Abstract
Pathological gambling is an impulse control disorder reported in association with dopamine agonists used to treat Parkinson’s disease. Although impulse control disorders are conceptualized as lying within the spectrum of addictions, little neurobiological evidence exists to support this belief. Functional imaging studies have consistently demonstrated abnormalities of dopaminergic function in patients with drug addictions, but to date no study has specifically evaluated dopaminergic function in Parkinson’s disease patients with impulse control disorders. We describe results of a [11C] raclopride positron emission tomography (PET) study comparing dopaminergic function during gambling in Parkinson’s disease patients, with and without pathological gambling, following dopamine agonists. Patients with pathological gambling demonstrated greater decreases in binding potential in the ventral striatum during gambling (13.9%) than control patients (8.1%), likely reflecting greater dopaminergic release. Ventral striatal bindings at baseline during control task were also lower in patients with pathological gambling. Although prior imaging studies suggest that abnormality in dopaminergic binding and dopamine release may be markers of vulnerability to addiction, this study presents the first evidence of these phenomena in pathological gambling. The emergence of pathological gambling in a number of Parkinson’s disease patients may provide a model into the pathophysiology of this disorder.
Keywords: Parkinson’s disease, dopamine, impulse control disorders, pathological gambling, PET, functional imaging
Introduction
Impulse control disorders (ICDs) are a diverse group of psychiatric disorders characterized by a loss of voluntary control over impulses or drives that produce repetitive, self-destructive behaviours. The range of disorders that comprise the ICDs is broad and, as currently defined by the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV-TR) (2000), includes diverse conditions such as compulsive eating, hypersexuality, trichilomania, kleptomania, intermittent explosive disorder and compulsive gambling.
ICDs have been variously conceptualized as states of imbalance resulting from excessive drive, impairment of inhibition or as disorders lying along the spectrum of behaviours defined by extremes of purely impulsive to compulsive behaviour (McElroy et al., 1992; Hollander and Wong, 1995a, b). Other investigators have highlighted the similarities between impulse control disorders and substance addiction, and have proposed that the ICDs should be included in a reward-based group that may best align with behavioural addictions (Holden, 2001; Grant et al., 2006; Potenza, 2006). On the basis of both its phenomenology and neurobiology, pathological gambling may be the ICD that most closely resembles chemical addiction (Potenza, 2006).
A number of ICDs have been reported in the setting of Parkinson’s disease treated with dopaminergic agents, including hypersexuality, compulsive shopping (Grosset et al., 2006; Lu et al., 2006; Voon et al., 2006a; Weintraub et al., 2006), compulsive eating (Nirenberg and Waters, 2006) and pathological gambling (Grosset et al., 2006; Lu et al., 2006; Voon et al., 2006b; Weintraub et al., 2006). Initial estimates of the prevalence of ICDs in Parkinson’s disease have ranged from 5.9% to 6.6%, and for pathological gambling in Parkinson’s disease from 3% to 8% (Avanzi et al., 2006; Grosset et al., 2006; Lu et al., 2006; Voon, et al., 2006b; Weintraub et al., 2006). This is in contrast to the estimated 1.6% prevalence of pathological gambling in the North American population as a whole (Shaffer et al., 1999).
Although ICDs do rarely occur in Parkinson’s disease treated with high doses of L-dopa (Klos et al., 2005; Avanzi et al., 2006), mono amine oxidase (MAO) inhibitor monotherapy (Shapiro et al., 2006) and deep brain stimulation (Smeding et al., 2007), these adverse effects appear to be primarily related to treatment with dopamine agonists with an unclear relationship to dosage (Grosset et al., 2006; Lu et al., 2006; Voon, et al., 2006b; Weintraub et al., 2006; McKeon et al., 2007; Evans and Butzkueven, 2007; Quickfall and Suchowersky, 2007; Tippmann-Peikert et al., 2007). The reported 0.7% prevalence of pathological gambling in Parkinson’s disease patients treated with levodopa therapy alone versus 13.7% of Parkinson’s disease patients on mono therapy with dopamine agonists may provide some indications of the relative contribution of dopaminergic agonists to the development of the disorder (Voon, et al., 2006a).
ICDs have been frequently conceptualized as lying within the spectrum of addiction, which itself is increasingly conceived of as an evolving process which may involve dysfunction of a number of behavioural subsystems (Volkow et al., 2007; Goodman, 2008). Over the last decade, functional imaging studies have revealed abnormalities of dopaminergic function in a number of the behavioural components implicated in addiction, including the modulation of expectation and reward, appetitive urge, craving and salience, behavioural impulsivity and gambling (Bergh et al., 1997; Kalivas and Volkow, 2005; Reuter et al., 2005; Riba et al., 2008; Rowe et al., 2008). In addicted individuals, reduced striatal dopamine activity has been associated with disrupted metabolism in prefrontal regions, particularly the orbitofrontal and anterior cingulate cortex, brain regions involved in motivation, drive and inhibitory control, respectively (Volkow et al., 1993). These observations are consistent with more recent studies showing that monetary rewards during gambling activates ventral striatum and ventromedial prefrontal cortex and that subjects with pathological gambling present a much blunted activation of these areas (Reuters et al., 2006). Similar abnormalities have also been observed in patients with Parkinson’s disease. In fact, 18F-FDG positron emission tomography (PET) studies have also revealed a significant metabolism impairment of orbitofrontal and anterior cingulate cortex in Parkinson’s disease patients during gambling task (Thiel et al., 2003). According to a more recent functional magnetic resonance imaging (fMRI) study, it seems that Parkinson’s disease patients present an impaired ability to adapt the function of the cingulate-striatal reward system from representation of actual reward to representation of expected reward (Rowe et al., 2008). There is evidence that while cingulate activation associated with reward expectation declined with severity of the disease, activation following actual rewards increased it. These abnormalities in Parkinson’s disease may facilitate a change in goal-directed behaviours from deferred predicted rewards to immediate actual rewards, particularly when on dopaminergic treatment. Different reports have described the ability of dopamine and dopaminergic agents to prime or increase subsequent dopamine release in the ventral striatum. This phenomenon appears associated with an increase in salience or desirability of these drugs that manifests as craving (Berke and Hyman, 2000; Robinson and Berridge, 2000). Evans and colleagues (2006), in a [11C] raclopride PET evaluation of Parkinson’s disease patients with dopamine dysregulation syndrome (a condition characterized by pathological craving for dopaminergic medications) have demonstrated that in response to a single dose of levodopa, patients with dopamine dysregulation released more dopamine in the ventral striatal circuits compared to control Parkinson’s disease patients. To date, no study has specifically evaluated dopaminergic function in Parkinson’s disease patients with pathological gambling. The emergence of pathological gambling in a relatively large number of Parkinson’s disease patients exposed to dopamine agonists may provide a model into the pathophysiology of this disorder in the general population. Since drugs with dopaminergic actions are known to cross-prime for the release of dopamine induced by other drugs within the same class and to up-regulate the incentive salience of a variety of stimuli with which they have become associated (Engber et al., 1989; Robinson and Berridge, 2000; Nocjar and Panksepp, 2002; Robinson and Berridge, 2003), the potential exists that an increase in striatal DA release may distinguish patients who develop pathological gambling on dopamine agonist therapy from those who do not.
To test this hypothesis, we initiated a [11C] raclopride PET study of pathological gambling associated with dopamine agonist use. Here, we describe striatal D2/D3 receptor binding and dopamine release in response to an active gambling task for monetary reward in Parkinson’s disease patients who developed pathological gambling while on dopamine agonists and compared to a group of Parkinson’s disease patients on agonist therapy who remained free of the disorder.
Methods
Subjects and experimental design
Seven Parkinson’s disease patients with pathological gambling and seven Parkinson’s disease patients without history of gambling participated in the study. Parkinson’s disease patients (Queen’s Square Brain Bank Criteria) were identified at the Movement Disorders Centre of the Toronto Western Hospital and matched for amount of dopaminergic medication intake, age and disease severity. The demographic characteristics of the two groups of patients are presented in Table 1.
Table 1.
Demographic characteristics of the Parkinson’s disease patients with and without pathological gambling
| Parkinson’s disease patients with pathological gambling (n = 7) | Parkinson’s disease patients without pathological gambling (n = 7) | P-value | |
|---|---|---|---|
| Gender | Five males, two females | Six males, one female | |
| Age | 47–72 years | 51–74 years | 0.5 |
| H & Y, mean (±SD) | 2 (±0.6) | 1.9 (±0.7) | 0.6 |
| PDRS (III), mean (±SD), off medication | 25.2 (±4.5) | 20.2 (±5.4) | 0.09 |
| Disease duration, mean years (±SD) | 7.4 (±3.2) | 5.6 (±2.5) | 0.15 |
| Current Total LEDD, mean (±SD) | 856 (±407) mg | 756 (± 400) mg | 0.6 |
| Current Dopamine agonist LEDD, mean (±SD) | 138 (±172) mg | 167 (±113) mg | 0.7 |
| MoCA, mean (±SD) | 26 (±3.2) | 28 (±2.3) | 0.2 |
| G-SAS, mean (±SD) | 34.8 (±5.6) | 4.6 (±6.3) | <0.001 |
H & Y = Hoehn and Yahr; UPDRS III = Unified Parkinson’s Disease Rating Scale, motor score; LEDD = levodopa equivalent daily dose: L-dopa dose + L-dopa dose × 1/3 if on entacapone + bromocriptine (mg) × 10 + cabergoline or pramipexole (mg) × 67 + ropinirole (mg) × 20 + pergolide (mg) × 100 + apomorphine (mg) × 8 (from Evans et al., 2004); MocA = Montreal Cognitive Assessment; G-SAS = Gambling Symptom Assessment Scale.
All patients with pathological gambling developed this on exposure to dopamine agonists (pramipexole n = 5 or ropinirole n = 2) independent of the timing of initiation of levodopa therapy. Calculation of a daily L-dopa equivalent daily dose (LEDD) for each patient was based on theoretical equivalence to L-dopa (Evans et al., 2004) as follows: L-dopa dose + L-dopa dose × 1/3 if on entacapone + bromocriptine (mg) × 10 + cabergoline or pramipexole (mg) × 67 + ropinirole (mg) × 20 + pergolide (mg) × 100 + apomorphine (mg) × 8. All individuals were screened for dementia prior to the experiment using the Montreal Cognitive Assessment (Nasreddine et al., 2005).
Subjects were studied after overnight withdrawal (12–18 h) of their anti-parkinsonian medications with [11C] raclopride PET to measure changes in striatal dopamine D2/D3 receptor binding during gambling. Each subject underwent two [11C] raclopride PET sessions within 2 weeks (total injected dose: 20 mCi), one during performance of a gambling task and one during a control task. The scan order was randomized across subjects, and scans were performed on two separate days.
This study was approved by the Research Ethics Committees for the Centre for Addiction and Mental Health and the University Health Network of the University of Toronto. All subjects provided written informed consent to participate.
Gambling task
During PET acquisition scans, participants performed a gambling task and control task (Fig. 1) viewed through video eyewear (DV920; Icuiti Corporation, New York, USA).
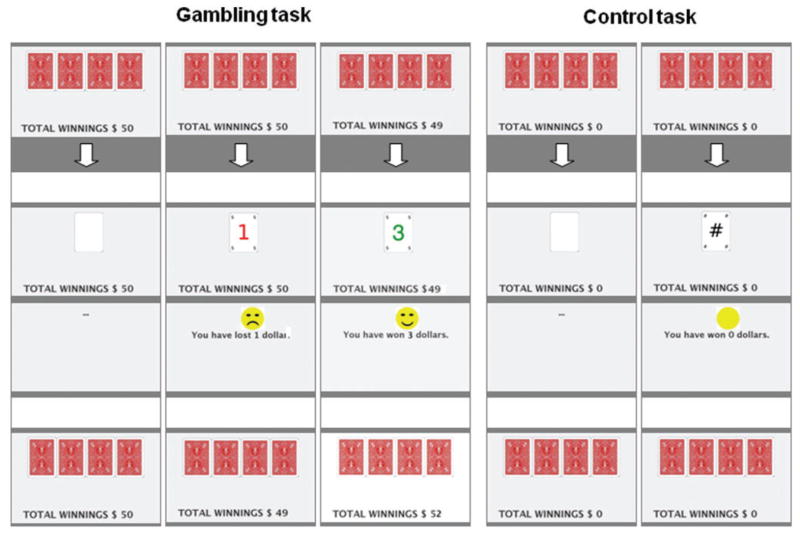
Figure 1.
Example of the gambling and control task performed during the PET scans by Parkinson’s disease patients with and without pathological gambling. The gambling task included both reward and penalty trials. Details are reported in the text.
For the gambling task subjects were asked to select with their right hand one of four cards (25% probability) displayed on the video screen. The gambling task included both reward and penalty trials in the amounts of +$1, +$3 or +$5 Canadian or −$1, −$3 or −$5 Canadian, respectively. On reward trials, a green number with the amount of the reward and a smiling face as a positive reinforcement cue was displayed for 1.5 s, and subjects were presented with the sound of a cash register door opening. On penalty trials, a red number with the amount of the penalty ($1, $3 or $5 Canadian) and a frowning face as a negative reinforcement cue was displayed for 1.5 s, along with an unpleasant sound of a horn. After each trial, an updated running total of the subjects’ current earnings was displayed. The subjects started from an initial holding of $50 Canadian. The card sequence was determined by a computerized random sequence generator providing a relative ratio of 3:1 reward versus penalty cards, ensuring a final winning total of $146 Canadian for each subject. Subjects were not told that their choices would have no influence on the outcome of the gambling task.
In the control task, subjects selected cards as for the gambling task but received neither a reward nor penalty. After three blank cards, a card displaying a meaningless symbol (#) was shown on the screen, and the subject was presented with a clicking sound. After each trial, an updated running total of subjects’ current earnings, set to zero, was displayed. The gambling paradigm employed in this study is only partially representative of gambling in real life and represents a necessary simplification of gambling in order to control for the variable degree of cognitive involvement and skill level that would otherwise vary dramatically between control Parkinson’s disease patients and experienced gamblers with pathological gambling.
Both tasks included three blocks of 140 trials each, for a total of 420 trials. Subjects were given a 5 min rest between each block. Both gambling and control tasks were started 5 min before the injection of the radiotracer and continued until all 420 trials were completed (40 min duration). Response times during each trial were measured to ensure that motor disability did not confound results and a two-way ANOVA with two factors (patients and tasks) × two levels (patients: with/without pathological gambling, tasks: gambling and control) design was performed.
PET
PET scans were obtained with a high-resolution PET CT, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN, USA) operating in 3D mode with an in-plane resolution of ~4.6 mm full width at half-maximum (FWHM). To minimize head movement, subjects were fitted with a custom-made thermoplastic facemask with a head-fixation system secured to the scanner platform (Tru-Scan Imaging, Annapolis). Before each emission scan, a scout view was obtained to determine accurate positioning of the subject and a low-dose (0.2 mSv) CT scan was acquired to correct for attenuation.
Ten mCi of [11C] raclopride was injected into the left antecubital vein over 60 s, and emission data were then acquired over 60 min in 28 frames of progressively increasing duration (fine 1 min frames, 20 2 min frames, three 5 min frames). Each subject underwent two [11C] raclopride PET sessions, one during performance of gambling task and one during performance of the control task in random order. High-resolution MRI scans (GE Sigma 1.5 T, T1-weighted images, 1 mm slice thickness) of each subject’s brain was acquired for co-registration and transformed into standardized stereotaxic space. After the realignment procedure for motion correction among the frames, motion corrected PET frames were summed, registered to the corresponding MRI (Woods et al., 1993) and transformed into standardized stereotaxic space using the transformation parameters previously determined for the MRI.
Voxelwise [11C] raclopride binding potential was calculated using a simplified reference tissue (cerebellum) method (Lammertsma and Hume, 1996; Gunn et al., 1997) to generate statistical parametric images of change in binding potential (Aston et al., 2000). This method uses the residuals of the least-squares fit of the compartmental model to the data at each voxel to estimate the standard deviation of the binding potential estimate, thus greatly increasing degrees of freedom. Only peaks falling within the striatum were considered. A reduction in [11C] raclopride binding potential indicated an increase in extra-cellular dopamine concentration (Dewey et al., 1993; Breirer et al., 1997).
A threshold level of t ≥ 4.1 was considered significant (P<0.05, two-tailed) corrected for multiple comparisons (Worsley et al., 1996), assuming a search volume equal to the entire striatum, an effective image filter of 6 mm FWHM, and 276 degrees of freedom (Aston et al., 2000). In each patient, binding potential values were extracted from a spherical region of interest (radius 2 mm) centred at the x, y and z coordinates of the statistical peak revealed by the parametric map. This region of interest corresponded to a volume of 56 mm3 (single voxel volume = 2.13 mm3). Differences in binding potentials were analysed with a two-way ANOVA with two factors (patients and tasks) × two levels (patients: with/without pathological gambling, tasks: gambling and control) design.
Striatal anatomical boundaries are based on the functional organization of limbic, associative and sensorimotor sub-compartments as proposed by Laruelle and colleagues (Mawlawi et al., 2001; Martinez et al., 2003). Coordinates listed below are expressed in Talairach space.
Results
Parkinson’s disease patients with pathological gambling did not differ from control Parkinson’s disease patients in age, disease severity, cognitive status or current LEDD of medications (Table 1).
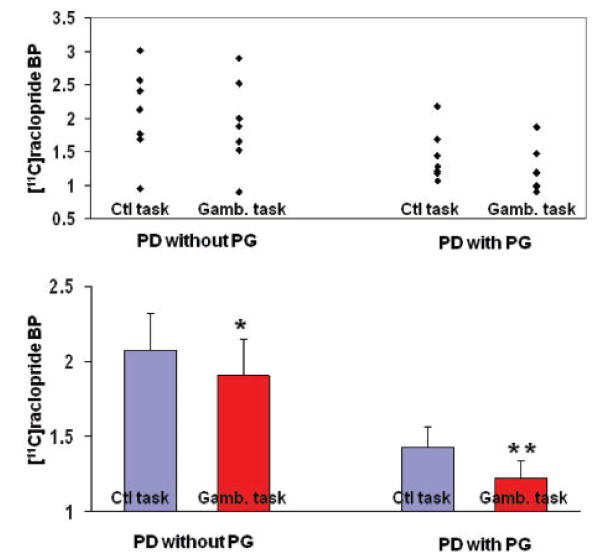
Parametric images revealed that patients with pathological gambling presented greater release of dopamine in the ventral striatum during performance of the gambling task (Fig. 2, bottom figure, Figs 3 and 4). In Parkinson’s disease patients with pathological gambling, [11C] raclopride binding potential extracted from a region of interest centered at the statistical peak revealed by the parametric map (X = −27, Y = 4, Z = −10; t = 4.9, P<0.05 corrected for multiple comparisons) was 1.42 ± 0.14 (mean ± SE) during control task and 1.22 ± 0.12 (mean ± SE) during gambling task (Fig. 4). In Parkinson’s disease patients without pathological gambling, [11C] raclopride binding (X = −27, Y = 0, Z = −6; t = 4.4; P<0.05 corrected for multiple comparisons) was 2.07 ± 0.25 (mean ± SE) during control task and 1.90 ± 0.24 (mean ± SE) during gambling task (Fig. 4). A two-way ANOVA analysis from region of interests centred at the statistical peak revealed by the parametric map (pathological gambling: X = −27, Y = 4, Z = −10; without pathological gambling: X = −27, Y = 0, Z = −6) revealed a significant main effect for patients [F(1, 6) = 6.827, P = 0.03], tasks [F(1, 6) = 48.737, P<0.0001] and patient/task interaction [F(1, 6) = 7.155, P = 0.03].
Figure 2.
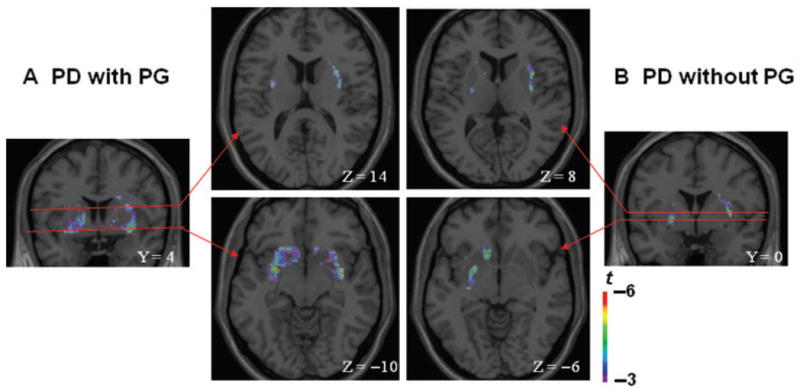
Coronal and axial sections of the statistical parametric map of the change in [11C] raclopride binding potential overlaid upon the average MRI in stereotaxic space. The figure displays the significant areas of striatal dopamine release during gambling as compared to control task in (A) Parkinson’s disease patients with pathological gambling (Y = 4; Z = −10; Z = 14) and (B) without pathological gambling (Y = 0; Z = −6; Z = 8) in the ventral striatum (Z = −10 and Z = −s6) (bottom figure) and dorsal striatum (Z = 14 and Z = 8) (top figure).
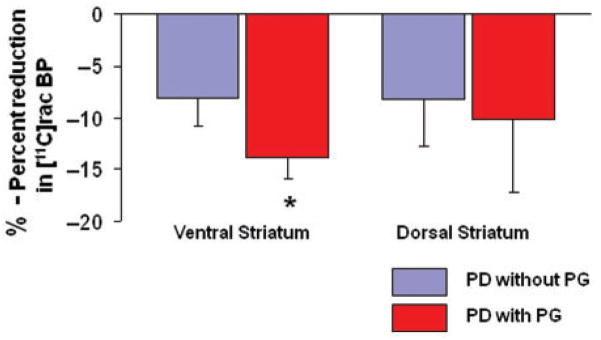
Figure 3.
Percent reduction in [11C] raclopride-binding potentials during gambling (as compared to control task) in Parkinson’s disease patients without pathological gambling (blue bars) and with pathological gambling (red bars). Binding potentials are extracted from a spherical region of interest (radius 2 mm) centered at the x, y and z coordinates of the statistical peak revealed by the parametric map at the level of ventral (pathological gambling: X = −27, Y = 4, Z = −10; without pathological gambling: X = −27, Y = 0, Z = −6) and dorsal (pathological gambling: X = 32, Y = 4, Z = 14; without pathological gambling: X = 32, Y = 0, Z = 8) striatum. *Paired t-test, P = 0.01.
Figure 4.
Individual values (top) and mean ± SE (bottom) of [11C] raclopride-binding potential from the ventral striatum extracted from the x, y and z coordinates described in Fig. 3 in Parkinson’s disease patients with pathological gambling (**t = 4.9) and without pathological gambling (*t = 4.4) during control (Ctl) and gambling (Gamb) task.
Thus, patients with pathological gambling presented greater decreases in [11C] raclopride binding in the ventral striatum during gambling (13.9%) than did control patients (8.1%) (paired t-test, P = 0.01) (Fig. 3). No significant correlation was observed between the amount of dopamine release and severity of gambling (G-SAS score) in Parkinson’s disease patients with pathological gambling.
Voxel-based analysis showed that while Parkinson’s disease patients with pathological gambling released DA bilaterally in the ventral striatum (356 voxels—2848 mm3), patients without pathological gambling released DA only unilaterally in the left ventral striatum (152 voxels—1216 mm3) (Fig. 2, bottom figure).
Another important observation was that at baseline, between group comparison of [11C] raclopride binding potentials obtained during the control task demonstrated that patients with pathological gambling had lower levels of D2 binding in ventral striatum than did control Parkinson’s disease patients (paired t-test, P = 0.02).
In the dorsal striatum (Fig. 2, top figure), comparison of [11C] raclopride binding potential between gambling and control task revealed that while binding potentials decreased in both groups of patients (with and without pathological gambling), differences between groups in this measure were less marked than in the ventral striatum and a two-way ANOVA analysis from regions of interest centred at the statistical peak revealed by the parametric map (pathological gambling: X = 32, Y = 4, Z = 14; without pathological gambling: X = 32, Y = 0, Z = 8) revealed no significant main effect for patients, tasks and patient/task interaction (Fig. 3). BP was reduced by 10.2% in Parkinson’s disease patients with pathological gambling (X = 32, Y = 4, Z = 14; t = 4.4, P<0.05 corrected for multiple comparisons; mean ± SE, control task: 1.86 ± 0.26; gambling task: 1.64 ± 0.25) and 8.2% in Parkinson’s disease patients without pathological gambling (X = 32, Y = 0, Z = 8; t = 4.3 P<0.05 corrected for multiple comparisons; mean ± SE, control task: 2.02 ± 0.25; gambling task: 1.80 ± 0.15).
Motor performance during the tasks, as measured by response times, was comparable, between the two groups of patients. A two-way ANOVA analysis revealed no significant main effects and interaction. In Parkinson’s disease with and without pathological gambling during gambling motor performance was 1912.9 ± 1291.92 ms and 2160.08 ± 1259.21 ms, respectively; in Parkinson’s disease with and without pathological gambling during control tasks was 2186.13 ± 1230.75 ms and 2131.98 ± 1214.41 ms, respectively.
Discussion
This study identified a number of differences in dopaminergic function between Parkinson’s disease patients who developed pathological gambling while being treated with dopamine agonists and control Parkinson’s disease patients who were matched for age, disease severity, cognitive performance and amount of dopaminergic intake. Patients with pathological gambling had greater reductions in binding in the ventral striatum during gambling than did control patients. [11C] raclopride is sensitive to competition from endogenously released dopamine in response to drugs or tasks that induce dopamine release. In this study, the observed decreases in binding potential are, therefore, likely secondary to dopamine release in response to the gambling task. Reduction in binding potential was observed bilaterally in the striatum of Parkinson’s disease patients with pathological gambling; whereas controls exhibited decreased binding only in the left striatum, an asymmetry that could reflect differences between groups in the neural processing of the gambling. Another interesting observation was that at baseline Parkinson’s disease patients with pathological gambling also had, as measured during the control task, lower D2 receptor binding in ventral striatum than control Parkinson’s disease patients without pathological gambling.
These results are broadly consistent with previous functional imaging evaluations of both behavioural and chemical addictions in the general population. In normal subjects, the activity and biochemistry of the brain does not appear to differ qualitatively when rewards derived from drugs of abuse or from behavioural stimuli. Monetary and sexual stimuli, all elicit the same patterns of striatal activation as drugs of abuse (Koepp et al., 1998; Knutson et al., 2001; Erk et al., 2002; Childress et al., 2008). However, patients with chemical addiction release more dopamine in ventral striatal circuits in response to their drugs of abuse (Volkow et al., 2006, 2007). The current study presents the first evidence for this phenomenon in pathological gambling and supports the categorization of this disorder within the spectrum of behavioural addictions. Some investigators have proposed that the increase in dopamine release seen in chemical addiction may reflect a sensitization of circuits that occurs when repeated exposure to the addictive stimulus bypasses normal mechanisms of habituation (Di Chiara and Bassareo, 2007). In this context, the increased release of dopamine we have observed in patients with pathological gambling could be interpreted as reflecting either a priming effect of repeated exposure to the gambling stimulus itself or to dopamine agonists, or to premorbid hypersensitivity of the ventral striatal circuits in the pathological gambling population.
Our finding of lower baseline values of D2/D3 binding in ventral striatum of patients with pathological gambling could be interpreted to reflect increased DA release in the basal state or, equally, lower baseline levels of D2/D3 receptors. This observation is likewise consistent with prior studies showing that low dopamine binding may mediate vulnerability to addiction. In non-addicted subjects, below baseline measures of striatal dopaminergic receptor availability predicts liking for methylphenidate and low striatal dopaminergic receptor availability has likewise been demonstrated in subjects with morbid obesity due to overeating (Volkow et al., 2002a, 2008). Results from studies in a number of animal models of addiction also support a role for low dopaminergic receptor availability mediating vulnerability to addiction (Nader et al., 2002, 2006; Dalley et al., 2007). Some authors have proposed the finding of low D2 receptor availability in addictions best fits within the framework of the ‘reward deficiency syndrome’, whereby a chronic hypo-dopaminergic state is proposed to render individuals vulnerable to addiction by triggering a drive for rewarding substances or behaviours to supplement deficient dopamine in reward circuits (Volkow et al., 2002b).
The major findings we have demonstrated in this study localize to the ventral striatum, although we have also observed dopamine release in the dorsal striatum in response to the gambling task in both pathological gambling and control groups. This result is consistent with the known importance of ventral striatal dopamine release in both normal reward and formation of addictions. A growing body of work, however, has also highlighted the importance of the dorsal striatum in the maintenance of established addictions and in the phenomenon of craving (Volkow et al., 2006). Increasingly a distinction between dopamine release in ventral and dorsal striatal circuits appears to be important in the addiction process, with possible implications for ICDs. Phasic release of dopamine from mesolimbic projections to the nucleus accumbens is thought to occur in response to errors in the predicted value of outcomes. The signal that dopamine encodes likely serves to continuously update the importance of any stimulus that predicts a reward and may thus be critical to the formation of addictions. But as addictions become progressively more ingrained and habitual, the locus of neural control appears to shift dorsally in the striatum, to regions conventionally associated with the performance and maintenance of habits (Chambers et al., 2003; Porrino et al., 2004; Everitt and Robbins, 2005). Thus the shift from the initiation to the consolidation of addiction may reflect an equivalent shift from limbic, to associative and sensorimotor corticostriatal circuits (Porrino et al., 2004).
One of the most salient observations relating to ICDs associated with dopaminergic medications is that they develop only in a subset of patients. This suggests an underlying susceptibility within the population that may be quite separate from the co-occurrence of additional diseases such as Parkinson’s disease—although a potential interaction between susceptibility to ICDs and comorbid conditions cannot be excluded. In this context, the emergence of ICDs on exposure to dopaminergic agents could be viewed as a form of pharmacological challenge, unmasking behavioural vulnerabilities latent in the general population. In support of this view, a number of cognitive and behavioural traits such as impulsivity and novelty seeking have been shown to differentiate patients with ICDs, in both the general population and in those patients with comorbid Parkinson’s disease (Blaszczynski et al., 1997; Jentsch and Taylor, 1999; Evans et al., 2005; Potenza, 2006; Voon et al., 2007). Such differences may point to underlying genetic variability conferring risk for ICDs, and indeed a number of allelic associations with both pathological gambling and drug addictions have been reported (Eisen et al., 1998; Potenza, 2005).
Mechanisms underlying the induction of ICDs by dopaminergic medications could potentially operate on multiple levels; by preferentially activating dopamine receptor subtypes, sensitizing receptors or by providing excessive dopaminergic stimulation to circuits regulating individual components of the addiction process, including motivation, reward, habit formation, and impulse control (Lawrence et al., 2003). The potential for dopaminergic agents to sensitize striatal circuits may be particularly significant in the context of the increased striatal release of dopamine we have observed in our study.
The extensive literature describes the ability of dopamine and dopaminergic agents to prime or increase subsequent dopamine release in the ventral striatum. This phenomenon appears associated with an increase in salience or desirability of these drugs that manifests as craving (Robinson and Berridge, 2000; Berke and Hyman, 2000). Likewise Evans and colleagues (2006) in an [11C] raclopride PET evaluation of Parkinson’s disease patients with dopamine dysregulation have demonstrated that in response to a single dose of L-dopa, patients with dopamine dysregulation release more dopamine in ventral striatal circuits compared to control Parkinson’s disease patients. This hypersensitivity correlated with the self reported compulsive ‘wanting’ of the drug but not liking for it. Drugs with dopaminergic actions are known to cross-prime for the release of dopamine induced by other drugs within the same class, and to upregulate the incentive salience of a variety of stimuli with which they have become associated. In rodent models of addiction, for example, repeated exposure to psychostimulants, such as amphetamine, increases the salience for other rewards such as food and sexual stimuli (Engber et al., 1989; Robinson and Berridge, 2000; Nocjar and Panksepp, 2002; Robinson and Berridge, 2003). Likewise in problem gamblers, amphetamine has been shown to increase motivation for gambling in a manner that is predicted by the severity of the subject’s gambling (Zack and Poulos, 2004).
Parallels between this phenomenon and the development of behavioural addictions in patients treated with dopaminergic agents are self evident. A prominent feature of all the ICDs relating to use of dopaminergic agents—compulsive gambling, compulsive shopping and hypersexuality—is an intense craving for the behaviours consistent with increased incentive salience (Robinson and Berridge, 2000). Patients often report being driven to perform their behaviours in spite of full awareness of the adverse consequences of doing so. In this context, the dopaminergic stimulation provided by dopamine agonists may mimic the sensitizing effects of excess dopamine in ventral striatal circuits during performance of rewarding behaviours and so triggering a form of behavioural addiction.
Another interesting observation of our study was that changes in binding encompassed different areas of the ventral striatum. According to previous imaging reports (Mawlawi et al., 2001; Martinez et al., 2003), these areas of the striatum are part of both limbic and associative circuits. This is not surprising, if we consider that gambling behaviour, besides engaging the reward system, requires also significant cognitive strategies often impaired in these patients (Volkow et al., 2004; Baler and Volkow, 2006). In fact gamblers, including those in this study, report the tendency to calculate odds on the basis of cards already revealed.
In contrast to other functional imaging studies (Evans et al., 2006; Reuter et al., 2006), we did not observe any correlation between striatal findings and severity of gambling in Parkinson’s disease patients with pathological gambling. It is possible that limitations intrinsic to our gambling task—which did not allow us to dissect different behavioural aspects (e.g. perseveration) or individual strategies—may have reduced the sensitivity of the analysis.
Reduction in binding potential was observed bilaterally in the striatum of Parkinson’s disease patients with pathological gambling; whereas controls exhibited decreased binding only in the left striatum. While such asymmetry could reflect differences between groups in the neural processing of the gambling, it is difficult to speculate whether this observation could be related to the underlying disease or be a feature of the reward network or combination of both. However, it is worth noting that similar changes in the left ventral striatum have also been reported in functional imaging studies performed in healthy subjects during performance of reward tasks (Zald et al., 2004; Riba et al., 2008).
While we acknowledge the fact that due to the blurred anatomical boundaries between dorsal and ventral striatal regions some of the radioactivity measured in the ventral striatum may result from contamination from counts originating in the adjacent dorsal striatum (Mawlawi et al., 2001), we believe that our measures taken to prevent head motion along with the applied motion correction have minimized the contribution of this factor. In addition, the striking difference in dopamine release observed in our study between dorsal and ventral striatum favours and supports the significant functional differences between these two dopaminergic regions.
Although the pathogenesis for the changes in dopaminergic function observed in our Parkinson’s disease patients with pathological gambling remains to be elucidated, the similarities between the findings in these patients and those with chemical addictions supports the classification of pathological gambling within the spectrum of behavioural addiction. Our findings may point to an underlying vulnerability in the general population that may provide insight into the pathophysiology of this and other ICDs and may suggest Parkinson’s disease as a potential model of dopaminergic disregulation to elucidate behaviour addiction.
Acknowledgments
We wish to thank the staff of the PET centre at CAMH and Movement Disorders Centre at the TWH for their assistance in carrying out the studies.
Funding
Ontario Problem Gambling Research Centre and CIHR (MOP-64423 to A.P.S.); CIHR New Investigator Research Award (to A.P.S.).
Abbreviations
- FWHM
full width at half-maximum
- ICD
impulse control disorders
- LWDD
L-dopa equivalent daily dose
- MAO
mono amine oxidase
- PET
positron emission tomography
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. DSM-IV-TR. [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomography neuro-receptor ligand data. Neuroimage. 2000;12:245–56. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Avanzi M, Baratti M, Cabrini S, Uber E, Brighetti G, Bonfà F. Prevalence of pathological gambling in patients with Parkinson’s disease. Mov Disord. 2006;21:2068–72. doi: 10.1002/mds.21072. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–66. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bergh C, Eklund T, Sodersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–5. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Steel Z, McConaghy N. Impulsivity in pathological gambling: the antisocial impulsivity. Addiction. 1997;92:75–87. [PubMed] [Google Scholar]
- Breirer A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94:2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–6. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Lin N, Lyons MJ, Scherrer JF, Griffith K, True WR, et al. Familial influences on gambling behaviour: an analysis of 3359 twin pairs. Addiction. 1998;93:1375–84. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- Engber TM, Susel Z, Juncos JL, Chase TN. Continuous and intermittent levodopa differentially affect rotation induced by D-1 and D-2 dopamine agonists. Eur J Pharmacol. 1989;168:291–8. doi: 10.1016/0014-2999(89)90790-5. [DOI] [PubMed] [Google Scholar]
- Erk S, Spitzer M, Wunderlich AP, Galley L, Walter H. Cultural objects modulate reward circuitry. Neuroreport. 2002;13:2499–503. doi: 10.1097/00001756-200212200-00024. [DOI] [PubMed] [Google Scholar]
- Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol. 2006;59:852–8. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- Evans AH, Lawrence AD, Potts J, Appel S, Lees AJ. Factors influencing susceptibility to compulsive dopaminergic drug use in Parkinson disease. Neurology. 2005;65:1570–4. doi: 10.1212/01.wnl.0000184487.72289.f0. [DOI] [PubMed] [Google Scholar]
- Evans AH, Butzkueven H. Dopamine agonist-induced pathological gambling in restless legs syndrome due to multiple sclerosis. Mov Disord. 2007;22:590–1. doi: 10.1002/mds.21303. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Grant JE, Brewer JA, Potenza MN. The neurobiology of substance and behavioural addictions. CNS Spectr. 2006;11:924–30. doi: 10.1017/s109285290001511x. [DOI] [PubMed] [Google Scholar]
- Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Grosset DG. Problematic gambling on dopamine agonists: Not such a rarity. Mov Disord. 2006;21:2206–8. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Holden C. ’Behavioral’ addictions: do they exist? Science. 2001;294:980–2. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- Hollander E, Wong CM. Body dysmorphic disorder, pathological gambling, and sexual compulsions. J Clin Psychiatry. 1995a;56 (Suppl 4):7–12. [PubMed] [Google Scholar]
- Hollander E, Wong CM. Obsessive-compulsive spectrum disorders. J Clin Psychiatry. 1995b;56 (Suppl 4):3–6. [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2005;11:381–6. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–8. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Evans AH, Lees AJ. Compulsive use of dopamine replacement therapy in Parkinson’s disease: reward systems gone awry? Lancet Neurol. 2003;2:595–604. doi: 10.1016/s1474-4422(03)00529-5. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lu C, Bharmal A, Suchowersky O. Gambling and Parkinson disease. Arch Neurol. 2006;63:298. doi: 10.1001/archneur.63.2.298-a. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–57. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Bostwick MJ, et al. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord. 2007;13:516–9. doi: 10.1016/j.parkreldis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Pope HJ, Keck PE, Aizley HG. The DSM-III-R impulse control disorders not elsewhere classified: clinical characteristics and relationship to other psychiatric disorders. Am J Psychiatry. 1992;149:318–27. doi: 10.1176/ajp.149.3.318. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–6. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Waters C. Compulsive eating and weight gain related to dopamine agonist use. Mov Disord. 2006;21:524–9. doi: 10.1002/mds.20757. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interaction with environmental variables. Behav Brain Res. 2002;128:189–203. doi: 10.1016/s0166-4328(01)00321-7. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–62. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, Eisen SA. Shared genetic contributions to pathological gambling and major depression in men. Arch Gen Psychiatry. 2005;62:1015–21. doi: 10.1001/archpsyc.62.9.1015. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Should addictive disorders include non-substance-related conditions? Addiction. 2006;101 (Suppl 1):142–51. doi: 10.1111/j.1360-0443.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Suchowersky O. Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism Relat Disord. 2007;13:535–6. doi: 10.1016/j.parkreldis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–8. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Riba J, Krämer UM, Heldmann M, Richter S, Münte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS One. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, et al. Parkinson’s disease and dopaminergic therapy–differential effects on movement, reward and cognition. Brain. 2008:2094–105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MA, Chang YL, Munson SK, Okun MS, Fernandez HH. Hypersexuality and paraphilia induced by selegiline in Parkinson’s disease: report of 2 cases. Parkinsonism Relat Disord. 2006;12:392–5. doi: 10.1016/j.parkreldis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am J Public Health. 1999;89:1369–76. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeding HM, Goudriaan AE, Foncke EM, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry. 2007;78:517–9. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss WD. Activation of basal ganglia loops in idiopathic Parkinson’s disease: a PET study. J Neural Transm. 2003;110:1289–301. doi: 10.1007/s00702-003-0041-7. [DOI] [PubMed] [Google Scholar]
- Tippmann-Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68:301–3. doi: 10.1212/01.wnl.0000252368.25106.b6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002a;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002b;78:610–24. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: Possible contributing factors. Neuroimage. 2008;42:1537–43. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 (Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006a;67:1254–7. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006b;66:1750–2. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- Voon V, Thomsen T, Miyasaki JM, de Souza M, Shafro A, Fox SH, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch Neurol. 2007;64:212–6. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63:969–73. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Amphetamine primes motivation to gamble and gambling-related semantic networks in problem gamblers. Neuropsychopharmacology. 2004;29:195–207. doi: 10.1038/sj.npp.1300333. [DOI] [PubMed] [Google Scholar]
- Zald DH, Boileau I, El-Dearedy W, Gunn R, McGlone F, Dichter GS, et al. Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci. 2004;24:4105–12. doi: 10.1523/JNEUROSCI.4643-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]