother who is clinically well with normal cortisol response to ACTH stimulation on two occasions.
Fig. 2.
Position and functional effects of DAX-1 mutants. a Cartoon of DAX-1 showing the position of T265R (highlighted) within a highly conserved region of helix 3 of the putative ligand-binding domain (LBD). A clustering of other missense mutations and single amino acid deletions has been reported here in patients with X-linked AHC (L262Q, R267P, V269del, shown in bold). b The effect of the T265R mutation on DAX1-mediated repressor activity was studied in a modified two-hybrid system. Mutant DAX-1 expression vectors (L262Q, T265R, R267P, V269del) were created with the carboxyl-terminal region of DAX1 (220–470) fused to the GAL4 DNA-binding domain. Transient transfection of this expression vector with a UASTKluc reporter shows 5-fold repression with wild-type DAX1, whereas naturally occurring mutations in the proximal cluster region of DAX1 show loss of repressor activity. Relative luciferase activity is expressed as a percentage of unopposed reporter activity (mean ± SEM) for four independent experiments, each performed in triplicate.
Functional Analysis of DAX-1 Repressor Activity
The effect of the T265R mutation on DAX-1-mediated repressor activity was studied in a modified two-hybrid system. Mutant DAX-1 expression vectors (including the T265R change, as well as previously reported changes L262Q, R267P, and V269del) were made using a site-directed mutagenesis kit (Invitrogen) with wild-type DAX1 cDNA as a template, as described previously [9, 23]. Transient gene expression assays were performed by co-transfecting empty, wild-type or mutant pBINDGAL4DAX1 vectors (5 ng) with a Renilla expression vector (7.5 ng) and UASTKluc reporter (100 ng) into tsa201 cells using Lipofectamine 2000 (Invitrogen) [9, 23]. Gene expression was quantified 24 h later using the Dual-Luciferase Reporter Assay System (Promega) and a BMG FLUOstar Optima 96-well plate reader (BMG). Results are expressed as mean ± SEM for four independent experiments, each performed in triplicate.
Functional analysis of the T265R mutant fused to GAL4 showed loss of DAX-1 repressor activity (fig. 2b). The degree of impairment was similar to other reported missense mutations within this region of DAX-1, and consistent in our experience with severe loss of DAX-1-mediated repressor activity [9, 23]. Similar results were seen using full-length DAX-1 T265R mutant as a repressor of SF1-mediated transcriptional activation [after 9, 23; data not shown].
Discussion
The family described provides an important insight into the diagnosis of patients with X-linked AHC. The proband presented with a fairly typical picture of salt wasting with failure to thrive in early childhood. The family history of a maternal uncle with documented adrenal failure formed a crucial component of the clinical picture, directing attention to AHC and leading to mutational analysis of DAX1 (NR0B1). It seems virtually certain that the maternal uncle who died aged 10 days with salt wasting was affected by AHC. It is of particular note that two maternal uncles died shortly after birth, suggesting that they too might have been suffering from the same condition, although such early presentation of adrenal failure is unusual, the earliest reported case of adrenal failure associated with a DAX1 mutation occurring at 2 days of age [6].
The threonine at position 265 of DAX-1 is highly conserved amongst many vertebrate species (fig. 2a), suggesting that this amino acid plays a key role in the structure of the DAX-1 protein. Missense mutations in DAX-1 are relatively rare, but the reported occurrence of several point mutations in this region (L262Q, R267P, and V269del) in multiple kindred suggests that this locus is one of the few mutational ‘hotspots’ [7, 23]. Furthermore, the novel T265R mutation reported in our family lies within a variant LXXLL motif that may be involved in nuclear receptor/co-factor interactions. However, previous studies have shown that mutations in this region can also impair nuclear localization of this transcription factor, or may disrupt the central hydrophobic core of the DAX-1 protein, so the mechanisms of its effects may be variable [22, 24, 25]. The disruptive consequences of the T265R mutation are confirmed by the absolute segregation of this mutation with adrenal failure in this kindred, as well as by in vitro assays of DAX-1 repressor function (fig. 2b). These studies show loss of DAX-1 repressor function comparable with other severe DAX-1 missense mutants and single amino acid deletions in this region.
Although genotype/phenotype correlations exist in some families with DAX1 mutations, phenotypic variability in the age at presentation can occur, as highlighted by the different ages at presentation in the kindred reported here [13]. Confirming the diagnosis at a molecular level can therefore help to identify at risk boys in future pregnancies within this kindred thus preventing salt-losing crises and/or recurrent hypoglycaemia with their associated morbidity and mortality. Although more than 100 patients with DAX1 mutations have now been described [7], variant features of this condition are emerging, some of which are highlighted by the case presented here. Mineralocorticoid insufficiency is a prominent feature of X-linked AHC and indeed some boys present with an isolated mineralocorticoid deficiency reminiscent of pseudohypoaldosteronism [11]. Our proband demonstrated clear evidence of mineralocorticoid deficiency at presentation during the third year of life with elevated renin levels, hyponatraemia, and hyperkalaemia, while there was a history of salt-losing crisis in the maternal uncle who died at 10 days of age. However, our index patient showed unusual sensitivity to 9α-fludrocortisone replacement once he was stabilized on treatment, with persistent hypertension in childhood. Even after reducing the mineralocorticoid dosage to lower levels than we would normally give, his blood pressure remains elevated. The reason for our patient proving to be so sensitive to mineralocorticoid replacement, with complete renin suppression in standard dosage, is unclear. Possible mechanisms include variability within the mineralocorticoid receptor and its downstream actions, differences in the activity of 11β-hydroxysteroid dehydrogenase enzyme systems, or a decreasing requirement for mineralocorticoid replacement with age. Our experience indicates that children on mineralocorticoid replacement need close supervision of blood pressure and that dose adjustments in 9α-fludrocortisone replacement may be required.
Another unusual feature reported here is of persistent mild testicular enlargement throughout childhood. Absent or arrested puberty due to disordered gonadotrophin release is a well-established feature of AHC due to DAX1 (NR0B1) mutations [4, 8]. Sexual precocity in AHC has been reported previously [14, 15], but in both these cases a decrease in testicular size and testosterone levels was seen following steroid replacement, indicating a potential ACTH-dependent pseudoprecocious puberty of testicular origin. In contrast, Argente et al. [16] described mild testicular enlargement in a boy with a DAX1 mutation who was stable on mineralocorticoid replacement. In our case, the testicular enlargement occurred over several years of steroid replacement during which ACTH suppression was well documented, while there was no evidence of true central precocious puberty, with a prepubertal GnRH test and undetectable testosterone levels, and no evidence of adrenal rests. Therefore, the testicular enlargement seems to be both ACTH- and gonadotrophin-independent. Long-term follow-up data from Dax1 (Ahch) knockout mice suggest that Dax-1 deletion may result in disruption of testicular developmental architecture and that cellular hyperplasia may occur with time [26]. No reports of similar findings have been published in humans, despite a slowly increasing number of reports of testicular histology in this condition in the literature [8, 10, 27]. This case highlights that mild testicular enlargement with or without signs of puberty may occur in this condition, and merits surveillance.
Established Facts.
X-linked adrenal hypoplasia congenita results from mutations in the nuclear receptor DAX-1 (NR0B1).
This condition typically presents with salt-losing primary adrenal failure in early infancy or childhood, and hypogonadotropic hypogonadism in adolescence.
Novel Insights.
Mild testicular enlargement in childhood may be found in some boys with this condition.
Sensitivity to mineralocorticoid replacement can vary, and close monitoring of blood pressure and plasma renin activity is necessary to individualize replacement doses.
Missense mutations in DAX-1 are helping to localize key domains of the protein that are necessary for nuclear localization, and protein stability and function.
References
- 1.Sikl H. Addison’s disease due to congenital hypoplasia of the adrenals in an infant aged 33 days. J Pathol Bacteriol. 1948;60:323–326. doi: 10.1002/path.1700600220. [DOI] [PubMed] [Google Scholar]
- 2.Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, Schwarz HP, Kaplan JC, Camerino G, Meitinger T, Monaco AP. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994;372:672–676. doi: 10.1038/372672a0. [DOI] [PubMed] [Google Scholar]
- 3.Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ERB, Meitinger T, Monaco AP, Sassone-Corsi P, Camerino G. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- 4.Habiby RL, Boepple P, Nachtigall L, Sluss PM, Crowley WF, Jameson JL. Adrenal hypoplasia congenita with hypogonadotropic hypogonadism: evidence that DAX-1 mutations lead to combined hypothalamic and pituitary defects in gonadotropin production. J Clin Invest. 1996;98:1055–1062. doi: 10.1172/JCI118866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalli E, Sassone-Corsi P. DAX-1 and the adrenal cortex. Curr Opin Endocrinol Diab. 1999;6:185–190. [Google Scholar]
- 6.Reutens AT, Achermann JC, Ito M, Ito M, Gu WX, Habiby RL, Donohoue PA, Pang S, Hindmarsh PC, Jameson JL. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999;84:504–511. doi: 10.1210/jcem.84.2.5468. [DOI] [PubMed] [Google Scholar]
- 7.Lin L, Gu W-X, Ozisik G, To WS, Owen CJ, Jameson JL, Achermann JC. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years’ experience. J Clin Endocrinol Metab. 2006;91:3048–3054. doi: 10.1210/jc.2006-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seminara SB, Achermann JC, Genel M, Jameson JL, Crowley WF., Jr X-linked adrenal hypoplasia congenita: a mutation in DAX1 expands the phenotypic spectrum in males and females. J Clin Endocrinol Metab. 1999;84:4501–4509. doi: 10.1210/jcem.84.12.6172. [DOI] [PubMed] [Google Scholar]
- 9.Tabarin A, Achermann JC, Recan D, Bex V, Bertagna X, Christin-Maitre S, Ito M, Jameson JL, Bouchard P. A novel mutation in DAX-1 causes delayed-onset adrenal insufficiency and incomplete hypogonadotropic hypogonadism. J Clin Invest. 2000;105:321–328. doi: 10.1172/JCI7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani G, De Menis E, Borretta G, Radetti G, Bondioni S, Spada A, Persani L, Beck-Peccoz P. DAX1 and X-linked adrenal hypoplasia congenita: clinical and molecular analysis in five patients. Eur J Endocrinol. 2006;154:685–689. doi: 10.1530/eje.1.02132. [DOI] [PubMed] [Google Scholar]
- 11.Wiltshire E, Couper J, Rodda C, Jameson L, Achermann JC. Variable presentation of Xlinked adrenal hypoplasia congenita. J Pediatr Endocrinol Metab. 2001;14:1093–1096. doi: 10.1515/jpem-2001-0804. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Shoji Y, Shoji Y, Haraguchi N, Takahashi I, Takadi G. Active hypothalamic-pituitary-gonadal axis in an infant with X-linked adrenal hypoplasia congenita. J Pediatr. 1997;130:488–491. doi: 10.1016/s0022-3476(97)70217-8. [DOI] [PubMed] [Google Scholar]
- 13.Achermann JC, Silverman BL, Habiby RL, Jameson JL. Presymptomatic diagnosis of X-linked adrenal hypoplasia congenita by analysis of DAX1. J Pediatr. 2000;137:878–881. doi: 10.1067/mpd.2000.108567. [DOI] [PubMed] [Google Scholar]
- 14.Domenice S, Latronico AC, Brito VN, Arnhold IJ, Kok F, Mendonca BB. Adrenocorticotropin-dependent precocious puberty of testicular origin in a boy with X-linked adrenal hypoplasia congenita due to a novel mutation in the DAX-1 gene. J Clin Endocrinol Metab. 2001;86:4068–4071. doi: 10.1210/jcem.86.9.7816. [DOI] [PubMed] [Google Scholar]
- 15.Katsumata N, Tanae A, Shinagawa T, Nimura A, Horikawa R, Tanaka T. Precocious puberty in patient with adrenal hypoplasia congenita (abstract P02-512). Proceedings of the 79th Annual Meeting of the Endocrine Society; Minneapolis. 1997. [Google Scholar]
- 16.Argente J, Ozisik G, Pozo J, Teresa Munoz M, Soriano-Guillen L, Jameson JL. A novel single base deletion at codon 434 (1301delT) of the DAX-1 gene associated with prepubertal testis enlargement. Mol Genet Metab. 2003;78:79–81. doi: 10.1016/s1096-7192(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani G, Ozisik G, Achermann JC, Romoli R, Borretta G, Persani L, Spada A, Jameson JL, Beck-Peccoz P. Hypogonadotropic hypogonadism as a presenting feature of late-onset X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2002;87:44–48. doi: 10.1210/jcem.87.1.8163. [DOI] [PubMed] [Google Scholar]
- 18.Ozisik G, Mantovani G, Achermann JC, Persani L, Spada A, Weiss J, Beck-Peccoz P, Jameson JL. An alternate translation initiation site circumvents an amino-terminal DAX-1 nonsense mutation leading to a mild form of X-linked adrenal hypoplasia congenita. J Clin Endocrinol Metab. 2003;88:417–423. doi: 10.1210/jc.2002-021034. [DOI] [PubMed] [Google Scholar]
- 19.Freeman JV, Cole TJ, Chinn S, Jones PRM, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson L, Thalange N, Cole T. Blood Pressure Centile Chart. Harlow Printing; South Shields: 2003. www.healthforallchildren.co.uk. [Google Scholar]
- 21.Tanner JM, Whitehouse RH, Cameron N, Marshall WA, Healy MJR, Goldstein H. Assessment of skeletal maturity and prediction of adult height (TW2 method) ed 2 Academic Press; London: 1983. [Google Scholar]
- 22.Zhang YH, Guo W, Wagner RL, Huang BL, McCabe L, Vilain E, Burris TP, Anyane-Yeboa K, Burghes AH, Chitayat D, Chudley AE, Genel M, Gertner JM, Klingensmith GJ, Levine SN, Nakamoto J, New MI, Pagon RA, Pappas JG, Quigley CA, Rosenthal IM, Baxter JD, Fletterick RJ, McCabe ER. DAX-1 mutations map to putative structural domains in a deduced three-dimensional model. Am J Hum Genet. 1998;62:855–864. doi: 10.1086/301782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achermann JC, Ito M, Silverman BL, Habiby RL, Pang S, Rosler A, Jameson JL. Missense mutations cluster within the carboxyl-terminal region of DAX-1 and impair transcriptional repression. J Clin Endocrinol Metab. 2001;86:3171–3175. doi: 10.1210/jcem.86.7.7660. [DOI] [PubMed] [Google Scholar]
- 24.Lehmann SG, Wurtz JM, Renaud JP, Sassone-Corsi P, Lalli E. Structure-function analysis reveals the molecular determinants of the impaired biological function of DAX-1 mutants in AHC patients. Hum Mol Genet. 2003;12:1063–1072. doi: 10.1093/hmg/ddg108. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann SG, Lalli E, Sassone-Corsi P. Xlinked adrenal hypoplasia congenita is caused by abnormal nuclear localization of the DAX-1 protein. Proc Natl Acad Sci USA. 2002;99:8225–8230. doi: 10.1073/pnas.122044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffs B, Meeks JJ, Ito M, Martinson FA, Matzuk MM, Jameson JL, Russell LD. Blockage of the rete testis and efferent ductules by ectopic Sertoli and Leydig cells causes infertility in DAX1-deficient male mice. Endocrinology. 2001;142:4486–4495. doi: 10.1210/endo.142.10.8447. [DOI] [PubMed] [Google Scholar]
- 27.Brown P, Scobie GA, Townsend J, Bayne RAL, Seckl JR, Saunders PTK, Anderson RA. Identification of a novel missense mutation that is as damaging to DAX-1 repressor function as a nonsense mutation. J Clin Endocrinol Metab. 2003;88:1341–1349. doi: 10.1210/jc.2002-021560. [DOI] [PubMed] [Google Scholar]