Abstract
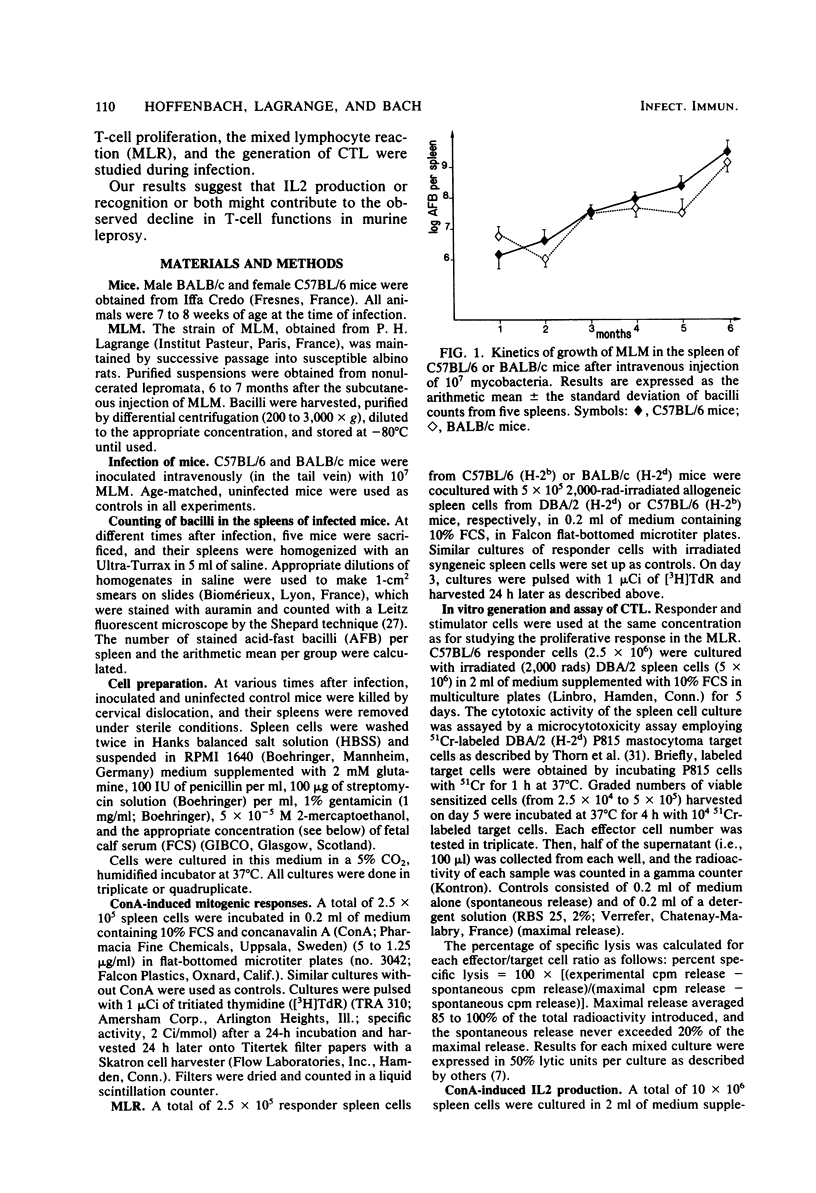
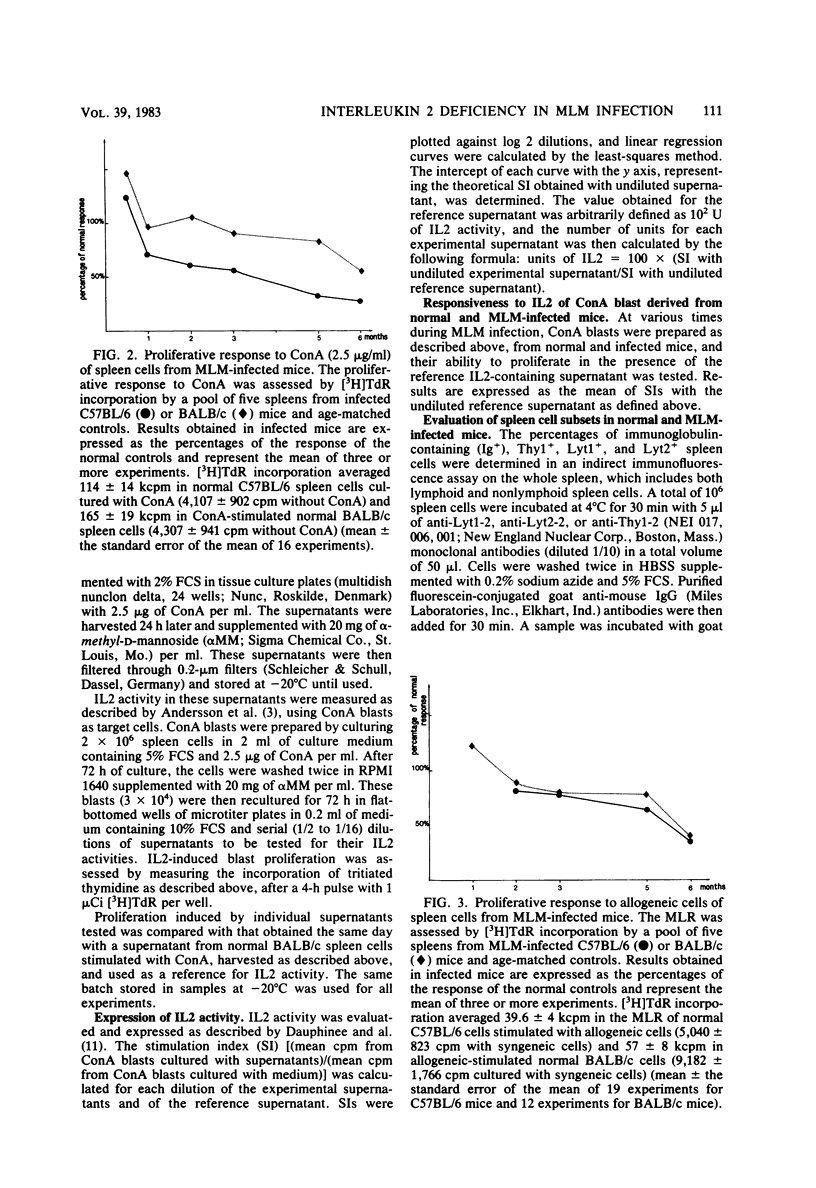
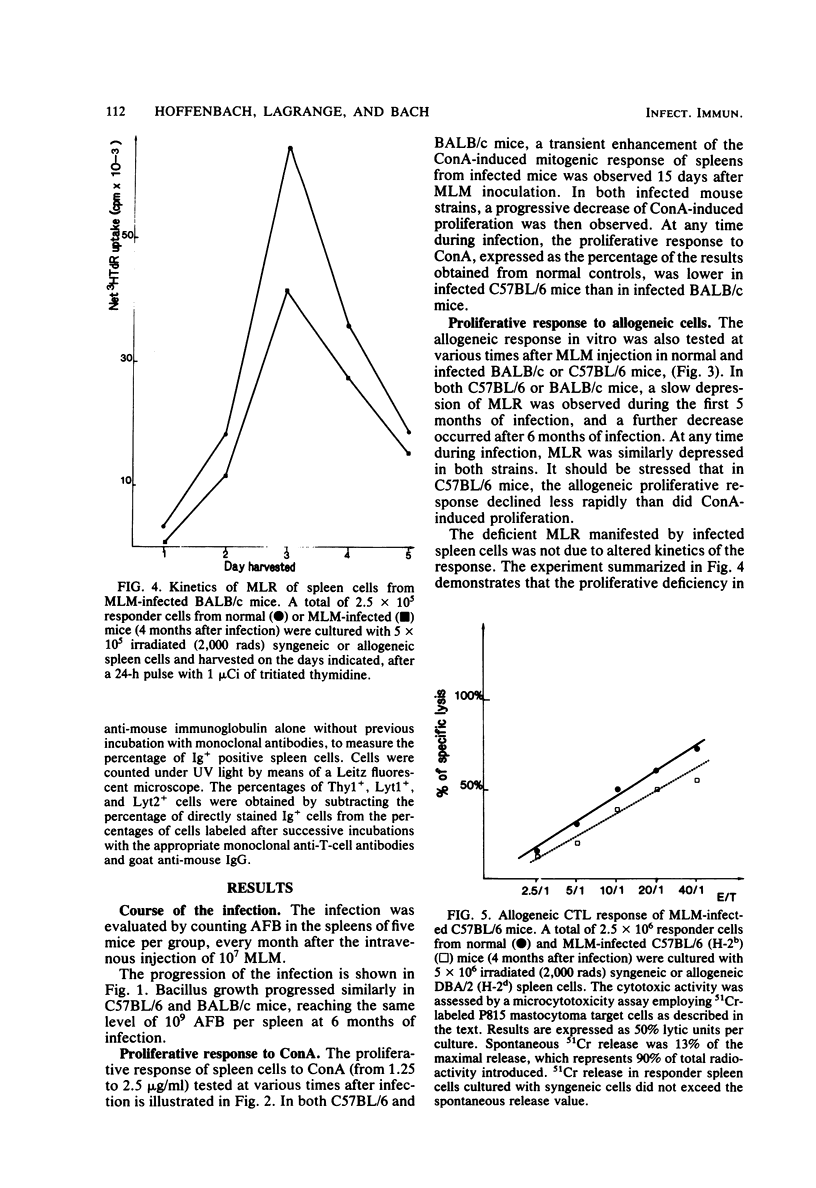
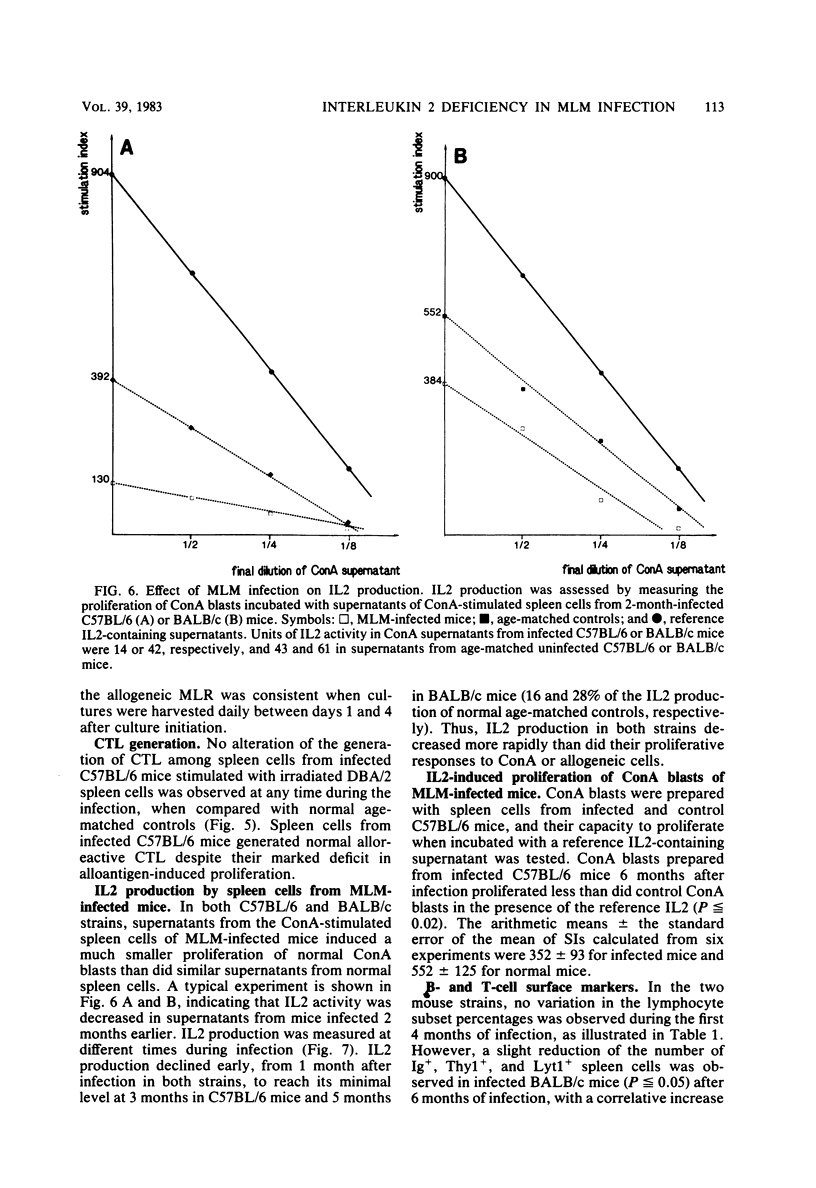
C57BL/6 and BALB/c mice were infected intravenously with 10(7) Mycobacterium lepraemurium (MLM). At various times after infection, spleen cells were tested for their capacity to proliferate in vitro in response to concanavalin A (ConA) and to allogeneic cells. The generation of alloreactive cytotoxic T lymphocytes was also studied. The mitogen- and allogeneic-cell-induced blastogenesis of splenocytes from MLM-infected C57BL/6 and BALB/c mice was shown to be depressed during infection. The maximal decrease occurred 6 months after infection. Conversely, no reduction in the ability to generate alloreactive cytotoxic T lymphocytes was observed even after 6 months of infection. At the same time, interleukin 2 (IL2) activity generated by ConA stimulation of infected splenocytes was measured in both strains. IL2 activity in the ConA-stimulated culture supernatants was decreased as early as 1 month after MLM inoculation as compared with supernatants from age-matched control mice. Thus, IL2 production by infected-mouse spleen cells was shown to decline earlier than their proliferative responses to ConA and to allogeneic cells. ConA-induced T-cell blasts from infected mice showed a reduced ability to proliferate when incubated with an IL2-containing reference supernatant from ConA-stimulated normal spleen cells. These data suggest that a defect in IL2 production and utilization might contribute to the impairment of T cell-mediated immunity observed in MLM-infected mice.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Grönvik K. O., Larsson E. L., Coutinho A. Studies on T lymphocyte activation. I. Requirements for the mitogen-dependent production of T cell growth factors. Eur J Immunol. 1979 Aug;9(8):581–587. doi: 10.1002/eji.1830090802. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Evans P. E., Filomeno A. R. Impairment of cell-mediated immune responses by infection with Mycobacterium lepraemurium. Infect Immun. 1977 Oct;18(1):157–164. doi: 10.1128/iai.18.1.157-164.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E. Leprosy: a model of immunological perturbation in chronic infection. J Infect Dis. 1978 Mar;137(3):341–354. doi: 10.1093/infdis/137.3.341. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: induction of immunity and immune paralysis to Mycobacterium lepraemurium in C57BL mice. Infect Immun. 1975 Oct;12(4):706–713. doi: 10.1128/iai.12.4.706-713.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Klimpel G. R., Okada M., Henney C. S. Inhibition of in vitro cytotoxic responses by BCG-induced macrophage-like suppressor cells. II. Suppression occurs at the level of a "helper" T cell. J Immunol. 1979 Jul;123(1):350–357. [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B. Local immune response to Mycobacterium lepraemurium in C3H and C57Bl/6 mice. Clin Exp Immunol. 1979 Dec;38(3):461–474. [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Lefford M. J., Patel P. J., Poulter L. W., Mackaness G. B. Induction of cell-mediated immunity to Mycobacterium lepraemurium in susceptible mice. Infect Immun. 1977 Dec;18(3):654–659. doi: 10.1128/iai.18.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Navalkar R. G., Patel P. J., Kanchana M. V. Studies on immune response to Mycobacterium lepraemurium. Evaluation of the cell-mediated immune response in mice. Int Arch Allergy Appl Immunol. 1980;62(4):423–432. [PubMed] [Google Scholar]
- Preston P. M. Macrophages and protective immunity in Mycobacterium lepraemurium infections in a 'resistant' (C57Bl) and a 'susceptible' (BALB/c) mouse strain. Clin Exp Immunol. 1982 Feb;47(2):243–252. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Revised nomenclature for antigen-nonspecific T cell proliferation and helper factors. J Immunol. 1979 Dec;123(6):2928–2929. [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Espinosa O., Casoluengo-Méndez M., Díaz G. V. Antibody-mediated immunity in CFW mice infected with Mycobacterium lepraemurium. Humoral immune response in murine leprosy. Clin Exp Immunol. 1976 Dec;26(3):381–387. [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Monticone V., Mills G., Paetkau V. Effects of costimulator on immune responses in vitro. J Immunol. 1978 Jun;120(6):1974–1980. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Fumoux F., Strobel M., Baret A., Michelson M. Augmentation de l'activité dismutasique dans les leucocytes spléniques de souris sensibles au BCG et à Mycobacterium lepraemurium. C R Seances Acad Sci III. 1981 Nov 16;293(10):575–578. [PubMed] [Google Scholar]
- Thorn R. M., Palmer J. C., Manson L. A. A simplified 51Cr-release assay for killer cells. J Immunol Methods. 1974 Mar;4(2):301–315. doi: 10.1016/0022-1759(74)90073-8. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Mochizuki D. Interleukin 2: a class of T cell growth factors. Immunol Rev. 1980;51:257–278. doi: 10.1111/j.1600-065x.1980.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T cells in mice heavily infected with mycobacteria. Immunology. 1980 Mar;39(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. The specificity of suppressor T cells induced by chronic Mycobacterium avium infection in mice. Clin Exp Immunol. 1981 Jan;43(1):10–19. [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Sljivić V. S., Brown I. N. Defect of macrophage function in the antibody response to sheep erythrocytes in systemic Mycobacterium lepraemurium infection. Nature. 1975 Jul 17;256(5514):206–208. doi: 10.1038/256206b0. [DOI] [PubMed] [Google Scholar]


