Abstract
To identify and characterize individual Ca2+ pumps, we have expressed an Arabidopsis ECA1 gene encoding an endoplasmic reticulum-type Ca2+-ATPase homolog in the yeast (Saccharomyces cerevisiae) mutant K616. The mutant (pmc1pmr1cnb1) lacks a Golgi and a vacuolar membrane Ca2+ pump and grows very poorly on Ca2+-depleted medium. Membranes isolated from the mutant showed high H+/Ca2+-antiport but no Ca2+-pump activity. Expression of ECA1 in endomembranes increased mutant growth by 10- to 20-fold in Ca2+-depleted medium. 45Ca2+ pumping into vesicles from ECA1 transformants was detected after the H+/Ca2+-antiport activity was eliminated with bafilomycin A1 and gramicidin D. The pump had a high affinity for Ca2+ (Km = 30 nm) and displayed two affinities for ATP (Km of 20 and 235 μm). Cyclopiazonic acid, a specific blocker of animal sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, inhibited Ca2+ transport (50% inhibition dose = 3 nmol/mg protein), but thapsigargin (3 μm) did not. Transport was insensitive to calmodulin. These results suggest that this endoplasmic reticulum-type Ca2+-ATPase could support cell growth in plants as in yeast by maintaining submicromolar levels of cytosolic Ca2+ and replenishing Ca2+ in endomembrane compartments. This study demonstrates that the yeast K616 mutant provides a powerful expression system to study the structure/function relationships of Ca2+ pumps from eukaryotes.
The role of Ca2+ in signaling and development is well recognized in eukaryotes (Bush, 1995; Clapham, 1995); however, the regulation of cytosolic and organellar Ca2+ in plants is still poorly understood. In plant cells the cytosol usually contains 30 to 600 nm free Ca2+ (Reed et al., 1992), whereas the cell wall and intracellular stores, such as the ER and vacuole, contain 1,000- to 10,000-fold higher concentrations. The Ca2+ gradients across the PM and the intracellular membranes are energized by primary Ca2+-ATPases and by H+-coupled Ca2+ antiport (Hirschi et al., 1996). A variety of signals induces transient increases in cytosolic Ca2+ because of the opening of specific Ca2+ channels (Bush, 1995). Recent studies with Ca2+-indicator dyes demonstrated that the perception of a nodulation signal results in Ca2+ waves or oscillations in root-hair cells of alfalfa (Ehrhardt et al., 1996). In frog oocytes inositol 1,4,5-trisphosphate induced repetitive Ca2+ waves; however, the frequency of the waves increased in cells overexpressing a SER Ca2+ pump (Camacho and Lechleiter, 1993). Because the SERCA pumps cytosolic Ca2+ into endomembrane compartments, intracellular Ca2+ pumps could be an important factor in controlling Ca2+ oscillations in plant and in animal cells.
Ca2+ is also essential for tip growth of pollen tubes. A recent study showed that the tip-focused intracellular [Ca2+] gradient oscillates with the same period as growth (Holdaway-Clarke et al., 1997). High levels of Ca2+ in intracellular compartments serve a variety of essential functions. This divalent cation is a cofactor for specific enzymes (Bush et al., 1989) and can bind to several chaperones in the ER (Bergeron et al., 1994). Thus, endoluminal Ca2+ supplied by Ca2+ pumps on the ER, Golgi, and secretory vesicles could affect processing and sorting and determine the ultimate fate of membrane and secreted proteins (Rudolph et al., 1989). Furthermore, nuclear cisternal Ca2+ controls nuclear pore permeability and thus regulates transport across the nuclear envelope (Perez-Terzic et al., 1997).
Although high-affinity, Ca2+-pumping ATPases have been identified in a variety of membranes, including the PM, ER, and tonoplast, the characterization of individual pumps separate from other related pumps has been difficult in many plant studies. Extensive biochemical studies demonstrated that plants have two major types of Ca2+ pumps with distinct properties. The PM-type Ca2+-ATPase is stimulated by calmodulin (Bonza et al., 1998), whereas the ER type is not (Bush, 1995; Hwang et al., 1997). However, unlike the PM-bound Ca2+ pumps from animals, the PM-type Ca2+-ATPase is localized to several membranes in plants (Askerlund, 1997; Hwang et al., 1997), suggesting a family of calmodulin-stimulated pumps. Several genes encoding Ca2+-ATPase homologs from plants have been isolated. Based on similarities of the deduced amino acid sequence with animal Ca2+ pumps, LCA1 from tomato (Wimmers et al., 1992) and a gene from rice (Chen et al., 1997) encoded ER-type Ca2+ pumps. PEA1 from Arabidopsis (Huang et al., 1993) and BCA1 from cauliflower (Malmstrom et al., 1997) encoded a PM-type Ca2+-ATPase homolog. Except for BCA1, the biochemical activities of these gene products have not been demonstrated.
To study individual Ca2+ pumps, we recently identified two distinct Ca2+-ATPases by functional expression of two plant genes in a yeast triple mutant. One cDNA (ECA1) encoded an ER Ca2+-ATPase homolog from Arabidopsis (Liang et al., 1997), and another gene (ACA2, accession no. AF025842) encoded a PM-type Ca2+-ATPase with a unique N-terminal regulatory domain (Harper et al., 1998). ECA1 encoded a 116-kD polypeptide that was more homologous to animal SERCA than to PM-type Ca2+ pumps. When ECA1 was expressed in a yeast triple mutant defective in both a Golgi and a vacuolar Ca2+ pump (pmr1pmc1cnb1) (Cunningham and Fink, 1994), growth of this mutant in Ca2+-depleted medium was restored. Furthermore, ECA1 could be phosphorylated in a Ca2+-dependent manner in vitro similarly to phosphoenzyme intermediates formed in the reaction cycle of Ca2+-ATPases. Therefore, ECA1 encoded a Ca2+-dependent ATPase (Liang et al., 1997); however, we were unable to demonstrate Ca2+-pumping activity because of high activity from the Ca2+ antiporter (Vcx1).
Here we provide the first biochemical characterization, to our knowledge, of a plant Ca+ pump functionally expressed in yeast. We show that ECA1 encodes a high-affinity plant Ca2+ pump that is blocked by cyclopiazonic acid. ECA1 shares many similarities with animal SER-type Ca2+-ATPases; however, it is unique in its insensitivity to thapsigargin. We also demonstrate that a yeast triple mutant provides a powerful expression system with which to study individual Ca2+ pumps from heterologous systems.
MATERIALS AND METHODS
Yeast Strain, Plasmid, and Growth Medium
Yeast (Saccharomyces cerevisiae) strain K616 (MATa pmr1::HIS3 pmc1::TRP1 cnb1::LEU2, ura3; Cunningham and Fink, 1994) is often referred to as the triple mutant. The full-length cDNA of the ECA1 gene (accession no. U96455) from Arabidopsis was constructed into the yeast expression vector p426Gal1 under the control of the Gal-inducible promoter (Liang et al., 1997). The mutant K616 was transformed with this construct or the empty vector using the lithium acetate method (Chen et al., 1992). Transformants were selected on SC-URA. The growth medium consisted of 6.7 g/L yeast nitrogen base without amino acids, 2 g/L drop-out mixture without uracil, and 2% Gal (Rose et al., 1990).
Yeast Growth
To measure the growth of mutant K616 strains transformed with either ECA1 or with vector alone, cells at the late-log phase were harvested by centrifugation and suspended in 10 mL of SC-URA that contained 1 mm Ca2+. The cell suspension was used to inoculate 20 mL of SC-URA (pH 6.2) to an initial A600 of 0.01. Either 10 mm Ca2+ or varying concentrations of EGTA was added to the medium to control free-Ca2+ levels. Growth at 30°C was monitored by the change in A600 of 0.8-mL samples for 2 d.
Isolation of Membrane Vesicles
Vesicles were isolated after cell disruption using the glass-bead method (Liang et al., 1997) with some modification. Transformants were inoculated into 25 mL of SC-URA and incubated overnight at 30°C. The seed culture was diluted into 250 to 300 mL of SC-URA/Gal and incubated until the A600 reached 1.5 to 2.0. Cells were pelleted at 4000g for 5 min, washed with 10 mL of distilled water, and pelleted. To isolate vesicles for transport studies, 2 mm MgCl2 was included in all of the solutions to facilitate separation of the ER from the vacuolar vesicles (see below). The cell pellet was suspended in 10 mL of glass-bead buffer and pelleted. The glass-bead buffer consisted of 10% Suc, 25 mm Hepes-BTP, pH 7.5, 2 mm MgCl2, 2 mm DTT, and 1 mm EGTA.
Typically, 3 to 4 mL of cells was resuspended in 1 volume of glass-bead buffer plus 1 mm PMSF, 10 mm benzamidine, 5 μg/mL pepstatin, 5 μg/mL leupeptin, and 0.5% BSA, and split into two Corning tubes (50 mL). An equal volume of glass beads (Sigma) was added and the mixture was vortex mixed four times for 30 s each. The lysate was centrifuged at 5,000g for 5 min and the supernatant was saved. The pellet was suspended in 1 volume of glass-bead buffer plus protease inhibitors, vortex mixed, and centrifuged as described above. Then, 2 to 3 mL of the pooled supernatant was layered onto a step gradient containing 6 mL each of 25% and 45% Suc in 20 mm Hepes-BTP (pH 7.0), 1 mm DTT, 2 mm MgSO4, 0.2 mm PMSF, and 5 mm benzamidine, and centrifuged (model SW 28 centrifuge, Beckman) at 108,000g for 2 h. Membranes at the 26%/45% Suc interface were collected and diluted 6- to 8-fold in a suspension solution containing 25 mm Hepes-BTP (pH 7.0), 1 mm DTT, 2 mm MgSO4, and protease inhibitors. After the sample was centrifuged at 108,000g for 50 min, the pellet was suspended in the same solution and stored at −80°C. The protein concentration was determined with the Bio-Rad reagent.
To determine the distribution of ECA1 in yeast membranes, microsomes were isolated in the presence or absence of Mg2+. About 0.5 mL of cells from 50 mL of overnight culture was suspended in 1 volume of glass-bead buffer with either 2 mm MgSO4 or 2 mm EDTA. The glass-bead buffer included 0.5 mm PMSF, 2 mm benzamidine, 5 μg/mL pepstatin, 5 μg/mL leupeptin, and 0.5% BSA. Cells were disrupted with the buffer as described above. The lysate was centrifuged at 5,000g for 5 min and the supernatant was saved. The pellet was suspended in glass-bead buffer, vortexed, and centrifuged as described above. The supernatants were pooled and pelleted at 108,000g for 50 min. The microsomal pellet was resuspended in 0.8 mL of the above solution without BSA and layered onto a step gradient with 1.2 mL each of 12%, 15%, 18%, 21%, 24%, 27%, 30%, 33%, 36%, 39%, 42%, and 45% Suc. The Suc solutions contained 25 mm Hepes-BTP, pH 7.0, 1 mm DTT, 0.1 mm PMSF, and 2 mm benzamidine with either 2 mm MgSO4 or 2 mm EDTA. After the sample was centrifuged at 110,000g for 16 h, 0.75-mL fractions were collected and stored at −80°C.
45Ca2+ Uptake
Ca2+ uptake into membrane vesicles was measured by the filtration method. Typically, transport was initiated with 3 mm ATP in a reaction mixture (250 μL) containing 250 mm Suc, 25 mm Hepes/BTP (pH 7.0), 10 mm KCl, 0.4 mm NaN3, 3 mm MgSO4, 100 μm EGTA, and 10 μm 45CaCl (3000 Ci/mmol, NEN-Dupont) so the final specific activity was 1 to 2 μCi/2.5 nmol Ca2+ per reaction. Under these conditions, the calculated free-Ca2+ concentration is about 0.1 μm (Bers et al., 1994). For measuring ΔpH-independent Ca2+-pumping activity, 0.5 μm bafilomycin A1 and 5 μm gramicidin D were routinely included. After incubation at 22°C, 220 μL was run through a filter (0.45-μm pore size, Millipore) moistened with a rinse solution containing 250 mm Suc, 2.5 mm Hepes-BTP (pH 7.0), and 0.2 mm CaCl2. The filter was washed with 5 mL of a cold rinse solution. The 45Ca2+ radioactivity associated with the filter was determined by liquid-scintillation counting. Active transport was determined as the difference between activity in the presence and absence of ATP, and is expressed as nanomoles of Ca2+ per milligram of protein. All inhibitors were preincubated with membranes at 22°C for 15 min before the reaction was started. To determine the Km for Ca2+, the reaction mixture contained 500 μm EGTA and various amounts of Ca2+ to give the desired range of the free-Ca2+ concentration (10 nm to 2 or 3 μm).
Electrophoresis, Immunostaining, and Calmodulin Overlay
These procedures were described previously (Hwang et al., 1997; Liang et al., 1997) and are briefly described in the figure legends. After SDS-PAGE, proteins were transferred to Immobilon-P membranes (Millipore) in 25 mm Tris, pH 8.3, 192 mm Gly, and 15% methanol. To determine the calmodulin binding, the blot was blocked for 1 h with 1% BSA in TBS/CaMg (50 mm Tris, pH 7.5, 0.2 m NaCl, and 50 mm MgCl2, with or without 0.5 mm CaCl2). Then the blot was incubated with 100 ng/mL biotinylated calmodulin in TBS/CaMg for 2 h at 22°C and washed twice in TBS/CaMg plus 0.05% Tween 20. After the sample was incubated with streptavidin conjugated to alkaline phosphatase, color was developed with 5-bromo-4-chloro-3-indoyl phosphate and nitroblue tetrazolium (Sigma). To detect nonspecific binding, electroblotted proteins were incubated with biotinylated calmodulin in the presence of 1 mm EGTA. Bovine calcineurin (Sigma) was used as a positive control.
Chemicals
Erythrosin B, cyclopiazonic acid, and gramicidin D were obtained from Sigma, and thapsigargin was purchased from LC Service Co. (Woburn, MA). Bafilomycin A1 was a gift from Dr. Karlheinz Altendorf (University of Osnabrueck, Germany). All other chemicals were of a reagent grade.
RESULTS
An Arabidopsis Ca2+-ATPase ECA1 Increased Yeast Mutant K616 Growth on Ca2+-Depleted Medium
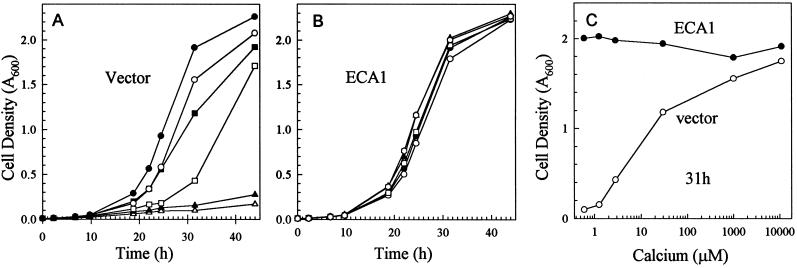
The yeast mutant K616 defective in both the Golgi (Pmr1) and the vacuolar Ca2+ (Pmc1) pumps was unable to grow on SC-URA plates containing 10 mm EGTA; therefore, we tested the growth rates of mutants expressing an Arabidopsis Ca2+-ATPase, ECA1, as a function of Ca2+ concentration. Although the control K616 mutant transformed with vector alone had a doubling time of 5 h in medium containing 1 to 10 mm Ca2+, growth was severely retarded as the free-Ca2+ concentration was lowered to 0.3 μm by 20 mm EGTA (Fig. 1A). In contrast, mutants transformed with the ECA1 gene had a doubling time of about 5 h when external Ca2+ was <1 μm (Fig. 1B). The initial free-Ca2+ concentration was estimated with the Max-Chelator program (Bers et al., 1994) based on the amount of EGTA added to the medium, which contained 1 mm Ca2+. At 31 h after inoculation, the relative density of ECA1-transformed mutants was 10- to 20-fold higher than that of the control mutants grown in medium containing approximately 0.3 μm Ca2+. The differential growth rate was consistently observed even when the medium pH was buffered to 6.0 (not shown). Therefore, expression of an Arabidopsis Ca2+-ATPase enhanced yeast mutant growth in submicromolar levels of Ca2+ to a rate nearly comparable to that of wild-type strains (not shown).
Figure 1.
ECA1 enhanced growth of yeast mutant K616 in Ca2+-depleted medium. A and B, Time course of yeast growth. Mutants transformed with vector (A) or ECA1 (B) were suspended in SC-URA (pH 6.2) to a final A600 of 0.01 and incubated at 30°C. The medium was either used directly (○) or supplemented with 10 mm CaCl2 (•) or 2 (▪), 5 (□), 10 (▴), or 20 (▵) mm EGTA. The turbidity of the culture was measured at 600 nm over time. C, Effect of free-Ca2+ concentration on growth of mutant K616 harboring vector control (○) or ECA1 (•). A600 was determined after 31 h of growth.
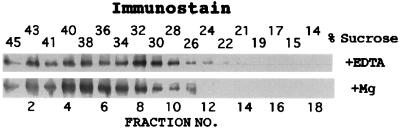
To determine the subcellular location of ECA1 in yeast, microsomes were isolated from ECA1-transformed mutants and fractionated by a Suc-density gradient. The fractions were analyzed by SDS-PAGE, blotted, and immunostained with a polyclonal antibody against the carboxyl terminus of ECA1. In the presence of EDTA, ECA1 was broadly distributed with an apparent peak at approximately 32% Suc. When Mg2+ was present during cell isolation and in gradient fractionation, ECa1p was concentrated at 38% to 40% Suc (Fig. 2). As EDTA chelates Mg2+ and dissociates ribosomes from the ER, the shift in ECA1 suggested that this polypeptide was associated with the ER. This interpretation agrees with other studies in which yeast ER was found at 40% Suc (Antebi et al., 1992). However, overexpression of this protein could result in localization to other endomembranes, such as the Golgi. To investigate the transport properties of ECA1, vesicles were routinely isolated from the interface of a 26%/45% Suc step gradient containing Mg2+.
Figure 2.
ECA1 was associated with ER vesicles from yeast. Microsomes were isolated from the ECA1-transformed mutant and fractionated with a Suc gradient containing 2 mm EDTA (top) or 2 mm MgCl2 (bottom). Proteins (1–5 μg/lane) were separated by 7% SDS-PAGE, blotted, and immunostained with polyclonal antibodies against ECA1 (1:10,000).
The Control K616 Mutant Has High H+/Ca2+Antiport Activity
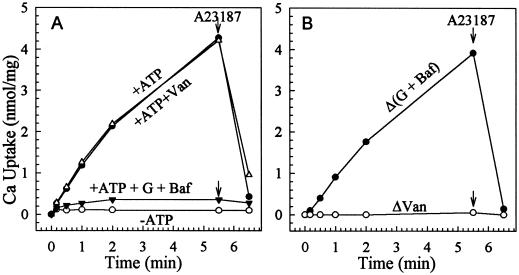
We first tested for background Ca2+ transport in vesicles isolated from the control mutant K616 transformed with vector alone. At a free-Ca2+ concentration of approximately 0.1 μm, ATP increased 45Ca2+ associated with vesicles. The Ca2+ was released by the ionophore A23187, indicating that it had accumulated against a concentration gradient. Transport was resistant to sodium vanadate, a P-type cation-pumping ATPase inhibitor (Nechay, 1984), but was decreased by gramicidin D, bafilomycin A1, or both (Fig. 3). Since bafilomycin specifically inhibits proton pumping by the vacuolar H+-ATPase and gramicidin dissipates proton gradients, the results demonstrated that more than 95% of Ca2+ transport was ΔpH dependent (Fig. 3). Thus, nearly all of the Ca2+ uptake was driven by the H+/Ca2+-antiport activity of the Vcx1 (Cunningham and Fink, 1996). Either gramicidin or bafilomycin alone inhibited 80% to 90% of the ΔpH-dependent Ca2+ uptake (data not shown); however, both compounds were needed to achieve maximum inhibition of antiport activity.
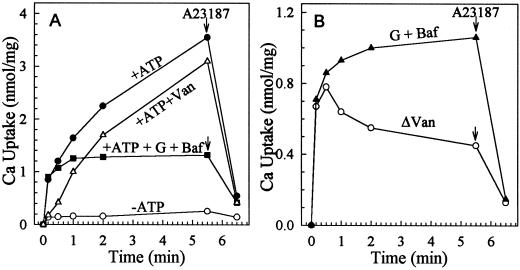
Figure 3.
Active 45Ca2+ uptake into vesicles of control mutant is ΔpH dependent. Microsomal vesicles were isolated from the vector-transformed K616 strain. Uptake was assayed in a mixture containing 0.1 μm free Ca2+. A, Total uptake. Activity was assayed in the absence (−ATP, ○) or presence (+ATP) of 3 mm ATP with or without inhibitors (•). When added, the concentrations of sodium vanadate (Van, ▵) or gramicidin D and bafilomycin A1 (G + Baf ▾) were 200 or 5 and 0.5 μm, respectively. B, ΔpH-dependent (ΔpH, •) and vanadate-sensitive (ΔVan, ○) Ca2+ transport. ΔpH or Δ(G + Baf) refers to the difference between total activity (+ATP) and that measured with bafilomycin and gramicidin (+ATP + G + Baf). ΔVan refers to the difference between total activity (+ATP) and that measured in the presence of vanadate (+ATP + Van). The arrows indicate addition of 1 μm ionophore A23187.
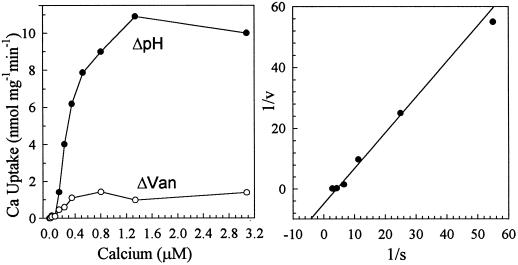
The ΔpH-dependent Ca2+ transport had an apparent affinity for Ca2+ of 350 ± 50 nm (n = 5; Fig. 4), which is lower than the published Km for Ca2+ of 10 to 100 μm reported for wild-type yeast (Ohsumi and Anraku, 1983; Dunn et al., 1994). A small component (<10%) of the vesicle-associated Ca2+ appeared to be vanadate sensitive when free [Ca2+] was >0.1 μm; however, this component was mainly due to ATP-dependent binding because the Ca2+ was not released by the ionophore A23187 (not shown).
Figure 4.
Ca2+ affinity of ΔpH-dependent Ca2+ transport. Vesicles were isolated from mutant K616 transformed with vector. Left, Uptake was determined at 40 s after 3 mm ATP was added to a reaction medium containing 10 nm to 3 μm free Ca2+. ΔpH-dependent Ca2+ transport (ΔpH, •) was determined as activity that was inhibited by 5 μm gramicidin and 0.5 μm bafilomycin. Vanadate-sensitive Ca2+ transport refers to activity inhibited by 200 μm sodium vanadate (ΔVan, ○). Right, Lineweaver-Burk plot. ΔpH-dependent uptake has a Km for Ca2+ of 350 ± 50 nm (n = 5).
ECA1-Catalyzed Ca2+-Pump Activity Is ΔpH Independent
To determine whether ECA1 encoded a functional Ca2+ pump, membrane vesicles were isolated from ECA1-transformed mutants and assayed for 45Ca uptake. In contrast to the findings shown in Figure 3, sodium vanadate consistently inhibited ATP-driven Ca2+ uptake partially (Fig. 5A). Furthermore, a component (50%) of the Ca2+ accumulated was resistant consistently to a combination of 5 μm gramicidin and 0.5 μm bafilomycin A1 (Fig. 5). Thus, the transport component that was ΔpH independent and vanadate sensitive resembled activity from a Ca2+ pump.
Figure 5.
Vanadate-sensitive and ΔpH-independent Ca2+-pumping activity. Vesicles were isolated from the ECA1-transformed mutant K616. 45Ca uptake was assayed as described in Figure 3. A, Total uptake. Uptake was determined without (−ATP, ○) or with ATP in the absence (+ATP, •) or presence of 200 μm sodium vanadate (+Van, ▵) or 5 μm gramicidin and 0.5 μm bafilomycin (G + Baf, ▪). B, ΔpH-independent (•) and vanadate-sensitive (ΔVan, ○) uptake were similar initially. The initial rate was approximately 4 nmol mg−1 protein min−1. The arrows indicate the addition of 1 μm ionophore A23187.
Ca2+ uptake driven by the antiport, but not by the pump, was enhanced 4-fold and 3-fold by 10 mm potassium oxalate and 10 mm potassium phosphate, respectively (Table I). Oxalate stimulation of Ca2+ uptake is thought to be caused by formation of Ca2+ oxalate precipitate inside the vesicles, thus decreasing the magnitude of the Ca2+ chemical gradient. Formation of Ca2+ phosphate precipitate in vesicles will result in a similar stimulatory effect of Ca2+ uptake. The differential stimulation of anions suggested that an oxalate carrier colocalized to the same vesicle membrane as the antiporter and that the pump, in spite of overexpression, resided on another membrane that was not permeable to oxalate or phosphate.
Table I.
Potassium oxalate and potassium phosphate stimulated Ca2+/H+-antiport but not Ca2+-pump activity
| Potassium Salt | Net
Ca2+ Uptake
|
||
|---|---|---|---|
| Total | Pump | Antiport | |
| nmol/mg (-fold) | |||
| None | 1.92 (1.00) | 0.98 (1.00) | 0.94 (1.00) |
| +Potassium oxalate | 5.00 (2.63) | 1.04 (1.06) | 3.96 (4.21) |
| +Potassium phosphate | 3.95 (2.06) | 0.90 (0.92) | 3.05 (3.25) |
Net ATP-dependent 45Ca2+ uptake at 6 min was determined with 0.1 μm Ca in the reaction mixture as described in Figure 5. Uptake was measured in the absence (Total) or presence (Pump) of 5 μm gramicidin and 0.5 μm bafilomycin. Antiport activity was determined as the difference between “total” and “pump” activity. Potassium oxalate or potassium phosphate was added to a final concentration of 10 mM. Data represent the averages of two experiments.
Although the initial rate of Ca2+ uptake by the pump (4 nmol mg−1 min−1) was 3- to 4-fold higher than that of the antiporter (1 nmol mg−1 min−1), net uptake by antiport activity was 3- to 4-fold higher than that by the pump. This difference could be due to one or more of the following: (a) differential driving force, (b) differential transport rate, or (c) differential Ca2+ leakage from the compartments via channels. These results further support the idea that the pump and antiport reside in separate compartments.
Although pump activity could be determined as either ΔpH-independent or vanadate-inhibitable Ca2+ activity, net uptake of the vanadate-sensitive component declined over time (Fig. 5B). It is possible that the high-capacity Vcx1 competed with the pump for free Ca2+ in the absence of bafilomycin and thus depleted the free-Ca2+ concentration in the medium. Therefore, subsequent ECA1-pump activity was measured as ΔpH-independent transport in the presence of gramicidin and bafilomycin A1.
High Affinity for Ca2+ and ATP
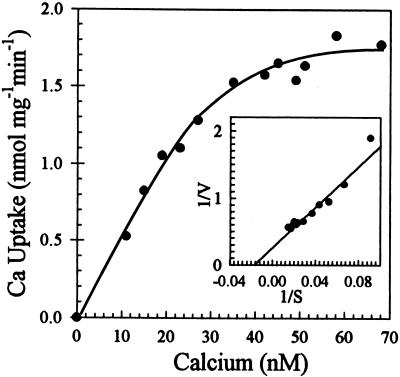
The initial rate of pumping was measured as bafilomycin- and gramicidin-resistant Ca2+ uptake at 40 s when uptake was nearly linear with incubation time (Fig. 5A). Free-Ca2+ concentration was controlled by varying the total Ca2+ added in the presence of 0.5 mm EGTA to give a final free-ion concentration ranging from 10 to 2000 nm (not shown). Surprisingly, the average Km for Ca2+ determined from five independent experiments was 30 ± 10 nm (Fig. 6). Thus, this ER-type Ca2+-ATPase has a high affinity for Ca2+ and reached a maximum velocity when Ca2+ was approximately 0.1 μm.
Figure 6.
ECA1 catalyzed high-affinity Ca2+-pumping activity. Ca2+ transport into vesicles isolated from the ECA1-transformed mutant K616 was performed as described in Figure 4, except that the free-Ca2+ concentration was buffered from 10 to 70 nm. Lineweaver-Burk plot (inset) showed an apparent Km for Ca2+ of 30 ± 10 nm (n = 5).
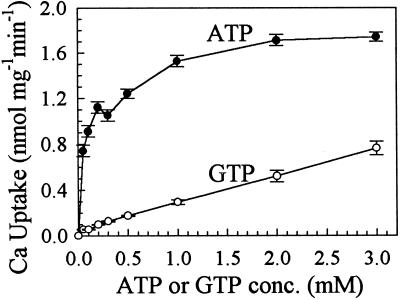
The initial rate of Ca2+ transport showed a biphasic dependence on ATP (Fig. 7), which is analogous to animal Ca2+ pumps. All assays were conducted at 40 s, when the ATP concentration had not changed significantly. These data are consistent with a model of two ATP-binding sites with Km values of 20 and 235 μm, respectively. GTP also energized Ca2+ pumping; however, we did not detect evidence for biphasic kinetics. The affinity of the pump for GTP was low, and the estimated Km for GTP was greater than 1.5 mm.
Figure 7.
High affinity for ATP. Vesicles were isolated from ECA1-transformed mutants for ATP- or GTP-dependent 45Ca transport. Reaction mixtures contained 0.1 μm free Ca2+, 5 μm gramicidin, 0.5 μm bafilomycin, and 25 μm to 3 mm ATP (•) or GTP (○). Uptake was measured at 40 s. Apparent ATP Km values were 20 and 235 μm. The results are the averages of three experiments. conc., Concentration.
Inhibitors of the ECA1 Pump
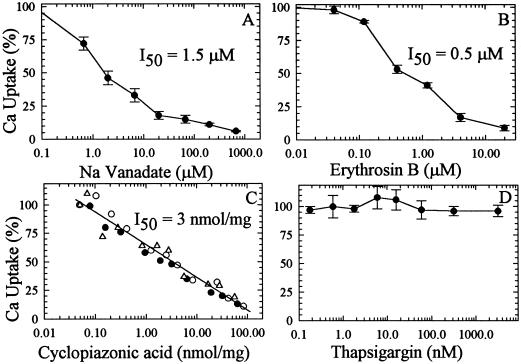
The initial rate of ECA1-catalyzed Ca2+ uptake was inhibited 50% by 1.5 μm sodium orthovanadate (Fig. 8A), a diagnostic inhibitor of P-type ATPases. Erythrosin B at about 0.5 μm inhibited Ca2+-pump activity by 50% (Fig. 8B). Erythrosin B is a halogenated derivative of fluorescein that could modify a Lys residue close to the ATP-binding site, causing inhibition of ATP binding (Mignaco et al., 1996).
Figure 8.
Inhibition of the ECA1 pump by vanadate and cyclopiazonic acid but not by thapsigargin. Vesicles were preincubated with each inhibitor or DMSO for 15 min at room temperature in the absence of ATP. To start the reaction, ATP was added to a mixture containing 0.1 μm free Ca2+ and various concentrations of sodium vanadate (A), erythrosin B (B), cyclopiazonic acid (C), or thapsigargin (D). The transport was stopped at 40 s. Control activity (100%) was 1.8 nmol Ca2+ mg−1 protein min−1. Data are the averages of three experiments. I50, Inhibitor concentration for 50% displacement.
Cyclopiazonic acid, an indole tetramic acid, has been reported to be a specific inhibitor of SER-type Ca2+-ATPase (Seidler et al., 1989). Pump activity from ECA1 was blocked 50% by 3 nmol cyclopiazonic acid mg−1 protein (Fig. 8C). This value is comparable to the concentration required to block SERCA. However, thapsigargin, a potent and specific inhibitor of SER-type Ca2+ pumps, had no effect on Ca2+ transport at concentrations up to 3 μm (or 66 nmol mg−1 protein; Fig. 8D).
No Effect by Calmodulin
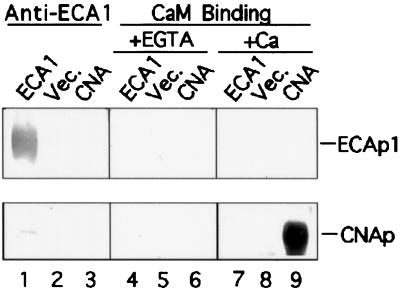
Because calmodulin-stimulated Ca2+ transport has been detected in the ER fraction from carrot cells (Hsieh et al., 1991; Hwang et al., 1997), we tested the effect of calmodulin on pump activity. Net Ca2+ uptake at 5 min was 0.65 nmol mg−1 protein with or without 1 μm calmodulin, suggesting that calmodulin did not stimulate this pump. To test whether ECA1 bound calmodulin, membranes were isolated and the proteins were separated by SDS-PAGE, blotted, and probed with biotinylated calmodulin (Fig. 9). As a positive control, we showed that calmodulin bound the 60-kD calcineurin A subunit in a Ca2+-dependent manner (Fig. 9, lanes 6 and 9). ECA1 was detected by immunostaining only in ECA1-transformed, but not in control, mutants (Fig. 9, lanes 1 and 2). However, calmodulin failed to bind any protein at approximately 116 kD from either membrane preparation. Thus, the ER-type Ca2+ pump encoded by ECA1 was not directly regulated by calmodulin.
Figure 9.
ECA1 did not bind calmodulin. Membranes were isolated from cells transformed with either vector alone (Vec.) or ECA1. Vesicle protein (5 μg) from the 25%/45% Suc interface was analyzed by SDS-PAGE, blotted onto an Immobilon-P membrane, and probed with an antibody against ECA1 (lanes 1–3). Another blot was probed with biotinylated calmodulin in the absence (+1 mm EGTA, lanes 4–6) or presence of (+Ca2+, lanes 7–9) 0.5 mm Ca2+. The bovine calcineurin A subunit (CNA; 0.2 μg protein/lane) is a calmodulin-binding protein.
DISCUSSION
A Functional Plant Ca2+ Pump Expressed in Yeast
Here we provide the first biochemical characterization, to our knowledge, of a functional Ca2+ pump encoded by a plant gene, ECA1. The pump properties were determined after the gene was expressed in a yeast mutant strain, K616, that lacked both the Golgi (Pmr1) and the vacuolar (Pmc1) Ca2+ pumps (Cunningham and Fink, 1994; Catty et al., 1997). The advantages of the triple mutant are: (a) the growth rate of the mutant on Ca2+-depleted medium provides an in vivo assay for functional identification of active Ca2+ pumps, (b) the control mutant is devoid of any background Ca2+-pump activity (Fig. 3) or Ca2+-dependent phosphoproteins (Liang et al., 1997), and (c) any heterologous gene encoding a Ca2+ pump could be overexpressed with a strong inducible promoter. Thus, the triple mutant provides an extremely valuable expression system for determining the nature and structure/function relationships of individual Ca2+ pumps from other eukaryotes.
Despite these advantages, Ca2+-pump activity is frequently masked by high H+/Ca2+-antiport activity in the triple mutant. Cunningham and Fink (1994, 1996) found that the double mutant pmr1pmc1 was not viable unless calcineurin function was disrupted. Evidence suggested that calcineurin acted as a negative regulator of VCX1 in wild-type strains. Loss of calcineurin function resulted in a triple mutant K616 (pmr1pmc1cnb1) that could tolerate high levels of Ca2+, apparently by activating the H+/Ca2+ antiport. Three strategies were used to separate pump activity from the vacuolar H+/Ca2+-antiport activity. First, vesicles enriched in ECA1 were collected from a 26%/45% Suc interface to minimize vacuoles at 22% Suc (Antebi and Fink, 1992; Sorin et al., 1997). Second, pump activity was assayed after inhibition of H+/Ca2+-antiport activity with proton ionophore, gramicidin, and a specific vacuolar H+-ATPase inhibitor, bafilomycin. Third, the free-Ca2+ concentration was reduced to 0.1 μm to reduce antiport activity and to minimize Ca2+ binding to membranes.
A High-Affinity Ca2+ Pump
The most striking feature of the ECA1 pump is its high affinity for Ca2+. The pump showed a Km for Ca2+ of about 30 nm (Fig. 6) relative to the Km of 350 nm of the H+/Ca2+ antiport. Transport was dependent on both Mg2+ (data not shown) and ATP (Fig. 5), and 1 to 3 mm was required for maximum pump activity (Fig. 7). It is interesting that the ECA1 pump displayed two affinities for ATP, 20 and 235 μm. In animal Ca2+ pumps the high-affinity site of about 2 μm is the hydrolytic site, whereas the low-affinity site ranging from 100 to 300 μm accelerates the reaction (Schatzmann, 1989). It is not clear whether the two sites are separate or whether one site changes in affinity in the reaction cycle.
Inhibition by Cyclopiazonic Acid but Not by Thapsigargin
Although sodium vanadate inhibited Ca2+ transport with a 50% inhibition of approximately 1.5 μm (Fig. 8A), it had little or no effect on the initial rate of phosphorylation (F. Liang, unpublished data). These results indicate that vanadate does not block the early steps in the reaction cycle. The reaction cycle of animal SERCA can move in the direction of ATP synthesis. In this mode, vanadate inhibits the Pi-dependent formation of E2P by competing for the phosphate-binding site (Schatzmann, 1989). Thus, vanadate inhibits Ca2+ transport, which depends on completion of many reaction cycles.
However, the ECA1 pump is insensitive to thapsigargin (Fig. 8D), a specific inhibitor of SERCA (Sagara and Inesi, 1991). It binds to SERCA with a one-to-one stoichiometry and a subnanomolar affinity on the third transmembrane segment (Norregaard et al., 1994). During turnover thapsigargin slowly inhibits activity by binding to the Ca2+-deprived intermediate at each cycle, leading to total inactivation. Thus, in a 1-mL reaction mixture containing 1 μg of sarcoplasmic reticulum protein, 4 pmol of thapsigargin (4 nm) will completely inhibit 4 pmol of the SERCA pump (Sagara and Inesi, 1991). We have estimated the relative amount of the Arabidopsis ER Ca2+-ATPase expressed in yeast membranes. The steady-state level of phosphoenzyme formed at 300 nm ATP (when rate was near maximum) was 120 to 150 pmol mg−1 membrane protein (F. Liang, unpublished data). With a molecular mass of 116 kD, the plant Ca2+ pump represents at least 1.4% to 1.7% of the membrane protein. Thus, the plant Ca2+ pump is 15- to 20-fold overexpressed in yeast membranes relative to native Ca2+-ATPases, which make up approximately 0.01% of plant membranes (Chen et al., 1993). However, 3 μm thapsigargin, which corresponds to 66 nmol mg−1 membrane protein, did not inhibit ECA1 pumping. Thus, this isoform of plant ER-type Ca2+ pump does not bind to thapsigargin, although the third transmembrane segment of ECA1 shared 67% identity with the thapsigargin-interaction site of SERCA. The difference between plant and animal ER-type Ca2+ pumps might be because thapsigargin is a plant-derived sesquiterpene lactone.
It is interesting that the plant ECA1 pump is inhibited by another specific SERCA inhibitor, cyclopiazonic acid, although the mode of action is still unclear. Cyclopiazonic acid inhibited sarcoplasmic reticulum Ca2+-ATPase stoichiometrically, but the indole tetramic acid at 1000 nmol mg−1 had no effect on the PM Ca2+-ATPase or other ion-pumping ATPases (Seidler et al., 1989). Binding of cyclopiazonic acid decreases the enzyme affinity for ATP 10-fold, and recent studies suggested that cyclopiazonic acid and ATP do not compete for the same binding site (Plenge-Tellechea et al., 1997). Both phosphoenzyme formation and Ca2+ transport of the plant ECA1 pump are inhibited with a 50% inhibition of approximately 3 nmol mg−1 (Fig. 8C). Thus, binding of cyclopiazonic acid could reduce the affinity of ECA1 for ATP and consequently decrease the initial rate of phosphoenzyme formation (Liang et al., 1997) and Ca2+ transport (Fig. 8C).
Function of ECA1 in Arabidopsis
It is interesting that the localization of ECA1 on the ER and perhaps the Golgi of Arabidopsis plants (Liang et al., 1997) is similar to its expression on the ER and on endomembrane compartments of yeast. Furthermore, ECA1 is localized on membranes distinct from the vacuolar H+/Ca2+ antiport in yeast (Table I), suggesting that a potential ER retrieval signal, KXKXX, at the carboxyl terminus of the plant pump is recognized in yeast. If so, the function of the plant Ca2+ pump in yeast may reflect in part its native role in plants. ECA1 could restore pmr1 mutant growth on Ca2+-depleted medium (Liang et al., 1997), indicating that it resembled the function of the native yeast Golgi Ca2+ pump (Sorin et al., 1997). However, ECA1 could pump other divalent cations, including Mn2+, because expression of ECA1 restored the growth of a yeast pmr1 mutant on Mn2+-containing medium (Liang et al., 1997). It is interesting that plants treated with cyclopiazonic acid showed aggregation of ER membranes (Busch and Sievers, 1993). Furthermore, gravitropic responses are inhibited in cress roots treated with cyclopiazonic acid (Sievers and Busch, 1992). After overexpression of a rice ER-type Ca2+-ATPase, the GA3 requirement for α-amylase induction and secretion in aleurone protoplasts was bypassed (Chen et al., 1997). These results support the idea that: (a) luminal Ca2+ supplied by ER Ca2+-pumping ATPase could play a role in protein processing and vesicle trafficking of the ER and Golgi (Beckers and Balch, 1989), and (b) ER Ca2+ pumps modulate cytoplasmic Ca2+ waves induced by various stimuli. Thus, like yeast cells, the normal growth and development of plant cells are dependent on active high-affinity Ca2+ pumps on the ER and Golgi.
ACKNOWLEDGMENTS
We would like to thank K.W. Cunningham (Johns Hopkins University) and J.F. Harper (The Scripps Research Institute) for providing yeast strains and an antibody against Arabidopsis ECA1/ACA3 protein, respectively.
Abbreviations:
- BTP
1,3-bis[tris(hydroxymethyl)methylamino]propane
- PM
plasma membrane
- SC-URA
synthetic complete medium minus uracil
- SER
sarcoplasmic/ER
- SERCA
SER Ca2+ ATPase
Footnotes
This work was supported in part by Department of Energy grant no. DE-95ER20200 and by Maryland Agricultural Experiment Station grant no. MD-J-151 (to H.S.).
LITERATURE CITED
- Antebi A, Fink GR. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P. Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower. Plant Physiol. 1997;114:999–1007. doi: 10.1104/pp.114.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CJ, Balch WE. Calcium and GTP essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989;108:1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron JJ, Brenner MB, Thomas DY, Williams DB. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Bers D, Patton C, Nuchitelli R. A practical guide to the study of Ca2+ in living cells. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- Bonza C, Carnelli A, De Michelis MI, Rasi-Caldogno F. Purification of the plasma membrane Ca2+-ATPase from radish seedlings by calmodulin-agarose affinity chromatography. Plant Physiol. 1998;116:845–851. doi: 10.1104/pp.116.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch MB, Sievers A. Membrane traffic from endoplasmic reticulum to the Golgi apparatus is disrupted by an inhibitor of the Ca2+-ATPase in the ER. Protoplasma. 1993;177:23–31. [Google Scholar]
- Bush DR. Calcium regulation in plant cells and its role in signaling. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- Bush DS, Sticher L, van Huystee R, Wagner D, Jones RL. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem. 1989;264:19392–19398. [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993;260:226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- Catty P, de Kerchove d'Exaerde A, Goffeau A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 1997;409:325–332. doi: 10.1016/s0014-5793(97)00446-8. [DOI] [PubMed] [Google Scholar]
- Chen DC, Yang BC, Kuo T-T. One-step transformation of yeast in stationary phase. Curr Genet. 1992;21:83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- Chen F-H, Ratterman DM, Sze H. A plasma membrane-type Ca2+-ATPase of 120 kilodaltons on the endoplasmic reticulum from carrot (Daucus carota) cells. Properties of the phosphorylated intermediate. Plant Physiol. 1993;102:651–661. doi: 10.1104/pp.102.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chang M, Wang B, Wu B. Cloning of a Ca2+-ATPase gene and the role of cytosolic Ca2+ in the gibberellin-dependent signaling pathway in aleurone cells. Plant J. 1997;11:363–371. doi: 10.1046/j.1365-313x.1997.11030363.x. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T, Gable K, Beeler T. Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem. 1998;273:1099–1106. doi: 10.1074/jbc.273.2.1099. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clake TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK. Pollen tube growth and the intracellular cytoplasmic calcium gradient oscillate in phase while extracellular calium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.1105/tpc.9.11.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh W-L, Pierce WS, Sze H. Calcium-pumping ATPases in vesicles from carrot cells. Plant Physiol. 1991;97:1535–1544. doi: 10.1104/pp.97.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Berkelman T, Franklin AE, Hoffman NE. Characterization of a gene encoding a Ca2+-ATPase-like protein in the plastid envelope. Proc Natl Acad Sci USA. 1993;90:10066–10070. doi: 10.1073/pnas.90.21.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between endoplasmic reticulum-type and plasma membrane-type Ca2+ pumps. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–9584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom S, Askerlund P, Palmgren MG. A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Lett. 1997;400:324–328. doi: 10.1016/s0014-5793(96)01448-2. [DOI] [PubMed] [Google Scholar]
- Mignaco JA, Barrabin H, Scofano HM. Effects of photo-oxidizing analogs of fluorescein on the sarcoplasmic reticulum Ca2+-ATPase. Functional consequences for substrate hydrolysis and effects on the partial reactions of the hydrolytic cycle. J Biol Chem. 1996;271:18423–18430. doi: 10.1074/jbc.271.31.18423. [DOI] [PubMed] [Google Scholar]
- Nechay BR. Mechanism of action of vanadium. Annu Rev Pharmacol Toxicol. 1984;24:501–24. doi: 10.1146/annurev.pa.24.040184.002441. [DOI] [PubMed] [Google Scholar]
- Norregaard A, Vilsen B, Andersen JP. Transmembrane segment M3 is essential to thapsigargin sensitivity of the sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1994;269:26598–26601. [PubMed] [Google Scholar]
- Ohsumi Y, Anraku Y. Calcium transport driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1983;258:5614–5617. [PubMed] [Google Scholar]
- Perez-Terzic C, Jaconi M, Clapham DE. Nuclear calcium and the regulation of the nuclear pore complex. Bioessays. 1997;19:787–792. doi: 10.1002/bies.950190908. [DOI] [PubMed] [Google Scholar]
- Plenge-Tellechea F, Soler F, Fernandez-Belda F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+-ATPases by cyclopiazonic acid. J Biol Chem. 1997;272:2794–2800. doi: 10.1074/jbc.272.5.2794. [DOI] [PubMed] [Google Scholar]
- Reed N, Allan WTG, Knight H, Knight MR, Malho R, Russel A, Shacklock PS, Trevawas AJ. Imaging and measurement of cytosolic free calcium in plant and fungal cells. J Microsc. 1992;166:57–86. [Google Scholar]
- Rose MD, Winston F, Hieter P (1990) Methods in Yeast Genetics: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 178–180
- Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Sagara Y, Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991;266:13503–13506. [PubMed] [Google Scholar]
- Schatzmann HJ. The calcium pump of the surface membrane and of the sarcoplasmic reticulum. Annu Rev Physiol. 1989;51:473–485. doi: 10.1146/annurev.ph.51.030189.002353. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- Sievers A, Busch MB. An inhibitor of the Ca2+-ATPases in the sarcoplasmic and endoplasmic reticula inhibits transduction of the gravity stimulus in cress roots. Planta. 1992;188:619–622. doi: 10.1007/BF00197057. [DOI] [PubMed] [Google Scholar]
- Sorin A, Rosas G, Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- Wimmers LE, Ewing NN, Bennett AB. Higher plant Ca2+-ATPase: primary structure and regulation of mRNA abundance by salt. Proc Natl Acad Sci USA. 1992;89:9205–9209. doi: 10.1073/pnas.89.19.9205. [DOI] [PMC free article] [PubMed] [Google Scholar]