Summary
Intraviral protein-protein interactions are critical for virus survival in the host. Discovery of such interactions is important to understand molecular mechanisms of viral replication and pathogenesis. The development of a cell-based assay that can be employed to examine systematically viral protein interactions is described. The method, known as the Split Luciferase Complementation Assay (SLCA), is based on the principle that N- and C-terminal domains of luciferase alone do not emit luminescence; however, if fused to interacting proteins the two non-functional halves can be brought into close enough proximity through a specific protein-protein interaction to restore the functions of the enzyme and emit detectable light. The well-studied influenza B polymerase acidic protein (PA) and basic protein 1 (PB1) interaction was used as a model system to develop the assay. Consistent with previous studies, a strong PA-PB1 interaction was demonstrated in the assay. The PA-PB1 interaction was also disrupted by single amino acid mutations in the N-terminal domain of PB1 that is responsible for binding PA. The described SLCA is highly specific and easy to perform, and thus may be useful for studying protein-protein interactions in viral diseases.
Keywords: viral disease, protein-protein interaction, Split Luciferase Complementation Assay
Virus infection is an intracellular event that depends on a highly connected, dynamic, and regulated network of protein-protein interactions (PPIs). It is generally believed that protein-protein interactions can be divided into two major categories in the context of virus infection: intraviral (i.e., virus-virus) and virus-host interactions. Intraviral protein-protein interactions are essential to virus replication and occur normally among viral structural proteins, which play an important role in different stages of virus life cycle such as assembly and release. Virus-host interactions involving both structural and non-structural viral proteins are believed to serve more complex roles in virus replication and pathogenesis. By hijacking cellular machineries through protein interactions, viruses can evade host immunity and replicate in the hosts, causing diseases. Virus-host interactions are also commandeered to support viral replication and transmission processes such as virus entry, virus genome transcription, and virus release. Protein interactions are at the heart of virus infections and the associated diseases. Thus, a study of protein interactions and the molecular principles that underlie them is critical towards the understanding of virus replication and pathogenesis. Viral protein-protein interactions can be also targeted for the development of antiviral therapies in the future.
Several approaches with high-throughput potential have been developed to study protein-protein interactions. Of these, the yeast-two-hybrid (Y2H) system is viewed as the standard assay for the study of protein-protein interactions (Bailer Haas, 2009). This system has been used previously to study viral protein-protein interactions in cells (Calderwood et al., 2007; de Chassey et al., 2008; Fossum et al., 2009; Rozen et al., 2008; Uetz et al., 2006). The Y2H system utilizes a reporter gene which expresses only two fusion proteins that interact appears to require the fusion proteins to be imported to the nucleus, which may limit the sensitivity for probing interactions which occur in the cytoplasm. Additional concern is that the discrepancy has been demonstrated for identified interactions in comparative studies using Y2H systems (Bailer Haas, 2009). Limitations of Y2H highlight a need for development of other protein-protein interaction assays that can be used independently to study protein interactions or as an alternative approach to validate the biological significance of an interaction identified by Y2H.
One such approach is the Split Luciferase Complementation Assay (SLCA), where a luciferase protein is reconstituted when two fusion proteins interact (Cissell et al., 2009; Fujikawa Kato, 2007; Kaihara et al., 2003; Luker Piwnica-Worms, 2004; Luker et al., 2004; Paulmurugan Gambhir, 2003; Paulmurugan, Umezawa, Gambhir, 2002). The assay is based on the principle that N- and C-terminal domains of luciferase alone do not emit luminescence; however, if fused to interacting proteins the two non-functional halves can be brought into close enough proximity to form a functional enzyme as a result of appropriate specific protein interactions (Fig. 1A). Thus, through SLCA, the specific protein-protein interactions can be detected quantitatively by measuring reassembled luciferase activity in a microplate luminometer. Due to its sensitive and highly dynamic nature for detecting protein-protein interactions, this assay is becoming an increasingly popular tool used to study protein-protein interactions in cell biology and plant science (Cissell et al., 2009; Fujikawa Kato, 2007; Kaihara et al., 2003; Luker Piwnica-Worms, 2004; Luker et al., 2004; Paulmurugan Gambhir, 2003; Paulmurugan et al., 2002). An additional advantage is that unlike split-GFP assay (also known as bimolecular fluorescent complementation assay, BiFC), the protein complexes formed through SLCA are largely reversible so the interactions detected are dynamic in nature (Paulmurugan et al., 2004). This assay has been used previously to study the homodimerization of herpes simplex virus 1 thymidine kinase (Massoud, Paulmurugan, Gambhir, 2004). In the current work, we sought to apply the Split Luciferase Complementation Assay to the detection of intraviral protein-protein interactions using a well-studied influenza B PA-PB1 interaction as a model system. Previous studies have shown that the N-terminal 25 amino acid residues of influenza B PB1 (PB11–25B) are sufficient for binding of influenza B PA protein (Deng et al., 2011; Wunderlich et al., 2010; Wunderlich et al., 2009).
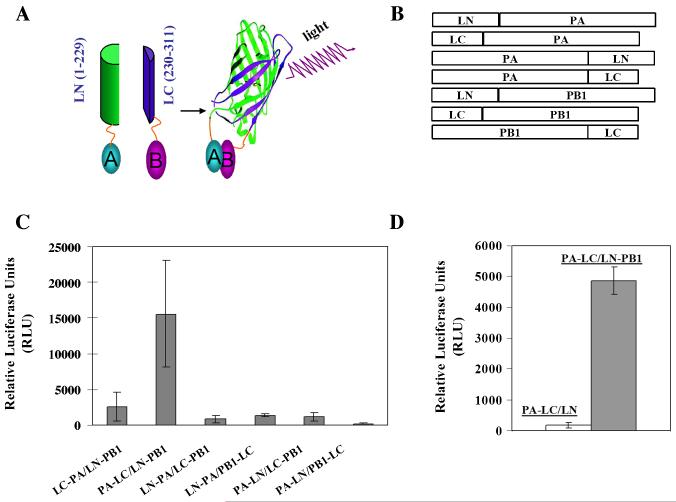
Figure 1. Split luciferase complementation-based strategy for detection of protein-protein interaction.
(A) Schematic representation of the assay principal for the detection of protein-protein interactions. Proteins A and B are fused to N- and C-terminal fragments of luciferase, respectively. In the absence of an interaction between A and B, the luciferase halves remain non-functional. Following interaction between A and B, a functional luciferase is reconstituted which exhibits emission of light upon the addition of appropriate substrate. LN, N-terminal 229 residues of Renila luciferase; LC, C-terminal 82 residues of Renila luciferase. (B) Schematic representation of constructs used in the split luciferase complementation assay (SLCA). All constructs were verified by sequencing in both directions. (C) SLCA detects protein-protein interaction. COS-1 cells in a 12-well cell culture plate were transfected with indicated pairs of fusion constructs (0.5μg each plasmid). At 48 h post transfection, cells were lysed to determine reconstituted Renilla luciferase reporter activity in a luciferase assay system (Promega) coupled with a BioTek Synergy 2 Multi-detection Microplate Reader (BioTekInstruments) according to the manufacturer’s recommendations. All experiments were measured in duplicate and repeated in triplicate. (D) SLCA specificity. COS-1 cells in a 12-well plate were transfected with PA-LC with empty LN vector or LN-PB1. At 48 h post transfection, the luciferase activities as a result of the association of LN and LC fragments through protein-protein interactions were measured as described above. Results represent the mean of three independent experiments plus standard deviation.
Renilla luciferase (RL) was selected as an enzyme for the assay and a split approach of Renilla luciferase was employed involving an N-terminal fragment consisting of amino acids 1-229 (termed LN) and a C-terminal fragment consisting of amino acids 230-311 (termed LC) (Fig. 1A). This design has been validated previously in an SLCA for the detection of protein-protein interactions (Fujikawa Kato, 2007). Considering that the orientation of fusion proteins may sterically affect the association of the RL fragments subsequently inhibiting enzymatic activity, fusion constructs representing the influenza B PA and PB1 proteins were generated in multiple configurations (Fig. 1B). To express influenza B viral polymerase proteins, the eukaryotic expression vector pPRE was used under the control of both the cytomegalovirus (CMV) immediate-early promoter and the bovine growth hormone polyadenylation signal as described previously (Patnaik et al., 2002; Wang et al., 2010). Three steps were required to generate various expression vectors used in the SLCA study. First, N-terminal fragment (residues 1-229 of Renilla luciferase) were amplified from the Renilla luciferase expression vector pGL4.76 (Promega) and inserted into the pPRE vector by digestion with EcoRI/NotI and then re-ligated. The resultant vector was designated pLN. Second, the same method was used to generate the vector pLC for expression of the C-terminal fragment (residues 230-311 of Renilla luciferase). Third, complete open-reading frames encoding PA and PB1 were amplified from an influenza B/Lee/40 reverse genetic system (Deng et al., 2011) and fused via a protein linker (GGGS x 2) to the N-terminus or C-terminus of the LN or LC fragments, respectively, which resulted in two groups of fusion plasmids: the PA group (PA-LN, PA-PC, LN-PA, and LC-PA) and the PB1 group (PB1-LC, LN-PB1, and LC-PB1). Primers and sequence information for each construct is available upon request. Initial screening of all possible combinations of SLCA PA/PB1 pairs for reconstituted RL enzymatic activity resulted in the identification of an interacting PA/PB1 pair (PA-LC and LN-PB1), which exhibited the highest activity (~15000RLU) (Fig. 1C). Thus, the PA-LC and LN-PB1 pair became the focus of our study.
COS-1 cells were cultured in Dulbecco’s minimal essential medium (DMEM) supplemented with 10% fetal bovine serum and grown on 12-well plates. Cells were then transfected using TransIT-LT1 (Mirus) following the procedures outlined by the manufacturer with PA-LC and LN-PB1 or LN empty vector (0.5 μg each plasmid). At 48 h post-transfection, cell lysates were collected to measure luciferase activity as a function of protein-protein interactions using a luciferase assay system (Promega) following the manufacturer’s recommendations. Transfection with both PA-LC and empty LN vectors served as a specificity control. As demonstrated in Fig. 1D, transfections involving PA-LC/LN constructs resulted in very low background signals (~120RLU), while transfections with a PA/PB1 pair (PA-LC/LN-PB1) produced ~4800RLU. ~40-fold increase of luciferase activity against the background observed in the PA-LC/LN-PB1 pair indicated that SLCA is robust and specific in detecting PA-PB1 interaction.
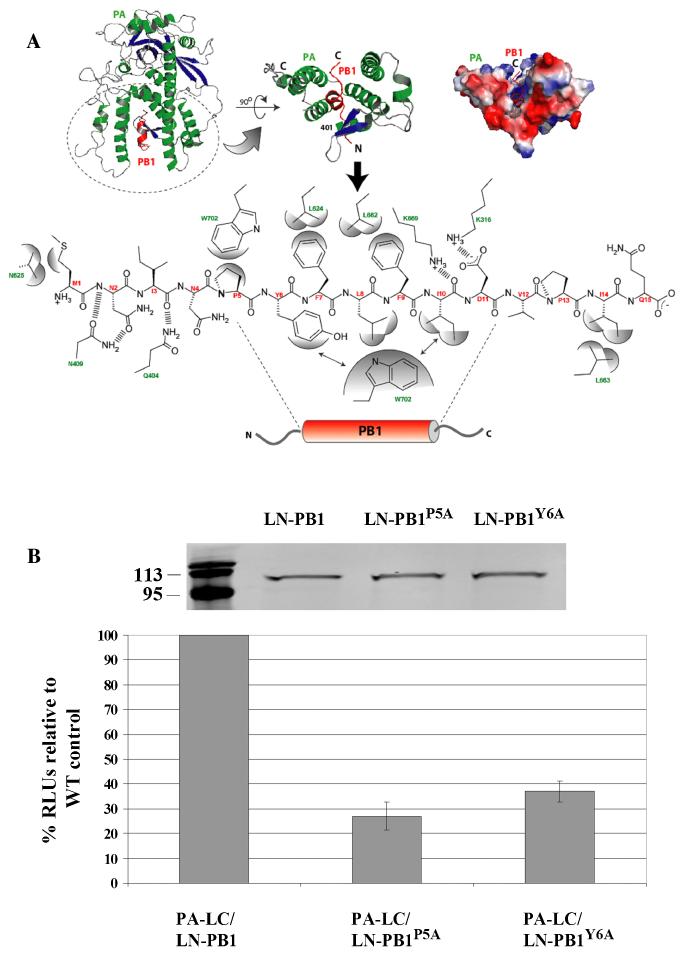
This approach was then validated by targeting the PA-PB1 interaction interface. To designa rational mutation that disrupts the PA-PB1 interface, we took advantage of a recently solved high-resolution structure of the influenza A RNA polymerase (PA-PB1) complex, and used it as a template to model the influenza B PA-PB1 interface (He et al., 2008; Obayashi et al., 2008). Due to the lack of any deletions or insertions and high sequence identity (~33 and ~60% for PA and PB1, respectively) between influenza A and B virus polymerase proteins (data not shown), the influenza B PA-PB1 interaction interface can be readily modeled on the influenza A virus template (Fig. 2A). Examining the modeled PA-PB1 interface revealed that some of the key residues within the N-terminal domain of PB1 (i.e., PA-binding domain) that contribute to the PA-PB1 interface are identical between the two viruses (Fig. 2A). For example, three nonpolar helical residues (P5, L8, and F9) in the influenza A PB1 N-terminal domain that have been shown to be critical in the helical stability and provide apolar contacts with PA are present at the same positions in the influenza B PB1 protein (He et al., 2008; Obayashi et al., 2008). P5 and Y6 were chosen as targeted residues for site-specific mutagenesis because the structural and biological importance of these two residues in the influenza A PA-PB1 interaction has been well-elucidated (He et al., 2008; Obayashi et al., 2008). As demonstrated in Fig. 2B, mutation of the P5 or Y6 residue to an alanine residue had no obvious effect on fusion protein expression but inhibited PB1 binding to PA by approximately 65% (Y6A) and 73% (P5A), respectively, further indicating the assays’ specificity in detecting PA-PB1 interaction of influenza B virus.
Figure 2. Modeled interface of influenza B PA-PB1 complex and validation of SLCA approach in detecting PA-PB1 interaction.
(A) Influenza B PA-PB1 complex (top panel) with PA (green) and PB1 (red) highlighting the interface (top panel) with the interacting residues (PB1, red and PA, green) detailed in the bottom panel. (B) Validation of SLCA approach. COS-1 cells were transfected with either a WT PA/PB1 construct (PA-LC/LN-PB1) or mutant construct pairs (PA-LC/LN-PB1P5A and PA-LC/LN-PB1Y6A) which contained a substitution of an alanine for proline at position 5 or for tyrosine at position 6 in PB1 N-terminal domain responsible for binding PA. At 48 h posttransfection, cells were harvested and then lysed for characterization of protein expression by a western-blot assay using an anti-Renila Luciferase antibody as well as by measurement of RL activity. The RLUs of transfected cells by WT PA/PB1 pair was arbitrarily set at 100%. The RLUs of mutant PA/PB1 pairs were normalized by comparison with WT pair. Numbers on the left indicate the migration positions of molecular mass markers (in kilodaltons). Results represent the mean of three independent experiments plus standard deviation.
In summary, a simple and specific split luciferase complementation assay was developed which can be used for detecting viral protein-protein interactions. SLCA is straightforward and can be performed easily using a luminometer and standard molecular biology and cell culture reagents. With the potential automation in a high-throughput screening format, this assay can be used alone or in conjunction with Y2H or other assays to study viral protein-protein interactions and to reveal the intricate networks that viruses employ to benefit their replication in the host. This information is useful for the design novel antiviral agents to treat viral diseases and overcome drug resistance associated with current antiviral drugs.
ACKNOWLEDGMENTS
We thank Elizabeth Kolb for editing the manuscript and thank members of the Li lab for helpful discussions and critical reviews of the manuscript. We thank Ruben Donis at the CDC for providing Flu B reagents. We acknowledge the use of the SDSU-Functional Genomics Core Facility supported in part by NSF/EPSCoR Grant No. 0091948, the Center of Excellence in Drought Tolerance through the South Dakota 2010 Initiative and the South Dakota Agri. Exp. Station. Research in the S.C. laboratory is supported by the South Dakota Agri. Exp. Station and the South Dakota 2010 Research Center, BCAAP (Biological Control and Analysis of Applied Photonics) Fund (3SG163 to S.C.). This work was supported by the South Dakota Agricultural Experiment Station (3AH203 to F.L.) and Public Health Service grants (AI078177) from NIAID to F.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bailer SM, Haas J. Connecting viral with cellular interactomes. Curr Opin Microbiol. 2009;12:453–459. doi: 10.1016/j.mib.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood MA, Venkatesan K, Xing L, Chase MR, Vazquez A, Holthaus AM, Ewence AE, Li N, Hirozane-Kishikawa T, Hill DE, Vidal M, Kieff E, Johannsen E. Epstein-Barr virus and virus human protein interaction maps. Proc Natl Acad Sci U S A. 2007;104:7606–7611. doi: 10.1073/pnas.0702332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cissell KA, Rahimi Y, Shrestha S, Deo SK. Reassembly of a bioluminescent protein Renilla luciferase directed through DNA hybridization. Bioconjug Chem. 2009;20:15–19. doi: 10.1021/bc8003099. [DOI] [PubMed] [Google Scholar]
- de Chassey B, Navratil V, Tafforeau L, Hiet MS, Aublin-Gex A, Agaugue S, Meiffren G, Pradezynski F, Faria BF, Chantier T, Le Breton M, Pellet J, Davoust N, Mangeot PE, Chaboud A, Penin F, Jacob Y, Vidalain PO, Vidal M, Andre P, Rabourdin-Combe C, Lotteau V. Hepatitis C virus infection protein network. Mol Syst Biol. 2008;4:230. doi: 10.1038/msb.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Wang D, Xiang X, Gao X, Hardwidge PR, Kaushik R, Wolff T, Chakravarty S, Li F. Nuclear localization of influenza B polymerase proteins and their binary complexes. Virus Res. 2011 doi: 10.1016/j.virusres.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum E, Friedel CC, Rajagopala SV, Titz B, Baiker A, Schmidt T, Kraus T, Stellberger T, Rutenberg C, Suthram S, Bandyopadhyay S, Rose D, von Brunn A, Uhlmann M, Zeretzke C, Dong YA, Boulet H, Koegl M, Bailer SM, Koszinowski U, Ideker T, Uetz P, Zimmer R, Haas J. Evolutionarily conserved herpesviral protein interaction networks. PLoS Pathog. 2009;5:e1000570. doi: 10.1371/journal.ppat.1000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa Y, Kato N. Split luciferase complementation assay to study protein-protein interactions in Arabidopsis protoplasts. Plant J. 2007;52:185–195. doi: 10.1111/j.1365-313X.2007.03214.x. [DOI] [PubMed] [Google Scholar]
- He X, Zhou J, Bartlam M, Zhang R, Ma J, Lou Z, Li X, Li J, Joachimiak A, Zeng Z, Ge R, Rao Z, Liu Y. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature. 2008;454:1123–1126. doi: 10.1038/nature07120. [DOI] [PubMed] [Google Scholar]
- Kaihara A, Kawai Y, Sato M, Ozawa T, Umezawa Y. Locating a protein-protein interaction in living cells via split Renilla luciferase complementation. Anal Chem. 2003;75:4176–4181. doi: 10.1021/ac0300800. [DOI] [PubMed] [Google Scholar]
- Luker KE, Piwnica-Worms D. Optimizing luciferase protein fragment complementation for bioluminescent imaging of protein-protein interactions in live cells and animals. Methods Enzymol. 2004;385:349–360. doi: 10.1016/S0076-6879(04)85019-5. [DOI] [PubMed] [Google Scholar]
- Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101:12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud TF, Paulmurugan R, Gambhir SS. Molecular imaging of homodimeric protein-protein interactions in living subjects. FASEB J. 2004;18:1105–1107. doi: 10.1096/fj.03-1128fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi E, Yoshida H, Kawai F, Shibayama N, Kawaguchi A, Nagata K, Tame JR, Park SY. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature. 2008;454:1127–1131. doi: 10.1038/nature07225. [DOI] [PubMed] [Google Scholar]
- Patnaik A, Chau V, Li F, Montelaro RC, Wills JW. Budding of equine infectious anemia virus is insensitive to proteasome inhibitors. J Virol. 2002;76:2641–2647. doi: 10.1128/JVI.76.6.2641-2647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Gambhir SS. Monitoring protein-protein interactions using split synthetic renilla luciferase protein-fragment-assisted complementation. Anal Chem. 2003;75:1584–1589. doi: 10.1021/ac020731c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Massoud TF, Huang J, Gambhir SS. Molecular imaging of drug-modulated protein-protein interactions in living subjects. Cancer Res. 2004;64:2113–2119. doi: 10.1158/0008-5472.can-03-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Umezawa Y, Gambhir SS. Noninvasive imaging of protein-protein interactions in living subjects by using reporter protein complementation and reconstitution strategies. Proc Natl Acad Sci U S A. 2002;99:15608–15613. doi: 10.1073/pnas.242594299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen R, Sathish N, Li Y, Yuan Y. Virion-wide protein interactions of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2008;82:4742–4750. doi: 10.1128/JVI.02745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P, Dong YA, Zeretzke C, Atzler C, Baiker A, Berger B, Rajagopala SV, Roupelieva M, Rose D, Fossum E, Haas J. Herpesviral protein networks and their interaction with the human proteome. Science. 2006;311:239–242. doi: 10.1126/science.1116804. [DOI] [PubMed] [Google Scholar]
- Wang D, Harmon A, Jin J, Francis DH, Christopher-Hennings J, Nelson E, Montelaro RC, Li F. The lack of an inherent membrane targeting signal is responsible for the failure of the matrix (M1) protein of influenza A virus to bud into virus-like particles. J Virol. 2010;84:4673–4681. doi: 10.1128/JVI.02306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Juozapaitis M, Manz B, Mayer D, Gotz V, Zohner A, Wolff T, Schwemmle M, Martin A. Limited compatibility of polymerase subunit interactions in influenza A and B viruses. J Biol Chem. 2010;285:16704–16712. doi: 10.1074/jbc.M110.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich K, Mayer D, Ranadheera C, Holler AS, Manz B, Martin A, Chase G, Tegge W, Frank R, Kessler U, Schwemmle M. Identification of a PA-binding peptide with inhibitory activity against influenza A and B virus replication. PLoS One. 2009;4:e7517. doi: 10.1371/journal.pone.0007517. [DOI] [PMC free article] [PubMed] [Google Scholar]