Abstract
Background
The brain biochemical changes of social anxiety have not been clarified although there have been a limited number of MR spectroscopic studies which utilized metabolite/creatine ratios. Present study aimed to explore the alteration of absolute metabolite concentration in social anxiety disorder using quantitative MR spectroscopy.
Materials and Methods
With a 3.0T MR scanner, single voxel MR spectroscopy (stimulated echo acquisition mode, TR/TE/TM = 2000/20/16 ms) was performed in the left dorsolateral prefrontal cortex and related regions of nine medication-free patients with social anxiety disorder and nine controls. Absolute metabolite concentration was calculated using tissue water as the internal reference and corrected for the partial volume of cerebrospinal fluid.
Results
In the left dorsolateral prefrontal cortex, the N-acetyl aspartate/creatine ratio of patients was significantly higher than that of controls, and this was due to the decrease of creatine concentration instead of the increase of N-acetyl aspartate concentration. Furthermore, the creatine concentration of the left dorsolateral prefrontal cortex was negatively correlated with the scores of Liebowitz social anxiety scale.
Conclusions
The alteration of creatine level in the left dorsolateral prefrontal cortex suggests abnormal energy metabolism and correlates with symptom severity in social anxiety disorder. And metabolite concentration is preferable to metabolite/creatine ratio for the investigation of individual, absolute metabolite changes in this region of social anxiety disorder.
Introduction
Social anxiety disorder (SAD) is among the most common anxiety disorders and is among the most common psychiatric disorders. The prevalence of SAD in China is reported to be 3.2–8.15% [1], [2]. SAD patients experience impairment in their work, home, and social relationships. Moreover, it is reported that 69%–92% of SAD patients have at least one kind of other mental disorders including other anxiety disorders (the most common complication), major depressive disorders, and substance abuse disorders [3].
Magnetic resonance spectroscopy (MRS) allows in-depth investigation of metabolic changes in specific brain regions. Only a few MRS studies of SAD [4]–[8] have been reported, most of which used metabolites/creatine (Cr) ratios to represent absolute metabolite changes based on the assumption that Cr level remained stable under various conditions. Elevated glutamine/Cr in whole brain and the thalamus was reported and regarded as the proof of overactivity of glutamatergic system [4]. Increased choline (Cho)/Cr in cortical gray matter was also reported and interpreted as accelerated activity of phospholipase-C [5]. N-acetyl aspartate (NAA)/Cr was found to be elevated in anterior cingulate cortex (ACC) but reduced in occipital cortex, and NAA/Cr in ACC was positively correlated with the severity of anxiety symptoms [8].
Recent quantitative MRS studies, however, have demonstrated abnormal changes of Cr level in other psychiatric disorders [9]–[11]. In depressive patients NAA, Cho and myo-inositol to Cr ratios were found to be significantly lower than in controls, whereas it was attributed to the increase of Cr concentration instead of the decrease of other metabolite concentrations [12]. Thus it is also necessary to clarify whether Cr is altered in SAD patients. Besides, information concerning other metabolic changes expressed in metabolite ratios (like NAA/Cr, Cho/Cr) also needs to be updated with absolute concentrations. And this is where we set out to perform this quantitative MRS study.
Previous studies revealed functional abnormalities of SAD patients in prefrontal cortex, ACC, and limbic/paralimbic regions which comprised corticolimbic circuitry and participated in the genesis of fear and anxiety [13]–[15]. In addition, thalamus and striatum abnormalities were also found in SAD patients, suggesting that these nuclei may also be involved in the pathogenesis of SAD [16], [17]. Interestingly, the left hemisphere seems to be more associated with fear conditioning [18], [19]. So in the present study we located the Volume of Interests (VOI) in the left dorsolateral prefrontal cortex (DLPFC), ACC, bilateral putamens, and left thalamus.
Materials and Methods
Subjects
Nine SAD patients and nine healthy controls from the same community were included in this study. SAD was diagnosed by experienced psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV, American Psychiatric Association). To be included, patients must be medication free, and not comorbid with any other psychiatric or neurologic disorders, or any other contraindications to MR imaging. Each subject was evaluated by the psychiatrists with Liebowitz Social Anxiety Scale (LSAS) to assess the severity of symptom. This study was approved by the local ethics committee of West China Hospital, and written informed consent was obtained from each subject.
Demographic characteristics of the participants were summarized in Table 1.
Table 1. Demographic characteristics of the participants.
| Sample size | Male/female | Age(years) | Avoidance part of LSAS score | Fear part of LSAS score | Total LSAS score | |
| SAD | 9 | 5/4 | 21.6±2.5 | 27.3±5.9 | 30.0±6.3 | 57.3±11.5 |
| Controls | 9 | 5/4 | 21.2±2.0 | 14.7±3.2 | 12.0±5.7 | 26.7±6.0 |
MR Spectroscopy
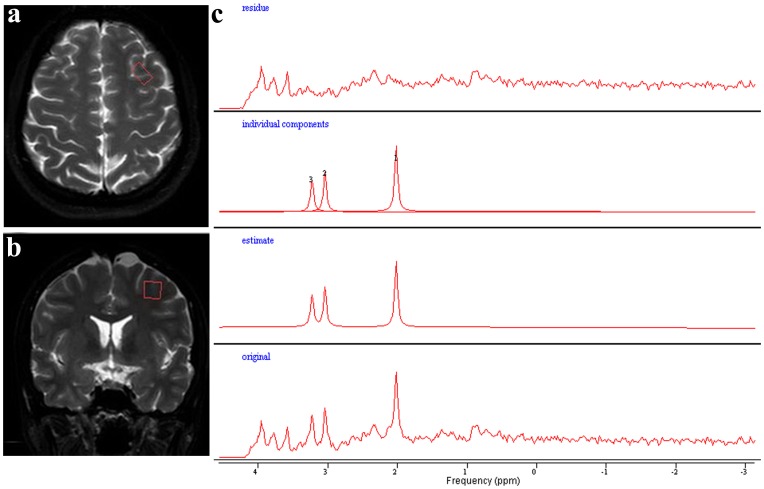
All the MRI and MRS examinations were performed on a 3.0 T MR scanner (Philips Achieva, Netherlands). The traditional MR imaging sequences included fast spin-echo T2-weighted images (TR/TE 4000/100 ms) and spin-echo T1-weighted images (TR/TE 500/15 ms) in three orthogonal planes. Single-voxel MR spectroscopy was performed using stimulated echo acquisition mode (STEAM) sequence. Both unsuppressed tissue water and metabolite with water suppression spectra were acquired, with the following parameters: TR/TE/TM = 2000/20/16 ms, spectral bandwidth = 2000 Hz, data points = 1024, number of signals averaged = 128 for metabolites and tissue water, and scanning time = 4 minutes and 56 seconds. VOI was placed in the left DLPFC (Fig. 1a, b), ACC, the left and right putamens and the left thalamus. The Mean±SD VOI volume was 3.5±0.2, 3.4±0.3, 2.8±0.2, 2.9±0.2, 3.2±0.3 cubic centimeters, respectively. Shimming, frequency adjustment, and water suppression were automatically accomplished before data acquisition.
Figure 1. MRS data acquisition and post-processing.
(a) and (b) are the transversal and sagittal view of the brain, respectively. The red rectangles indicate the volume of interest representing the left dorsolateral prefrontal cortex (DLPFC) from where the spectrum (c) is acquired. (c) is the spectrum of the left DLPFC. From bottom to the top are the original spectrum, the estimated spectrum yielded by ‘Advanced Method for Accurate, Robust and Efficient Spectral fitting (AMARES)’ package, the individual peak components and the residual spectrum.
Raw spectral data were exported and processed using jMRUI 3.0 (www.mrui.uab.es/mrui). Phase correction and 3 Hz Lorentzian apodization were first performed, and then tissue water and metabolite (including NAA, Cr and Cho) peak areas were obtained using ‘Advanced Method for Accurate, Robust and Efficient Spectral fitting (AMARES)’ package (Fig. 1c). Prior knowledge including chemical shift (NAA at 2.02 ppm, Cr at 3.03 ppm and Cho at 3.22 ppm with a deviation range of ±0.05 ppm for all), lineshape (Gaussian lineshape was used for all), linewidth (the initial value set for simulation was 4 Hz and was allowed to vary within a range of 1–8 Hz) was incorporated into the fitting algorithm. Soft constraints were applied to all the simulations. And then, absolute metabolite concentration was calculated using tissue water as the internal reference [20]–[22]. Tissue water concentration used was 35 mol/kg wet weight [23]–[25], and the T1 and T2 relaxation constants of tissue water and metabolites were obtained from reported values under the same field strength and the same sequence (STEAM) [26]–[28].
To correct the partial volume effect of cerebrospinal fluid (CSF) contained in the VOIs of ACC and left DLPFC, segmentation of the T2-weighted images was performed as we previously described [29]. The volume of CSF in each slice was calculated by multiplying their areas with slice thickness, and their summation gave total volume of CSF parts within the voxel. We assumed that CSF parts contributed to tissue water rather than to metabolite, and the corrected metabolite concentration was calculated as follows: Corrected metabolite concentration = metabolite concentration × VOI volume/(VOI volume − CSF volume).
Statistical Analysis
Statistical analysis was performed using the PASW Statistics 18.0 software package (IBM company, USA). The normal distribution and the homogeneity of variance of data were first verified. Then comparisons of metabolite/Cr ratios and each absolute metabolite concentration of the five VOIs between SAD subjects and controls were carried out with independent t-tests, assuming that there was no interaction between different brain regions and metabolites. In each VOI linear correlation analyses were performed between absolute Cr concentration and the total LSAS score, the avoidance part of the LSAS score (the sum of the scores reflecting the avoidance behavior), and the fear part of the LSAS score (the sum of the scores reflecting the fear emotion), respectively. Significance was set at p<0.05.
Results
The NAA/Cr peak area ratio of the left DLPFC of SAD patients was significantly higher than that of controls. The absolute Cr concentration in the left DLPFC of SAD patients was mildly, but significantly lower than that of controls; while the absolute NAA concentration of the left DLPFC was not significantly different. No significant difference between patients and controls was found for other peak area ratios or absolute concentrations, or in other regions. Detailed absolute metabolite concentrations and metabolite/Cr peak area ratios of each VOI were summarized in Table 2.
Table 2. Absolute metabolite concentrations and metabolite/creatine peak area ratios of each volume of interest (VOI).
| VOI | Metabolites | Metabolite/Cr Peak Area Ratios and Absolute Concentrations# | |||
| SAD(mean±SD) | control(mean±SD) | t value | P value | ||
| Left DLPFC | NAA * | 15.573±1.571 | 16.194±1.560 | −0.842 | 0.412 |
| Cho * | 2.373±0.597 | 2.757±0.657 | −1.296 | 0.213 | |
| Cr * | 11.217±1.297 | 13.392±2.220 | −2.539 | 0.022 | |
| NAA/Cr | 1.398±0.160 | 1.230±0.167 | 2.186 | 0.044 | |
| Cho/Cr | 0.209±0.034 | 0.207±0.034 | 0.149 | 0.883 | |
| ACC | NAA * | 14.839±2.081 | 15.173±3.564 | −0.236 | 0.817 |
| Cho * | 4.186±1.057 | 3.710±0.929 | 0.942 | 0.362 | |
| Cr * | 14.737±3.223 | 14.836±2.276 | −0.069 | 0.946 | |
| NAA/Cr | 1.034±0.184 | 1.019±0.159 | 0.176 | 0.863 | |
| Cho/Cr | 0.292±0.080 | 0.253±0.063 | 1.060 | 0.307 | |
| Left Putamen | NAA | 13.398±1.552 | 12.871±1.612 | 0.719 | 0.482 |
| Cho | 2.596±0.483 | 3.006±0.664 | −1.565 | 0.136 | |
| Cr | 13.730±1.630 | 13.834±1.769 | −0.132 | 0.896 | |
| NAA/Cr | 0.987±0.158 | 0.950±0.207 | 0.438 | 0.667 | |
| Cho/Cr | 0.191±0.038 | 0.216±0.027 | −1.595 | 0.129 | |
| Right Putamen | NAA | 12.310±1.786 | 12.809±1.692 | −0.608 | 0.551 |
| Cho | 2.164±1.320 | 2.557±0.735 | −0.779 | 0.447 | |
| Cr | 13.303±2.234 | 13.913±2.063 | −0.602 | 0.556 | |
| NAA/Cr | 0.947±0.198 | 0.931±0.138 | 0.191 | 0.851 | |
| Cho/Cr | 0.154±0.078 | 0.184±0.047 | −0.977 | 0.343 | |
| Left Thalamus | NAA | 14.960±2.404 | 14.209±4.071 | 0.506 | 0.620 |
| Cho | 2.761±0.840 | 2.293±0.792 | 1.228 | 0.236 | |
| Cr | 10.402±2.136 | 11.053±3.687 | −0.487 | 0.633 | |
| NAA/Cr | 1.475±0.296 | 1.362±0.399 | 0.715 | 0.484 | |
| Cho/Cr | 0.269±0.080 | 0.215±0.073 | 1.489 | 0.155 | |
Corrected for the partial volume effect of cerebrospinal fluid.
The unit of the absolute concentration is mol/kg wet weight.
Abbreviations: DLPFC-Dorsal Lateral Prefrontal Cortex; ACC-Anterior Cingulate Cortex.
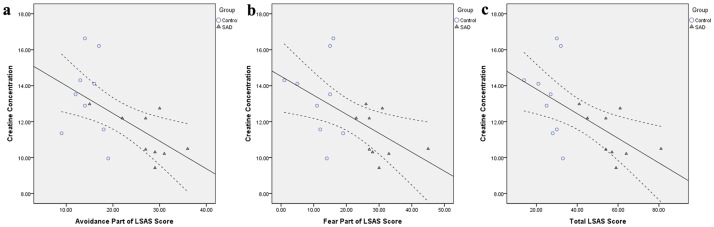
Correlation analysis revealed a significantly negative linear correlation between corrected Cr concentration of the left DLPFC and the avoidance part of the LSAS score (r = −0.589, p = 0.010), the fear part of the LSAS score (r = −0.553, p = 0.017), and the total LSAS score (r = −0.594, p = 0.007), respectively (Fig. 2). No significant correlation was found in other VOIs.
Figure 2. Correlation between creatine concentration and LSAS score.
Creatine concentration of the left DLPFC is negatively correlated with the avoidance part of the LSAS score (r = −0.589, p = 0.010) (a), the fear part of the LSAS score (r = −0.553, p = 0.017) (b), and the total LSAS score (r = −0.594, p = 0.007) (c), respectively.
Discussion
One of our major findings was the mild decrease of Cr concentration in the left DLPFC region, and the latter led to the increased of NAA/Cr ratio although NAA concentration was not significantly changed. Other studies using absolute quantification methods have also demonstrated Cr level, instead of remaining stable as assumed, was actually altered in depressive disorder [9], bipolar disorder [10], and panic disorder [11]. So metabolite/Cr ratio may lead to misinterpretation in case of a changed Cr level. Thus current study demonstrated that absolute metabolite concentration is preferable to metabolite/Cr ratios for unambiguous interpretation of absolute metabolite changes in the left DLPFC of SAD patients.
Cr serves as the reserves of high energy phosphates and as a buffer in adenosine triphosphate and adenosine diphosphate reservoir, and adenosine triphosphate can supply energy for cells to protect tissues from hypoxia-induced damage. Meanwhile, Cr can protect neurons against the toxicity of glutamate and β-amyloid [30], and Cr supplementation enhances brain function under normal and stress conditions [31]. Consistent with our finding, the decrease of Cr level was also reported in the centrum semiovale of patients with generalized anxiety disorder [32] and in the left amygdala of patients with borderline personality disorder (BPD) [9]. Castillo et al. [33], Schaller et al. [34] and Preedy et al. [35] claimed that Cr level was decreased in hypermetabolic states, and this was confirmed in tumors [13], [36] and BPD [9], [10]. Using both 18FDG-PET and MRS, previous studies reported simultaneous changes of enhanced 18FDG uptake and decreased Cr level in glioma [13] and in cachexia-inducing murine adenocarcinoma [36]. In the amygdalae of patients with BPD, decrease of Cr [9], overactivity [37], and hypermetabolism [10] have all been observed using MRS, functional MR imaging and positron emission tomography (PET), respectively in different studies. Likewise, our finding of decreased Cr level in the DLPFC may also suggest a regional hypermetabolic state. Although evidence currently available is not yet sufficient to establish a direct, causal link, findings from other relevant studies of SAD support this speculation: Tillfors et al. [38] observed increased cerebral blood flow in the same region of SAD patients using PET; Guyer et al. [39] performed functional MRI study and observed increased activity in their ventrolateral prefrontal cortex during provoked anticipatory anxiety in patients with SAD. The hypermetabolism and overactivity of DLPFC is possibly due to the up-regulation of the glutamatergic system. Glutamate is the major excitatory neurotransmitter in the central nervous system and excessive glutamate release is associated with fear-related learning and reactivity [40]. And increased glutamate/glutamine (Glx) level has been reported in the whole brain of SAD patients [4], [8]. Based on our findings and previous reports, a possible pathway for the genesis of SAD may be outlined, in which Cr may play a bridging role. That is, sustained anxiety state causes enhanced excitatory neurotransmitter release; the latter leads to the overactivity and hypermetabolism of DLPFC and subsequently overconsumption of Cr; and the decrease of Cr in turn makes neurons susceptible to the toxicity of glutamate and causes neural damage. However, the hypothesis still needs further studies to provide support particularly for the critical link between low Cr level and hypermetabolic state.
We did not find any inter-group difference of NAA and Cho concentration. In contrast, both Tupler et al. [5] and Phan et al. [8] reported abnormal changes of NAA/Cr (or NAA/Cho) and Cho/Cr. But only the latter calculated absolute concentration and made their results comparable to ours. Phan et al. claimed that the Cr level was not different between groups, thus the change of NAA/Cr (elevated in ACC and reduced in occipital cortex) and Cho/Cr (reduced in ACC and remained unchanged in occipital cortex) can represent the change of NAA and Cho levels. And they speculated that the change of NAA might reflect neuronal reorganization. Given that different VOIs have diverse changes (as demonstrated by Phan et al. [8] ), our arguments about NAA and Cho may derive from the difference of VOI. However the findings of both studies need to be replicated before any convincing conclusion can be make.
All the significant changes revealed in the present study involved the left DLPFC region. To the best of our knowledge, it is the first report of the DLPFC chemistry in patients with SAD. The DLPFC plays an essential role in mood regulation and integration of cognitive functions [41]. The DLPFC has wide reciprocal connections to the limbic structures and mediates the fear extinction by inhibiting the amygdala via ventromedial prefrontal regions [42]. Both morphological and functional changes have been found in DLPFC under various mental disorders [43]–[46]. In addition, the magnitude of increased activation of the left DLPFC is found to be correlated with the emotion regulation [47]. Consistently, we noted a significantly negative linear correlation between Cr concentration of the left DLPFC and the LSAS score. This indicates that Cr level may reflect the symptom severity of SAD. Our findings provide further evidence that deficits in DLPFC function may result in the emotional dysregulation [46].
The major limitation of this study is the limited number of subjects which make us maybe not able to find the difference in other VOIs. Further study with larger sample size may help to confirm our results. In addition, metabolite concentrations in this study is slightly higher than the reported data, possibly due to the contribution from macromolecules at short TE. Studies using techniques like ‘metabolite-nulling’ [48] should be conducted to evaluate their effect. We did not calculate tissue-specific metabolite concentration. However, previous studies have been able to calculate metabolite concentration of ‘pure’ gray or white matter using multi-voxel spectroscopy and tissue segmentation based on high resolution images [49], [50]. Although this method requires longer acquisition time and sacrifices the difference of several voxels, it may provide further insight into the underlying psychopathology of SAD.
In summary, Cr level decreased in the left DLPFC of SAD patients; therefore using absolute metabolite concentration would be a better strategy than using metabolite/Cr peak area ratio for the observation of individual metabolite changes. Decreased Cr level may result from a hypermetabolic state of the left DLPFC and is able to reflect the disease severity.
Acknowledgments
Dr. Qiyong Gong acknowledges the support from his CMB Distinguished Professorship Award (Award No. F510000/G16916411) administered by the Institute of International Education, USA. The authors would also like to thank Ms Hehan Tang and Mr Chunchao Xia for their assistances in data acquisition.
Funding Statement
This study was supported by the National Natural Science Foundation (Grant Nos. 81030027, 30900363, 81227002, 81220108013) and National Key Technologies R&D Program of China (Program No. 2012BAI01B03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lee S, Lee MT, Kwok K (2005) A community-based telephone survey of social anxiety disorder in Hong Kong. J Affect Disord 88: 183–186. [DOI] [PubMed] [Google Scholar]
- 2. Xiao R, Wu WL, Hu JM, Qiu CJ, Wang Q, et al. (2006) [Prevalence and risk factors of social anxiety disorder in high schools and universities in Chengdu]. Sichuan Da Xue Xue Bao Yi Xue Ban 37: 636–640. [PubMed] [Google Scholar]
- 3. Grachev ID, Apkarian AV (2000) Anxiety in healthy humans is associated with orbital frontal chemistry. Mol Psychiatry 5: 482–488. [DOI] [PubMed] [Google Scholar]
- 4. Pollack MH, Jensen JE, Simon NM, Kaufman RE, Renshaw PF (2008) High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry 32: 739–743. [DOI] [PubMed] [Google Scholar]
- 5. Tupler LA, Davidson JR, Smith RD, Lazeyras F, Charles HC, et al. (1997) A repeat proton magnetic resonance spectroscopy study in social phobia. Biol Psychiatry 42: 419–424. [DOI] [PubMed] [Google Scholar]
- 6. Miner CM, Davidson JR, Potts NL, Tupler LA, Charles HC, et al. (1995) Brain fluoxetine measurements using fluorine magnetic resonance spectroscopy in patients with social phobia. Biol Psychiatry 38: 696–698. [DOI] [PubMed] [Google Scholar]
- 7. Davidson JR, Krishnan KR, Charles HC, Boyko O, Potts NL, et al. (1993) Magnetic resonance spectroscopy in social phobia: preliminary findings. J Clin Psychiatry 54 Suppl: 19–25 [PubMed] [Google Scholar]
- 8. Phan KL, Fitzgerald DA, Cortese BM, Seraji-Bozorgzad N, Tancer ME, et al. (2005) Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport 16: 183–186. [DOI] [PubMed] [Google Scholar]
- 9. Hoerst M, Weber-Fahr W, Tunc-Skarka N, Ruf M, Bohus M, et al. (2010) Metabolic alterations in the amygdala in borderline personality disorder: a proton magnetic resonance spectroscopy study. Biol Psychiatry 67: 399–405. [DOI] [PubMed] [Google Scholar]
- 10. New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, et al. (2009) Laboratory induced aggression: a positron emission tomography study of aggressive individuals with borderline personality disorder. Biol Psychiatry 66: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gemar MC, Segal ZV, Mayberg HS, Goldapple K, Carney C (2007) Changes in regional cerebral blood flow following mood challenge in drug-free, remitted patients with unipolar depression. Depress Anxiety 24: 597–601. [DOI] [PubMed] [Google Scholar]
- 12. Schubert F, Gallinat J, Seifert F, Rinneberg H (2004) Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 21: 1762–1771. [DOI] [PubMed] [Google Scholar]
- 13. Alger JR, Frank JA, Bizzi A, Fulham MJ, DeSouza BX, et al. (1990) Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxyglucose PET. Radiology 177: 633–641. [DOI] [PubMed] [Google Scholar]
- 14. Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, et al. (2005) Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 57: 975–981. [DOI] [PubMed] [Google Scholar]
- 15. Grams AE, Brote I, Maderwald S, Kollia K, Ladd ME, et al. (2011) Cerebral magnetic resonance spectroscopy at 7 Tesla: standard values and regional differences. Acad Radiol 18: 584–587. [DOI] [PubMed] [Google Scholar]
- 16. Jonathan C.W Brooks, Neil Roberts, Kemp GJ (2001) A Proton Magnetic Resonance Spectroscopy Study of Age-related Changes in Frontal Lobe Metabolite Concentrations. Cerebral Cortex 11: 598–605. [DOI] [PubMed] [Google Scholar]
- 17. Kanowski M, Kaufmann J, Braun J, Bernarding J, Tempelmann C (2004) Quantitation of simulated short echo time 1H human brain spectra by LCModel and AMARES. Magn Reson Med 51: 904–912. [DOI] [PubMed] [Google Scholar]
- 18. Badre D, Wagner AD (2004) Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41: 473–487. [DOI] [PubMed] [Google Scholar]
- 19. Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, et al. (2001) Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441. [DOI] [PubMed] [Google Scholar]
- 20. Barker PB, Soher BJ, Blackband SJ, Chatham JC, Mathews VP, et al. (1993) Quantitation of proton NMR spectra of the human brain using tissue water as an internal concentration reference. NMR Biomed 6: 89–94. [DOI] [PubMed] [Google Scholar]
- 21. Christiansen P, Henriksen O, Stubgaard M, Gideon P, Larsson HB (1993) In vivo quantification of brain metabolites by 1H-MRS using water as an internal standard. Magn Reson Imaging 11: 107–118. [DOI] [PubMed] [Google Scholar]
- 22. Helms G (1999) Analysis of 1.5 Tesla proton MR spectra of human brain using LCModel and an imported basis set. Magn Reson Imaging 17: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 23. Isobe T, Matsumura A, Anno I, Yoshizawa T, Nagatomo Y, et al. (2002) Quantification of cerebral metabolites in glioma patients with proton MR spectroscopy using T2 relaxation time correction. Magn Reson Imaging 20: 343–349. [DOI] [PubMed] [Google Scholar]
- 24. Christiansen P, Toft PB, Gideon P, Danielsen ER, Ring P, et al. (1994) MR-visible water content in human brain: a proton MRS study. Magn Reson Imaging 12: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 25. Ernst T, Kreis R, Ross BD (1993) Absolute Quantitation of Water and Metabolites in the Human Brain. I. Compartments and Water. Journal of Magnetic Resonance, Series B 102: 1–8. [Google Scholar]
- 26. Li Y, Srinivasan R, Ratiney H, Lu Y, Chang SM, et al. (2008) Comparison of T(1) and T(2) metabolite relaxation times in glioma and normal brain at 3T. J Magn Reson Imaging 28: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mlynarik V, Gruber S, Moser E (2001) Proton T (1) and T (2) relaxation times of human brain metabolites at 3 Tesla. NMR Biomed 14: 325–331. [DOI] [PubMed] [Google Scholar]
- 28. Traber F, Block W, Lamerichs R, Gieseke J, Schild HH (2004) 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging 19: 537–545. [DOI] [PubMed] [Google Scholar]
- 29. Yue Q, Shibata Y, Isobe T, Anno I, Kawamura H, et al. (2009) Absolute choline concentration measured by quantitative proton MR spectroscopy correlates with cell density in meningioma. Neuroradiology 51: 61–67. [DOI] [PubMed] [Google Scholar]
- 30. Brewer GJ, Wallimann TW (2000) Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 74: 1968–1978. [DOI] [PubMed] [Google Scholar]
- 31. Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76: 329–343. [DOI] [PubMed] [Google Scholar]
- 32. Coplan JD, Mathew SJ, Mao X, Smith EL, Hof PR, et al. (2006) Decreased choline and creatine concentrations in centrum semiovale in patients with generalized anxiety disorder: relationship to IQ and early trauma. Psychiatry Res 147: 27–39. [DOI] [PubMed] [Google Scholar]
- 33. Castillo M, Kwock L, Mukherji SK (1996) Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 17: 1–15. [PMC free article] [PubMed] [Google Scholar]
- 34.Schaller B (2007) State-Of-The-Art Imaging in Stroke: Nova Publishers. [Google Scholar]
- 35.Preedy VR, Burrow GN, Watson R (2009) Comprehensive Handbook of Iodine: Nutritional, Biochemical, Pathological and Therapeutic Aspects: Elsevier Inc. [Google Scholar]
- 36. Penet MF, Gadiya MM, Krishnamachary B, Nimmagadda S, Pomper MG, et al. (2011) Metabolic signatures imaged in cancer-induced cachexia. Cancer Res 71: 6948–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazlett EA, Zhang J, New AS, Zelmanova Y, Goldstein KE, et al.. (2012) Potentiated Amygdala Response to Repeated Emotional Pictures in Borderline Personality Disorder. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tillfors M, Furmark T, Marteinsdottir I, Fredrikson M (2002) Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry 52: 1113–1119. [DOI] [PubMed] [Google Scholar]
- 39. Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, et al. (2008) Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry 65: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker DL, Davis M (2002) The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav 71: 379–392. [DOI] [PubMed] [Google Scholar]
- 41. Caetano SC, Fonseca M, Olvera RL, Nicoletti M, Hatch JP, et al. (2005) Proton spectroscopy study of the left dorsolateral prefrontal cortex in pediatric depressed patients. Neurosci Lett 384: 321–326. [DOI] [PubMed] [Google Scholar]
- 42. Hartley CA, Phelps EA (2010) Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ (2004) Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology 29: 952–959. [DOI] [PubMed] [Google Scholar]
- 44. Lyoo IK, Kim JE, Yoon SJ, Hwang J, Bae S, et al. (2011) The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry 68: 701–713. [DOI] [PubMed] [Google Scholar]
- 45. Frey BN, Stanley JA, Nery FG, Monkul ES, Nicoletti MA, et al. (2007) Abnormal cellular energy and phospholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disord 9 Suppl 1119–127. [DOI] [PubMed] [Google Scholar]
- 46. Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, et al. (2007) Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord 9: 810–819. [DOI] [PubMed] [Google Scholar]
- 47. Delgado MR, Nearing KI, Ledoux JE, Phelps EA (2008) Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mader I, Seeger U, Weissert R, Klose U, Naegele T, et al. (2001) Proton MR spectroscopy with metabolite-nulling reveals elevated macromolecules in acute multiple sclerosis. Brain : a journal of neurology 124: 953–961. [DOI] [PubMed] [Google Scholar]
- 49.Tal A, Kirov, II, Grossman RI, Gonen O (2012) The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR Biomed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, et al. (2006) Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55: 1219–1226. [DOI] [PubMed] [Google Scholar]