Abstract
Dopamine plays an important role in several brain functions and is involved in the pathogenesis of several psychiatric and neurological disorders. Neuroimaging techniques such as positron emission tomography allow us to quantify dopaminergic activity in the living human brain. Combining these with brain stimulation techniques offers us the unique opportunity to tackle questions regarding region-specific neurochemical activity. Such studies may aid clinicians and scientists to disentangle neural circuitries within the human brain and thereby help them to understand the underlying mechanisms of a given function in relation to brain diseases. Furthermore, it may also aid the development of alternative treatment approaches for various neurological and psychiatric conditions.
Keywords: brain imaging, dopamine, positron emission tomography, prefrontal cortex, transcranial magnetic stimulation
Dopamine (DA) is involved in many cerebral functions, for example, learning, reward, motor control, emotion, and executive functions. Given its pervasiveness, the dopaminergic system has been extensively targeted for the treatment of various psychiatric and neurological diseases, many of which are known to be associated with DA abnormalities, for example, Parkinson disease (PD), Huntington disease, depression, schizophrenia, and behavioral/chemical addiction. Nevertheless, how regional dopaminergic changes contribute to the various symptoms involved in these diseases is not yet clear.
Animal studies have revealed that there are three major dopaminergic pathways: nigrostriatal, mesolimbic, and mesocortical. Nigrostriatal DA plays a crucial role in cortico-striato-pallido-thalamo-cortical circuitry (Alexander and others 1986). It is thought that DA in this pathway facilitates the selection of an optimal response from competing motor/cognitive programs by balancing activity within and between a direct and an indirect pathway; that is, DA acts to excite the relevant motor/cognitive program while inhibiting irrelevant ones (Mink 1996, 2003). The mesolimbic and mesocortical dopaminergic pathways originate in the ventral tegmental area (VTA) of the midbrain and project to the limbic system (via the nucleus accumbens) and the frontal cortex, respectively. As such, the former is proposed to be important for reward-related learning and emotion (Haber and Knutson 2010) whereas the latter is involved in various executive cognitive functions (Floresco and Magyar 2006). Neuroimaging studies corroborate this functional network in the human (Egerton and others 2009) but are limited by the fact that they cannot determine if an area is an essential mediator of a particular brain function or one that is merely activated in tandem with other essential components (Walsh and Cowey 2000).
That said, the refined combination of interference techniques (e.g., transcranial magnetic stimulation [TMS]) with brain imaging (e.g., positron emission tomography [PET] and single-photon emission computation tomography [SPECT]) allows for this limitation to be overcome and allows for the investigation of the functional implications of neurochemical interactions within the human brain in vivo.
Traditionally, the striatal nodes of the nigrostriatal and mesolimbic pathways have been most extensively studied using the gold-standard dopaminergic receptor ligands [11C]raclopride for PET and [123I]IBZM for SPECT. The recent development of high-affinity DA receptor ligands such as [11C]FLB 457, [18F]fallypride, and [123I]epidepride has broadened the focus of neuroscientists to include extrastriatal regions within the mesolimbic and mesocortical dopaminergic pathways. This review attempts to summarize the animal experiments that provided the rationale for “perturbing and measuring” dopaminergic changes in the human brain. We will then give particular consideration to the most recent reports that have employed TMS methodology combined with displaceable PET agents in the human. We will also summarize the possible implications of this work and speculate on the future of brain stimulation/PET-ligand studies.
Measuring Dopamine Release in Experimental Animals
Microdialysis experiments conducted in experimental animals have documented that the prefrontal cortex (PFC) regulates DA release in the striatum (Del Arco and Mora 2008; Grace and others 2007). In particular, electrical stimulation applied for 20 min at 50 μA and 100 μA over the bilateral PFC increased striatal DA concentration by 38% and 69%, respectively (Taber and Fibiger 1995). Similarly, repetitive TMS (rTMS; 1000 pulses, 20 Hz, 50 pulses/train, 20 trains, 2.5-min intertrain interval) increased extracellular DA in the dorsal striatum (~70%; peak at 90 min after the end of rTMS) and nucleus accumbens (NAc; ~30%; peak at 120 min after the end of rTMS) in anesthetized rats (Keck and others 2002).
There are two main pathways through which the PFC can modulate dopaminergic levels: a corticonigral pathway (Karreman and Moghaddam 1996; Keefe and others 1993; Murase and others 1993) and a corticostriatal pathway (Keefe and others 1992). These two pathways present with significant differences. In the corticostriatal pathway, dopaminergic terminals are influenced by glutamate. In fact, glutamate receptor antagonists abolish PFC-regulated increase of striatal DA (Karreman and Moghaddam 1996; Taber and others 1995). Zangen and Hyodo (2002) reported increased glutamate (~55%) levels in addition to DA (~20%) in the NAc after frontal cortex stimulation (2 Hz, 200 pulses, 98% of maximum intensity). Therefore, PFC regulation over striatal DA release described in these electrical and TMS studies is likely performed via corticostriatal glutamatergic afferents to adjacent dopaminergic nerve terminals (Sesack and Pickel 1992) regulating tonic release of DA (West and others 2003). In conscious rats, DA release after PFC stimulation was considerably more sustained (>150 min) compared with anesthetized rats even after short rTMS (300 pulses; Keck and others 2002), suggesting that other mechanisms may play an important role in the awake state.
The PFC also regulates DA release via the corticonigral pathway (Karreman and Moghaddam 1996; Keefe and others 1993; Murase and others 1993), which is not influenced by glutamate but can be affected by tetrodotoxin, which blocks cell body firing and abolishes diffuse stress-induced DA release (Keefe and others 1993).
Models of DA regulation suggest that DA released in the synapse (phasic release) is not completely cleared by DA transporter (DAT) and may travel up to 7 to 8 μm outside the synapse, thereby influencing neighboring extra-synaptic receptors, especially high-affinity D2 receptors (Rice and Cragg 2008). This is even more true in conditions such as PD, in which DA released from the degenerating terminals, because of the loss of reuptake sites, tends to diffuse out of the synapse into the extracellular space acting on neighboring receptors of adjacent and nearby denervated sites (see review by Zigmond and others 1990). Thus, although there is clear evidence that phasic and tonic DA release interact and influence each other (Grace 1991), it is very likely that rTMS of the PFC may influence the striatal DA level via modulation of both modes of DA activity.
Animal studies have revealed that TMS can also induce dopaminergic changes outside the striatum in cortical regions (Ben-Shachar and others 1997; Keck and others 2000, 2002) via several mechanisms, including cortical afferents to the VTA (Carr and Sesack 2000), direct modulation of DA activity in the targeted tissue (Shaul and others 2003), and/or reciprocal cortico-cortical influence (Koch and others 2007; Koski and Paus 2000; Paus and others 2001). Administering rTMS (50 pulses, 20 Hz) over the rat brain increased DA concentration in the hippocampus (~18%) whereas it decreased DA levels in the frontal cortex (~26%) immediately after rTMS (i.e., acute effect of rTMS; Ben-Shachar and others 1997). Subsequent studies, although confirming the effect of rTMS (1000 pulses, 20 Hz, 50 pulses/train, 20 trains, 2.5-min intertrain interval) over the PFC, showing increased extracellular DA in the hippocampus (~150%; peak at 30 min after the end of rTMS) in anesthetized rats (Keck and others 2002), failed to show significant differences in frontal DA concentration during real and sham rTMS (Kanno and others 2004). Of particular interest was the observation that bursts of electrical stimulation of mesocortical axons increased frontal DA concentration to a greater extent compared with tonic stimulation (Bean and Roth 1991) and that rTMS-induced DA release was differently regulated in conscious animals compared with the anesthetized animals (Erhardt and others 2004; Keck and others 2002). Another important consideration was the stimulation intensity. It is commonly believed that higher stimulation intensities will produce a stronger effect. However, it has been shown that high-intensity (~149% motor threshold [MT]) stimulation, as well as low-intensity stimulation (~36% MT), did not modulate DA concentration. On the contrary, rTMS (500 pulses, 25 Hz, 25 pulses/train, 20 trains, 1-min intertrain interval) delivered at 110% MT increased dorsal striatal DA level (~30%) up to 100 min after the end of rTMS (Kanno and others 2004). Thus, based on these reports, it is important to keep in mind that stimulation modalities and level of vigilance may play an important role in rTMS-induced DA transmission in the human brain.
Table 1 provides a summary of the main features of those studies investigating the dopaminergic effect of TMS in animals.
Table 1.
Dopaminergic Effect of Brain Stimulation in Animals
| Article | Subjects (N) | Stimulation Parameters
|
Target Region | Methodology | Regional Changes | Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Method | Frequency | Intensity | Total pulses | ||||||
| Taber and Fibiger (1995) | Awake rats (6) | Electrical stimulation | 60 Hz | 50 μA 100 μA |
6420 | Bilateral PFC | Microdialysis | NAc | 38% ↑DA over 0–20 min 69% ↑DA over 0–20 min |
| Keck and others (2002) | Anesthetized rats (7) | rTMS (round coil) | 20 Hz | 130% RMT, 4.0 T | 1000 | Left frontal cortex | Microdialysis | Right dorsal HC | ~120% ↑DA over 0–30 min |
| Anesthetized rats (7) | Right NAc | ~30% ↑DA over 60–120 min | |||||||
| Anesthetized rats (6) | Right dorsal striatum | ~70% ↑DA over 30–120 min | |||||||
| Awake rats (6) | 300 | Right dorsal HC | ~120% ↑DA over 60–150 min | ||||||
| Awake rats (8) | Right NAc | ~40% ↑DA over 0–150 min | |||||||
| Zangen and Hyodo (2002) | Awake rats (8) | rTMS (round coil) | 2 Hz | 98% of max | 200 | Frontal cortex | Microdialysis | NAc | ~20% ↑DA, but returned to baseline at 30 min |
| Awake rats (8) | Caudal cortex | ~41% ↑DA, but returned to baseline at 30 min | |||||||
| Ben-Shachar and others (1997) | Rats (16–20) | rTMS (round coil) | 25 Hz | 2.3 T | 50 | Head | HPLC (decapitation at 10 s after rTMS) | HC Striatum Midbrain Frontal cortex |
18% ↑DA 25% ↑DA NS 26% ↓DA |
| Kanno and others (2004) | Awake rats (6) | rTMS (figure-of-eight coil) | 25 Hz | 60% of max (0.6 T), 110% RMT | 500 | PFC | Microdialysis | Right dorsolateral striatum | ~30% ↑DA over 0–110 min |
| Right PFC | NS | ||||||||
| 20% of max (0.2 T), 36% RMT | Right dorsolateral striatum | NS | |||||||
| 80% of max (0.8 T), 149% RMT | Right dorsolateral striatum | NS | |||||||
| Erhardt and others (2004) | Morphine-sensitized awake rats (8) | rTMS (round coil) | 20 Hz | 130% RMT, 4.0 T | 300 | Left frontal cortex | Microdialysis | Right NAc | ~55% ↑DA over 0–100 min |
| Control awake rats (8) | ~25% ↑DA, but returned to baseline at 40 min | ||||||||
| Ohnishi and others (2004) | Anesthetized monkeys (8) | rTMS (double coned coil) | 5 Hz | 35% of max | 2000 | Right M1 | [11C]raclopride PET | Left NAc Right NAc Right putamen |
8.2% ↓BP (↑DA) 8.12% ↓BP (↑DA) 10.51% ↑BP (↓DA) |
BP = binding potential; ↓ = decrease; DA = dopamine; HC = hippocampus; HPLC = high-performance liquid chromatography; ↑ = increase; NS = not significant; NAc = nucleus accumbens; PFC = prefrontal cortex; M1 = primary motor cortex; rTMS = repetitive transcranial magnetic stimulation; RMT = resting motor threshold. For microdialysis, the time shown in the Findings column reflects the time of measurement after the end of stimulation (e.g., 0 min refers immediately after the delivery of rTMS). For [11C]raclopride PET, the search region was limited to the striatum. Only those regions with a significant effect are shown in the table.
TMS and Striatal DA Imaging in Human
A recent consensus paper (Siebner, Bergmann, and others 2009) has categorized the timing of TMS relative to the acquisition of neuroimaging data based on the question to be tackled using the combined perturb-and-measure approach. In principle, TMS can be delivered while neuroimaging data are acquired (online approach). Alternatively, TMS may be applied offline before or after neuroimaging. This offline TMS-neuroimaging approach is technically easier and can be used to make causal interferences about the dopaminergic contribution of brain areas and related neural networks.
The long-lasting neurochemical and neurophysiological effects of brain stimulation observed in experimental animals combined with this offline approach have opened ample opportunities for basic neuroscience research and clinical application in humans. However, because of obvious evolutionary differences, significant controversies arise when one extrapolates findings from the animal to the human brain. To fully understand and exploit the usefulness of TMS in clinical settings, it is necessary to study TMS-induced neurochemical changes directly in the human brain. Obviously, direct measurements of DA levels are not feasible in human; however, PET/SPECT imaging allows one to indirectly quantify synaptic dopaminergic changes using different radioligands (Farde and others 1992; Seeman and others 1989).
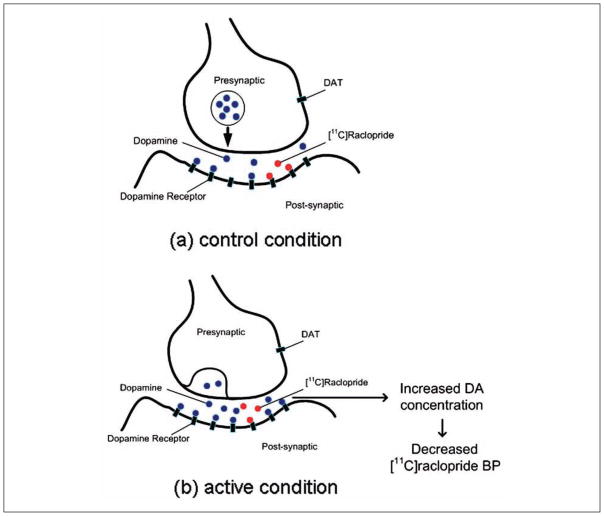
In displacement studies, for example, by comparing control versus active conditions, one can estimate modulations in synaptic DA concentration through changes in binding potential (BP), that is, intervention-induced DA release (Laruelle 2000; Fig. 1). In these displacement studies, [11C]raclopride and [123I]IBZM are the most commonly used radiotracers to quantify DA transmission in the striatum using PET and SPECT, respectively. For example, it has been demonstrated that various interventions including drugs (Dewey and others 1993; Laruelle and others 1997), behavioral tasks (Koepp and others 1998; Monchi, Ko, and others 2006), and rTMS (Strafella and others 2001, 2003, 2005) are able to induce significant and reproducible changes in [11C]raclopride binding, which is inversely proportional to synaptic DA transmission (Laruelle 2000).
Figure 1.
Illustration of the relationship between [11C]raclopride binding potential (BP) and synaptic dopamine (DA) concentration. (a) Assuming a baseline level of DA concentration, (b) DA released in the synapse during active conditions is associated with decreases in [11C]raclopride BP.
There are three major methods to estimate the ligand BP (Slifstein and Laruelle 2001): 1) equilibrium method, 2) graphical method, and 3) kinetic method. The sustained equilibrium method with bolus + infusion has been proposed as the most effective and accurate way to estimate BP changes (Slifstein and Laruelle 2001). However, the constant infusion of decaying ligand inevitably increases the amount of injected nonradioactive tracers, which will have a confounding effect on the accurate measure of BP for the short half-life tracers such as [11C]raclopride. In addition, intervention-induced increases of DA release may promote internalization of D2 receptors that lasts for more than 4 hours and may limit the efficacy of the counterbalancing of the active versus control condition (Laruelle 2000; Skinbjerg and others 2010). The graphical and kinetic methods with bolus injection of radioactive tracers can employ the dual scan paradigm. The graphical method does not make prior assumptions regarding compartmental models and thus is less exposed to kinetic modeling errors (Logan 2000). However, BP values can be significantly biased in the presence of statistical noise (Slifstein and Laruelle 2001), especially in voxel-based analysis. Various efforts have been made to overcome the noise-driven biases (Logan 2003; Logan and others 2011). As for the kinetic method, the simplified reference tissue model (SRTM) has been proposed to produce a reliable estimate of BP (Gunn and others 1997). In addition, combined with the residual t-test proposed by Aston and others (2000), this method provides some advantages by increasing sensitivity while reducing false-positive results. Therefore, the SRTM combined with the residual t-test may be the most suitable method to reliably and efficiently measure TMS-induced DA release. For a detailed review of the methodology of radioligand PET, see Slifstein and Laruelle (2001).
Targeting the motor or visual cortex with TMS is relatively easy, as these areas induce detectable motor-evoked potentials and phosphenes, respectively. However, the targeting of the other cortical areas (e.g., the dorsolateral prefrontal cortex [DLPFC]) requires a more complicated approach. Although one of the simpler ways to determine the location of the target is to use the international electroencephalography electrode position (Pascual-Leone and others 1991), a more sophisticated and precise technique is to use the frameless stereotaxy system (Paus and others 1997). With this method, one can identify a target area given in standardized stereotaxic space (Talairach and Tournoux 1988) in each individual based on his or her high-resolution structural MRI (Collins and others 1994).
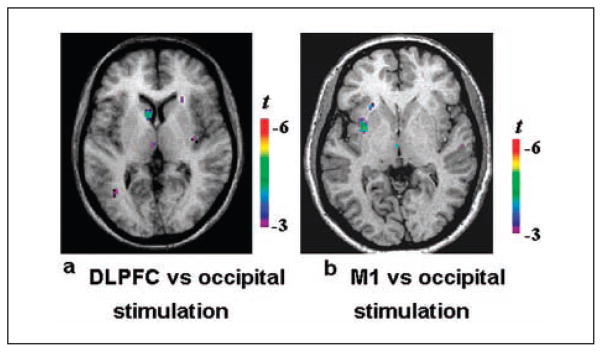
Using frameless stereotaxy for guiding the TMS coil (Paus and others 1997) and SRTM for [11C]raclopride BP estimation (Aston and others 2000; Gunn and others 1997), Strafella and others (2001, 2003) examined the functional organization and interaction of the corticostriatal pathway and found evidence of frontal-striatal control of DA release in the human brain. They demonstrated in young healthy subjects that rTMS (450 pulses, 10 Hz, 3 blocks, 15 trains/block, 10 pulses/train, 10-s intertrain interval, 10-min interblock interval) over the left DLPFC decreases [11C]raclopride BP in the ipsilateral caudate nucleus (7.36%) compared with the changes in BP after occipital cortex stimulation (control condition; Strafella and others 2001; Fig. 2a; Table 2). Later, the same group studied the effect of rTMS over the primary motor area (M1), which resulted in a decrease in [11C]raclopride BP in the ipsilateral putamen (9.49%) (9.49%; Strafella and others, 2003; Fig. 2b; Table 2). The explanation for those findings was that rTMS-induced activation of corticostriatal fibers led to focal DA release in the projection site of the stimulated area of cortex (Fig. 3a). The anatomical location of the cluster of DA release found following stimulation of those cortical areas corresponded exactly to the corticostriatal distribution reported in previous anatomical and physiological studies in monkeys (Jones and others 1977; Kemp and Powell 1970; Kunzle 1975; Takada and others 1998; Tokuno and others 1999; Whitworth and others 1991; Yeterian and Pandya 1991).
Figure 2.
[11C]raclopride positron emission tomography studies combined with repetitive transcranial magnetic stimulation. (a) Left dorsolateral prefrontal cortex (DLPFC) stimulation resulted in significant decrease of [11C]raclopride binding potential (BP) in the ipsilateral caudate nucleus compared with occipital stimulation (control). (b) Left M1 stimulation resulted in a significant decrease of [11C]raclopride BP in the ipsilateral putamen compared with occipital stimulation (control). Adapted from Strafella and others (2001, 2003).
Table 2.
Dopaminergic Effect of Brain Stimulation in Humans
| Article | Subject (N) | Stimulation Parameters
|
Target Region | Methodology | Regional Changes | Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Method | Frequency | Intensity | Total Pulses | ||||||
| Strafella and others (2001) | Awake healthy humans at rest (8) | rTMS (round coil) | 10 Hz | 100% RMT | 450 | Left DLPFC | [11C]raclopride PET | Left caudate | 7.36% ↓BP (↑DA) |
| Strafella and others (2003) | Awake healthy humans at rest (6) | rTMS (round coil) | 10 Hz | 90% RMT | 450 | Left M1 | [11C]raclopride PET | Left putamen | 9.49% ↓BP (↑DA) |
| Cho and Strafella (2009) | Awake healthy humans at rest (7) | rTMS (figure-of-eight coil) | 10 Hz | 100% RMT | 750 | Left DLPFC | [11C]FLB 457 PET | Left subgenual ACC Left pregenual ACC Medial OFC |
37.7% ↓BP (↑DA) 45.3% ↓BP (↑DA) 23.6% ↓BP (↑DA) |
| Ko, Monchi, Ptito, Bloomfield, and others (2008) | Awake healthy humans while performing behavioral tasks (10) | cTBS (figure-of-eight coil) | 50 Hz | 80% AMT | 900 | Left DLPFC | [11C]raclopride PET | Left caudate Right caudate Left putamen |
14.67% ↑BP (↓DA) 12.59% ↑BP (↓DA) 12.98% ↑BP (↓DA) |
| Right DLPFC | Striatum | NS | |||||||
| Strafella and others (2005) | Awake patients with PD (off-med) at rest (7) | rTMS (round coil) | 10 Hz | 90% RMT | 450 | More affected M1 Less affected M1 |
[11C]raclopride PET | Ipsilateral putamen | 9.47% ↓BP (↑DA) 12.99% ↓BP (↑DA) |
| Pogarell and others (2006, 2007) | Awake patients with depression (off-med) at rest (9) | rTMS (figure-of-eight coil) | 10 Hz | 100% RMT | 3000 | Left DLPFC | [123I]IBZM SPECT | Bilateral striatum | 9.6% ↓V3 (↑DA) |
| Awake patients with depression (off-med) at rest (4) | 25,500 (3 wk) | NS | |||||||
| Kuroda and others (2006) | Awake patients with depression (off-med) at rest (9) | rTMS (figure-of-eight coil) | 10 Hz | 100% RMT | 10,000 (2 wk) | Left DLPFC | [11C]raclopride (PET) | Striatum | NS |
| Khedr and others (2007) | Awake patients with PD (off-med) at rest (20) | rTMS (figure-of-eight coil) | 25 Hz | 100% RMT | 18,000 (1 wk) | Left and right M1-hand and lower limbs | DA level | Serum | 71.5% ↑DA |
| Shimamoto and others (2001) | Awake patients with PD (on-med) at rest (9) | rTMS (round coil) | 0.2 Hz | 100 ± 10% RMT | 480 (2 mo) | Left and right frontal | DA level Homovanillic acid level |
CSF CSF |
NS 19.9% ↓homovanillic acid level |
| Kim and others (2008) | Awake patients with PD (off-med) at rest (9) | rTMS (figure-of-eight coil) | 5 Hz | 90% RMT | 300 (2 d) | More affected M1 | [11C]raclopride (PET) | Contralateral caudate | 12.1% ↓BP (↑DA) |
AMT = active motor threshold; ACC = anterior cingulate cortex; BP = binding potential; CSF = cerebrospinal fluid; cTBS = continuous theta burst stimulation; ↓ = decrease; DA = dopamine; DLPFC = dorsolateral prefrontal cortex; ↑ = increase; NS = not significant; OFC = orbitofrontal cortex; PD =Parkinson disease; M1 = primary motor cortex; V3 = ratio of specific to nonspecific brain uptake; rTMS = repetitive transcranial magnetic stimulation; RMT = resting motor threshold. For [11C]raclopride PET and [123I]IBZM SPECT, the search region was limited to the striatum. For [11C]FLB 457, the search region was whole brain. Only those regions with significant effect are shown in the table. When no significant effect is observed in the search region, NS is reported.
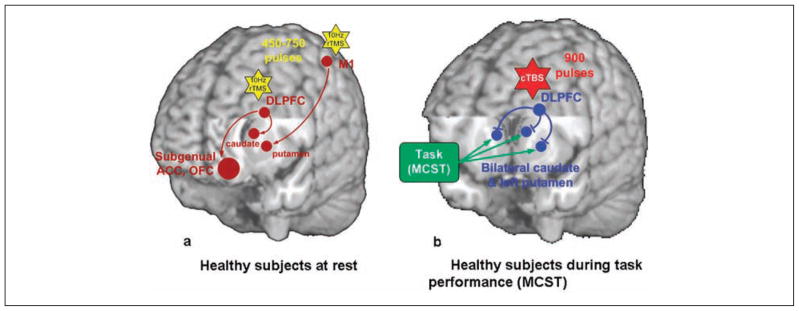
Figure 3.
Schematic diagrams of the dopaminergic effect of repetitive transcranial magnetic stimulation (rTMS). (a) In healthy subjects at rest, 10-Hz rTMS over the left dorsolateral prefrontal cortex (DLPFC) increased dopamine (DA) release in the ipsilateral caudate nucleus (Strafella and others 2001), anterior cingulate cortex (ACC), and medial orbitofrontal cortex (OFC; Cho and Strafella 2009), whereas M1 stimulation increased DA release in the ipsilateral putamen (Strafella and others 2003). The yellow stars represent the 10-Hz rTMS. The brown circles and arrows represent the stimulated site (i.e., DLPFC and M1) and increased DA release. (b) During task performance, continuous theta burst stimulation (cTBS) applied over the left DLPFC impaired task performance and disrupted striatal DA release in the bilateral striatum (Ko, Monchi, Ptito, Bloomfield, and others 2008). In contrast, right DLPFC stimulation during the same task had no significant effect. The green box and arrows represent the effect of performing the Montreal card sorting task (MCST), which is shown to increase DA release in the striatum (Monchi, Ko, and others 2006). The red star represents cTBS. The blue circles and arrows represent the stimulated site (i.e., DLPFC) and decreased DA release.
More recently, Pogarell and others (2006) showed that rTMS of the prefrontal regions (3000 pulses, 10 Hz, 100 pulses/train, 30 trains, 30-s intertrain interval, 100% RMT) in patients with depression induced changes in striatal [123I]IBZM BP (9.6%) that were surprisingly comparable with amphetamine-induced changes (8%; Pogarell and others 2007; Fig. 4a). However, it was unclear why there was bilateral [123I]IBZM BP reduction after unilateral stimulation (Pogarell and others 2006, 2007) whereas only ipsilateral BP changes were observed in previous [11C] raclopride PET studies performed in young healthy subjects (Strafella and others 2001, 2003). It is possible that the disease and the different stimulation paradigm may have played a significant role. In fact, one could predict that the rTMS effect may differ between patients and healthy subjects given obvious differences in the neurochemical, anatomical, and functional state between these two groups (Rigucci and others 2010). This is also emphasized by the different rTMS effects on mood and behavior between patients and healthy volunteers, for example, left DLPFC high-frequency stimulation ameliorated depressive symptoms in patients with major depression (Martin and others 2003; Pascual-Leone, Rubio, and others 1996), whereas it induced sadness in healthy volunteers (George and others 1996; Pascual-Leone, Catala, and others 1996) or produced no significant results (Michael and others 2003; Mosimann and others 2000). Likewise, low-frequency stimulation on the right DLPFC improved mood ratings in patients with depression (Fitzgerald and others 2003; Klein and others 1999), but no significant changes were observed in healthy volunteers (Grisaru and others 2001; Jenkins and others 2002).
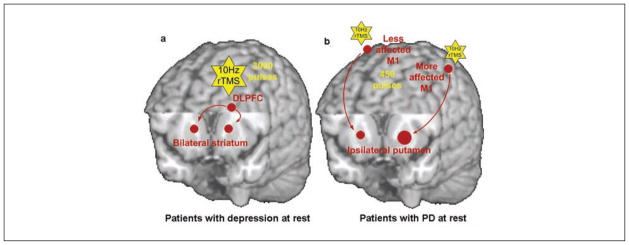
Figure 4.
Schematic diagrams of the dopaminergic effect of repetitive transcranial magnetic stimulation (rTMS) in patients. (a) In patients with depression (off medication), 10-Hz rTMS over the left dorsolateral prefrontal cortex (DLPFC) increased dopamine (DA) release in the bilateral striatum (Pogarell and others 2006). The yellow stars represent 10-Hz rTMS. The brown circles and arrows represent the stimulated site (i.e., DLPFC) and increased DA release. Here, Pogarell and others (2006) did not differentiate between the caudate nucleus and putamen in the single-photon emission computation tomography image analysis. (b) In patients with Parkinson disease (off medication, unilateral motor symptoms), 10-Hz rTMS over M1 in the symptomatic hemisphere induced a smaller amount of DA release in the ipsilateral putamen than in the asymptomatic hemisphere (Strafella and others 2005). However, the size of the significant cluster of change in [11C]raclopride binding in the symptomatic hemisphere was greater than in the asymptomatic hemisphere, which is represented by the larger brown circle.
In anesthetized monkeys, rTMS (2000 pulses, 5 Hz, 100 pulses/train, 20-s intertrain interval) over M1 reduced [11C]raclopride binding in the left and right striatum (Ohnishi and others 2004), consistent with the bilateral striatal DA modulation observed in patients (Pogarell and others 2006, 2007). Another intriguing observation of this primate study was the increased [11C]raclopride binding in the ipsilateral putamen. The authors argued that 5Hz-rTMS may have inactivated M1-putamen circuitry and decreased extracellular DA levels in anesthetized monkeys (Ohnishi and others 2004). These various observations from human (Strafella and others 2003) and primate studies (Ohnishi and others 2004) and between healthy subjects and patient groups (Pogarell and others 2006, 2007) strongly emphasize how underlying neurochemical changes and the functional state of neuronal circuits (e.g., disease or level of consciousness) along with different stimulation parameters may influence rTMS effects on striatal DA release.
TMS and Extrastriatal DA Imaging in Human
Extrastriatal DA plays an important role in human cognition/behavior and different neurological and psychiatric diseases (Abi-Dargham and others 2002; Brozoski and others 1979; Kaasinen and others 2000; Sawaguchi and Goldman-Rakic 1991). Recent advances in neuroimaging techniques have made it possible to study extrastriatal DA D2/3 receptors in the human by quantifying the binding, with high-affinity radiotracers such as [11C] FLB 457 (Chou and others 2000), [18F or 11C]fallypride (Riccardi and others 2006; Slifstein and others, 2004), and [123I]epidepride (Fujita and others 2000). To date, these ligands have been used to assess pharmacologic challenges (Aalto, Ihalainen, and others 2005; Aalto and others 2009; Cropley and others 2008; Hagelberg and others 2004; Montgomery and others 2007; Riccardi and others 2006; Slifstein and others 2010; Steeves and others 2010) and cognitive/behavioral (Aalto, Bruck, and others 2005; Christian and others 2006; Ko and others 2009) contributions to extrastriatal synaptic DA neurotransmission. Among these radiotracers, Narendran and others (2009) recently demonstrated the superior sensitivity and robustness of [11C]FLB 457 over [11C]fallypride in detecting changes in BP (induced by amphetamine) in different cortical areas. This was suspected to be due to the higher signal-to-noise ratio provided by [11C]FLB 457. However, a limitation of the [11C]FLB 457 radiotracer is that, because of its kinetic properties, it does not allow measurements of DA in the striatum. In contrast, fallypride as an 18F-labelled tracer has the advantage of imaging both striatal and extrastriatal DA (although scan duration can last up to 3 h, posing a problem of feasibility in patients), as well as being more widely available enabled by the longer half-life.
Recently, Cho and Strafella (2009) showed decreased cortical [11C]FLB 457 binding after DLPFC high-frequency stimulation (Figs. 3a and 5; Table 2). They stimulated both left and right DLPFC in young healthy subjects with 10 Hz rTMS (750 pulses/day, 10 pulses/train, 15 trains/block, 5 blocks, 10-s intertrain interval, 10-min interblock interval) on different days. The most interesting finding was that only left DLPFC stimulation decreased [11C]FLB 457 binding in the ipsilateral subgenual anterior cingulate cortex (ACC; BA 25/12, 37.7%), pregenual ACC (BA 32, 45.3%), and medial orbitofrontal cortex (OFC; BA 11, 23.6%). In contrast, right DLPFC stimulation did not induce any significant dopaminergic change. This hemispheric discrepancy may originate from the intrinsically asymmetric properties of the frontal lobes.
Figure 5.
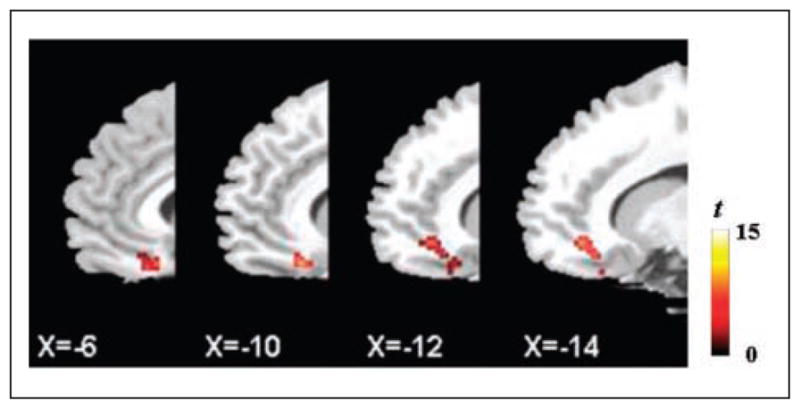
[11C]FLB 457 binding potential decreased in the ipsilateral subgenual anterior cingulate cortex (ACC), pregenual ACC, and medial orbitofrontal cortex after left dorsolateral prefrontal cortex stimulation versus control stimulation. Adapted from Cho and Strafella (2009).
Regional blood flow changes in the subgenual cingulate and OFC after rTMS applied over the left DLPFC has been previously reported to be associated with the efficacy of rTMS treatment in depression (Teneback and others 1999). DLPFC control over ACC/OFC DA may occur via a prefrontal projection to the VTA (Karreman and Moghaddam 1996; Murase and others 1993), which sends afferent dopaminergic projections to the ACC/OFC (Williams and Goldman-Rakic 1998). Alternatively, there may be cortico-cortical connections between these areas, as revealed by restored DLPFC activity in patients with treatment-resistant depression after deep brain stimulation (DBS) of the subgenual ACC (Mayberg and others 2005).
TMS and DA in Cognition
TMS has been widely used to create a virtual lesion to dissect the functional role of a given cortical area (Siebner, Hartwigsen, and others 2009; Walsh and Cowey 2000). Although it is well known that the effect of rTMS spreads well beyond the targeted areas, the potential implication of the dopaminergic influence over cognition and behavior is now receiving a great deal of interest (Leh and others 2010; Owen 2004; Zgaljardic and others 2003).
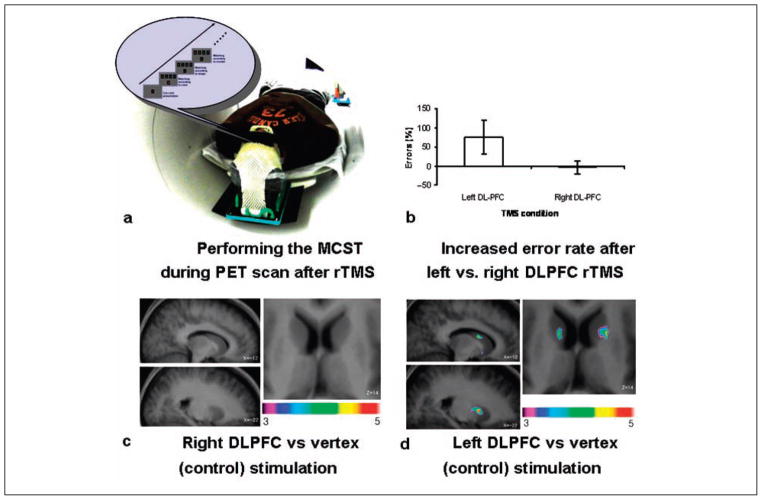
fMRI (Monchi, Petrides, and others 2006; Monchi and others 2007) and PET (Monchi, Ko, and others 2006) studies have confirmed that the prefrontostriatal network plays a significant role in executive functions, as suggested as well by the cognitive anatomical loop proposed by Alexander and others (1986). Executive function is recently being tapped using the Montreal card sorting task (MCST), a variant of the Wisconsin card sorting task (Milner 1963), which assesses patients’ ability to set shift (to switch between different rules to guide behavior). Previous neuroimaging studies have shown that executive processes tested with card-sorting tasks requiring planning and set shifting (e.g., MCST) may engage the DLPFC while inducing DA release in the striatum (Monchi, Ko, and others, 2006). However, functional imaging studies can only provide neuronal correlates of cognitive performance and cannot establish a causal relationship between observed brain activity and task performance. To investigate the contribution of the DLPFC during set shifting and its effect on the striatal dopaminergic system, we applied continuous theta burst stimulation (cTBS) to the left and right DLPFC. Our aim was to transiently disrupt its function and to measure MCST performance and striatal DA release during [11C]raclopride PET (Ko, Monchi, Ptito, Bloomfield, and others 2008).
Healthy subjects performed the MCST during a 60-min [11C]raclopride PET scan after cTBS. The cTBS protocol was designed so that it produced a long-term depression-like effect, mainly working through GAB-Aergic inhibition (Stagg and others 2009), known for its profound behavioral after effect following acute treatment (Huang and others 2005; Nyffeler and others 2006; Vallesi and others 2007).
Three sessions of 20-s cTBS (900 pulses per day, 80% active MT) were applied to the left and right DLPFC and vertex (control site) on different days followed by a [11C] raclopride PET scan while subjects performed the MCST. Interestingly, only left DLPFC stimulation increased the number of errors on the task and increased [11C]raclopride BP in the bilateral caudate nucleus and ipsilateral putamen (Ko, Monchi, Ptito, Bloomfield, and others 2008; Figs. 3b and 6; Table 2). This hemispheric asymmetry was in line with results of previous neuroimaging studies suggesting that the left DLPFC is more engaged during set-shifting processes. The right DLPFC, on the other hand, is mainly involved in the monitoring of working memory and less emphasized in the performance of this task (Ko, Monchi, Ptito, Petrides, and others 2008; Konishi and others 2002; Lie and others 2006; Monchi and others 2001, 2007). Similarly, the lateralized control of set shifting is supported by human lesion studies (Aron and others 2004; Richer and others 1993; Rogers and others 1998; Stuss and Alexander 2007).
Figure 6.
[11C]raclopride PET study with continuous theta burst stimulation (cTBS) on the Montreal card sorting task (MCST). (a) Subjects performed the MCST during [11C]raclopride PET scan after cTBS applied to the left or right dorsolateral prefrontal cortex (DLPFC) or vertex (control site) on different days. (b) Left DLPFC stimulation increased error making, but right DLPFC stimulation did not. Error rate was calculated as the percentage changes compared with control stimulation (vertex). (c) Right DLPFC stimulation did not induce any significant changes in [11C]raclopride binding potential (BP). (d) Left DLPFC stimulation reduced [11C]raclopride BP significantly in the bilateral caudate nucleus and the left putamen. Adapted from Ko, Monchi, Ptito, Bloomfield, and others (2008).
Because cTBS is considered to inhibit the cortical excitability of the targeted area (Huang and others 2005, 2007; Hubl and others 2008), we hypothesized that by inhibiting the left DLPFC and disrupting its function, we indirectly inhibited striatal DA neurotransmission during performance of executive tasks, which resulted in an increase of [11C] raclopride BP as compared with a control condition (i.e., vertex stimulation). Here, interestingly, changes in [11C] raclopride BP after cTBS were not topographically selective (i.e., there was a bilateral effect over the caudate nucleus and an ipsilateral effect in the putamen), unlike the more specific changes in DA release after 10-Hz rTMS (Strafella and others 2001, 2003). This is not surprising given the significant differences in stimulation parameters (10-Hz rTMS vs. cTBS) and functional state (rest vs. task performance). In fact, although ipsilateral DA modulation (Strafella and others 2001, 2003) suggests that prefontostriatal projection neurons were stimulated by 10-Hz rTMS (while the subject was at rest), bilateral DA modulation during an executive task (Ko, Monchi, Ptito, Bloomfield, and others 2008) following cTBS-induced inhibition of DLPFC suggests a local disruption of the underlying targeted area is processing relevant information of the task at hand.
TMS and Dopamine in Neurologic and Psychiatric Diseases
The ability to modulate brain activity and subsequent neurotransmitter release using TMS has obvious clinical implications (Dlabac-de Lange and others 2010; Elahi and others 2009; Floel and Cohen 2010; Guse and others 2010; Padberg and George 2009). In patients with major depression, Pogarell and others (2006) previously showed a reduction of [123I]IBZM BP (9.6%) in the striatum after left prefrontal stimulation (3000 pulses, 10 Hz, 100 pulses/train, 30 trains, 30-s intertrain interval, 100% RMT; Table 2; Fig. 4a). Four patients were investigated with the same protocol after 3 weeks of rTMS treatment sessions (1500 pulses/daily), but there was no significant interaction between acute rTMS-induced [123I]IBZM BP reduction and time (3 weeks), whereas all patients’ symptoms were improved at the end of 3 weeks of treatment (5%–82% using the Hamilton rating scale for depression; Table 2). Although the small sample size limits any definite conclusion, this pilot study opens the possibility of involvement of other neurochemical mechanisms including extrastriatal DA (Cho and Strafella 2009) or serotonin (Sibon and others 2007) in the therapeutic effects of rTMS in depression.
Kuroda and others (2006) failed to demonstrate in patients with depression any significant changes in [11C]raclopride BP after 10 daily sessions of rTMS (1000 pulses/day, 10 Hz, 50 pulses/train, 20 trains, 25-s intertrain interval, 100% RMT; Table 2). Unlike the previous studies, however, the patients were scanned 1 day after the treatment. Interestingly, electroconvulsive therapy (ECT; 6–7 sessions over 2–3 weeks), which has often been related to rTMS in term of its clinical effect (Fitzgerald 2004; Knapp and others 2008), decreased [11C]FLB 457 BP in the ACC (25.2%) even after 1 day from the last ECT session in patients with depression (Saijo and others 2010). In nonhuman primates, Landau and others (2011) demonstrated that presynaptic dopaminergic mechanisms (i.e., DAT and vesicular monoamine transporter measured by [11C]d-threomethylphenidate and [11C]+/− dihydrotetrabenazine, respectively) were also modulated by human clinical protocols of ECT (6 sessions) for up to 8 to 10 days. Although it must be emphasized that ECT may benefit depressed patients via different neurochemical mechanisms than rTMS (Lisanby and Belmaker 2000), these studies appear to indirectly suggest that the long-term clinical benefits of rTMS in depression may not necessarily be associated only with postsynaptic DA transmission but also with extrastriatal DA and/or pre-synaptic modulation.
In PD, there have been a few studies that indirectly indicate increased DA function after therapeutic rTMS (Table 2). For example, serum DA level was increased after six daily sessions of 25-Hz rTMS (3000 pulses/day, 100% RMT; Khedr and others 2007), whereas 0.2-Hz rTMS (60 pulses/day, once a week for 2 mo) decreased the level of homovanillic acid in cerebrospinal fluid in PD patients, a possible expression of reduced breakdown of DA (Shimamoto and others 2001). The Unified Parkinson’s Disease Rating Scale (UPDRS) scores were improved in both studies (Khedr and others 2007; Shimamoto and others 2001).
Using [11C]raclopride PET, Kim and others (2008) investigated the therapeutic effect of rTMS in PD patients (Table 2). Two daily sessions of rTMS (150 pulses/day, 5 Hz, 5 pulses/train, 15 trains, 2 blocks, 10-s intertrain interval, 10-min interblock interval, 90% RMT, post-TMS scanning started 5 min after TMS) induced a significant decrease of BP in the contralateral caudate nucleus (12.1%, but not ipsilateral to the stimulated hemisphere), with significant clinical benefits as measured by the motor section of the UPDRS (UPDRS III). The main limitation of this study was represented by the fact that a counterbalanced control condition was not included; thus (as suggested by the authors), a TMS-induced placebo effect in this case may account for the outcomes.
In fact, the placebo effect and expectation of a clinical benefit have been shown to be associated with release of DA in the striatum (de la Fuente-Fernandez and others 2001). Along this line, in one of the previous TMS studies (Strafella and others 2006), it was demonstrated that in patients with PD, the expectation of therapeutic benefit from rTMS, which actually was delivered only as sham rTMS (placebo-rTMS), induced diffuse changes in striatal [11C] raclopride BP as measured with PET. Placebo-rTMS induced a significant bilateral reduction in [11C] raclopride BP in the dorsal and ventral striatum as compared with the baseline condition. The changes in [11C] raclopride binding were more consistent in the hemisphere associated with the more affected side, supporting the hypothesis that the more severe the symptoms, the greater the drive for symptom relief and therefore the placebo response. Although those results confirmed earlier evidence that expectation induces dopaminergic placebo effects (de la Fuente-Fernandez and others 2001), they also suggest the importance of placebo-controlled studies for future clinical trials involving brain stimulation techniques.
In parkinsonian rats, acute rTMS (2000 pulses, 25 Hz, 200 pulses/train, 10 trains, 10- to 15-min intertrain intervals) increased tyrosine hydroxylase expressions and nigral neuronal survivals and ameliorated the parkinsonian symptoms to the normal level (Funamizu and others 2005). More recently, chronic low-frequency rTMS treatment (4 wk, 500 pulses/day, 0.5 Hz) alleviated dopaminergic cell loss and prevented striatal DA reduction in 6-OHDA lesioned rats (Yang and others 2010). As remarkable as these studies can be, the potential for neuroprotective effects of rTMS needs to be fully investigated.
In stroke (Floel and Cohen 2010), schizophrenia (Dlabac-de Lange and others 2010), and addiction (Daskalakis and others 2008), it has been proposed that the potential rTMS benefits may be mediated via dopaminergic modulation, but the therapeutic efficacy remained to be tested with in vivo dopaminergic imaging.
In addition to the therapeutic benefits, studies with acute rTMS combined with PET or SPECT may also help to elucidate the underlying neurochemical mechanisms of disease progression. For example, Strafella and others (2005) sought to investigate early PD patients with evidence of unilateral symptoms. They measured changes in the putamen in extracellular DA concentration following rTMS (450 pulses, 10 Hz, 3 blocks, 15 trains/block, 10 pulses/train, 10-s intertrain interval, 10-min interblock interval, 90% RMT) of the left and right primary motor cortex (Table 2). The main objective was to identify potential differences in corticostriatal DA release between the hemisphere clearly associated with contralateral motor symptoms and the presymptomatic stage of the other hemisphere in which, conceivably, functional compensatory mechanisms from surviving dopaminergic terminals were still preventing the appearance of PD symptoms. They demonstrated a larger cluster size of DA release when rTMS was applied over the primary motor cortex of the affected hemisphere (compared with the asymptomatic side; Fig. 4b). The authors suggested that these findings may represent the expression of the loss of functional segregation of cortical information to the striatum, in vivo, and may provide indirect evidence of abnormal corticostriatal transmission in early PD. There are a number of alternative explanations for those observations. Indeed, because of the partial loss of reuptake sites (Zigmond and others 1990), released DA diffuses out to more distant regions of the receptor population in the DA-denervated striatum. In addition, several electrophysiological studies have also shown that in conditions involving DA denervation, there exists a hyperactivity of corticostriatal glutamatergic transmission (Calabresi and others 1993, 2000; Lindefors and Ungerstedt 1990), with increased numbers of striatal neurons responding to cortical stimulation (Florio and others 1993). Therefore, because of such corticostriatal hyperactivity, rTMS could be responsible for an abnormal release of glutamate, which, by diffusing into the extrasynaptic space (Onn and others 2000), may activate larger areas of dopaminergic terminals in the symptomatic hemisphere.
Having said that, although the delivery of acute rTMS (i.e., one study session) has certainly provided interesting observations, cortical stimulation may be required to be chronically applied (e.g., with 2–3 weeks of repetitive sessions) to have clinically relevant outcomes. At the moment, preliminary research on the effect of chronic rTMS treatment has failed to reveal significant changes in [123I]IBZM/[11C]raclopride BP or correlation with its clinical benefits (Kim and others 2008; Kuroda and others 2006; Pogarell and others 2006, 2007). Nevertheless, various reviews and meta-analyses have emphasized the potential benefits of rTMS in diverse diseases with dopaminergic impairment (Dlabac-de Lange and others 2010; Elahi and others 2009; Floel and Cohen 2010; Guse and others 2010; Padberg and George 2009), and recent ECT studies suggest that long-term changes in the dopaminergic system may be observed at presynaptic sites (Landau and others 2011) and extrastriatal regions as well (Saijo and others 2010). Therefore, further controlled investigations with larger cohorts and different radiotracers are warranted.
Future Directions
Neuroreceptor imaging using PET/SPECT is highly recognized for its capability to investigate the neurobiology of diseases due to its unique ability to quantify in vivo neurochemical abnormalities with high spatial resolution.
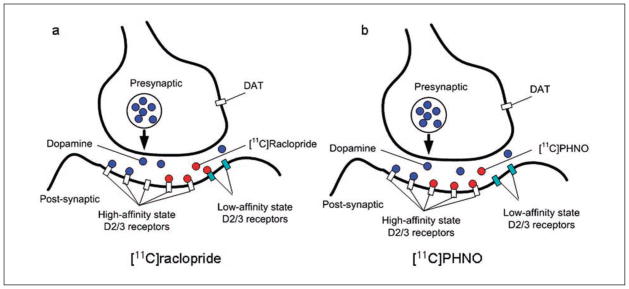
There is a continuing effort to develop new radioligands that tackle different neurochemical systems and bind to specific receptor subtypes. Such progress continues to increase knowledge of how different neurotransmitters contribute to brain function. For example, Sibon and others (2007) previously demonstrated that acute rTMS may affect serotonin synthesis capacity using [11C]-alpha-methyl-tryptophan. Also, the application of newly developed agonist-radioligands such as [11C]PHNO may contribute to increasing understanding of the involvement of high-affinity versus low-affinity states of DA receptors (Willeit and others 2006; Fig. 7). Indeed, D2/3 receptor antagonist-radioligands such as [11C]raclopride, by binding to both high- and low-affinity state DA receptors, may underestimate intervention-induced DA displacement. An agonist-radioligand such as [11C]PHNO, however, by binding mainly to DA receptors in the high-affinity state, may more accurately estimate physiologically relevant changes in D2/3 receptor availability (Leff 1995; Willeit and others 2006). In addition, the higher affinity toward D3-receptors of [11C]PHNO may unveil important underlying mechanisms of the dopaminergic effects of rTMS. D1-like receptors are also known to play an important role in high-level cognition, especially in the prefrontal area (Goldman-Rakic and others 2000; Sawaguchi and Goldman-Rakic 1991). Therefore, the development in the future of reliable D1 radiotracers will expand knowledge of the neurochemical control of cognition and behavior in both health and disease. In addition, the possibility of differentiating presynaptic versus postsynaptic receptor binding will provide new opportunities to clarify the underlying mechanisms of the rTMS effect on the dopaminergic systems.
Figure 7.
Illustration of different binding sites of dopamine antagonist and agonist radioligands in the synapse. (a) [11C]raclopride is a dopamine receptor antagonist that binds to dopamine receptors in both the high- and low-affinity states. (b) [11C]PHNO is a dopamine agonist that predominantly binds to dopamine receptors in the high-affinity state.
Traditionally, it has been widely accepted that low- and high-frequency rTMS has different and often opposite effects on neurophysiological parameters (Chen and others 1997; Pascual-Leone and others 1994) and local/remote cerebral blood flow (Knoch and others 2006; Rounis and others 2005; Speer and others 2000). Given that the used rTMS parameters (frequency, intensity, intertrain interval, priming, etc.) are critical deciding factors for clinical efficacy (Padberg and George 2009), it will be important to compare neurochemical changes after different (but consistent) rTMS paradigms across different centers. Here, we have summarized the dopaminergic effect of different rTMS protocols in animals (Table 1) and humans (Table 2; Figs. 3 and 4). We would like to emphasize the importance of caution when comparing studies with different conditions. For example, a stimulation protocol that has been shown to have a certain effect in awake humans at rest may produce different outcomes in patients, or in subjects during action performance, or in anesthetized animals. Therefore, further validation with various rTMS protocols in different subject populations needs to be performed before one can extrapolate to cognition and behavior the terminology of excitatory and inhibitory stimulation broadly adopted from high- and low-frequency rTMS, respectively, in human motor cortex neurophysiology.
Transcranial direct current stimulation (tDCS) will also receive extensive attention as a potential neuromodulatory tool (Zaghi and others 2009). Although it has been demonstrated that the effects of tDCS may be influenced indirectly by dopaminergic medication (Nitsche and others 2006; Terney and others 2008), the direct effect of tDCS on human dopaminergic levels has not yet been investigated. Considering the advantages offered by this promising technique (Zaghi and others 2009), further research on tDCS effects using dopaminergic imaging technology is warranted.
Another potential method for perturbing the dopaminergic system is DBS. DBS has been widely accepted as an alternative treatment for PD patients who have developed drug-related side effects such as motor fluctuations (Lang and others 2006; Moro and Lang 2006) and also for other neurological and psychiatric diseases such as dystonia (Hamani and Moro 2007), depression (Mayberg and others 2005), Tourette syndrome (Sassi and others 2010), obsessive-compulsive disorder (Mian and others 2010), and epilepsy (Lockman and Fisher 2009). Studies with healthy nonhuman primates reported changes in striatal and extrastriatal [18F]fallypride binding during DBS (Vandehey and others 2009). Human [11C]raclopride PET studies in PD patients (off medication), however, failed to observe any changes in striatal DA release following subthalamic nucleus DBS (Abosch and others 2003; Hilker and others 2003; Strafella and others 2003; Thobois and others 2003). As well, comparisons of preoperative and postoperative D2/3 BP have reported inconsistent findings (Hesse and others 2008; Nakajima and others 2003). Therefore, further research with different ligands (e.g., [11C]FLB 457) and larger cohorts of patients is necessary for a better understanding of the neurochemical changes induced by DBS.
Conclusion
DA plays an important role in learning, reward, motor control, emotion, and executive function in both health and disease. Thanks to the development of neuroimaging techniques such as PET and SPECT, it is now possible to quantify dopaminergic activity in the living human brain. The combination of these technologies with TMS has great potential in that it is capable of tackling questions regarding region-specific/function-specific neurochemical activity. Such studies may aid clinicians and scientists to dissect neural circuitries and thereby help them to understand the underlying mechanisms of a given function in relation to brain diseases. Furthermore, it may also aid the development of clinical applications for various neurological/psychiatric conditions.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: A.P.S. is supported by a grant from the Canadian Institutes of Health Research (MOP 64423) and Edmond J. Safra Philanthropic Foundation.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–7. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aalto S, Hirvonen J, Kaasinen V, Hagelberg N, Kajander J, Nagren K, et al. The effects of d-amphetamine on extrastriatal dopamine D2/D3 receptors: a randomized, double-blind, placebo-controlled PET study with [11C]FLB 457 in healthy subjects. Eur J Nucl Med Mol Imaging. 2009;36:475–83. doi: 10.1007/s00259-008-0969-9. [DOI] [PubMed] [Google Scholar]
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182:375–83. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abosch A, Kapur S, Lang AE, Hussey D, Sime E, Miyasaki J, et al. Stimulation of the subthalamic nucleus in Parkinson’s disease does not produce striatal dopamine release. Neurosurgery. 2003;53:1095–102. doi: 10.1227/01.neu.0000088662.69419.1b. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–73. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Aston JA, Gunn RN, Worsley KJ, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage. 2000;12:245–56. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Bean AJ, Roth RH. Extracellular dopamine and neurotensin in rat prefrontal cortex in vivo: effects of median fore-brain bundle stimulation frequency, stimulation pattern, and dopamine autoreceptors. J Neurosci. 1991;11:2694–702. doi: 10.1523/JNEUROSCI.11-09-02694.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shachar D, Belmaker RH, Grisaru N, Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm. 1997;104:191–7. doi: 10.1007/BF01273180. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–32. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci. 2000;23:S57–63. doi: 10.1016/s1471-1931(00)00017-3. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons; implications for Parkinson’s disease. Brain. 1993;116(Pt 2):433–52. [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral pre-frontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou YH, Halldin C, Farde L. Effect of amphetamine on extrastriatal D2 dopamine receptor binding in the primate brain: a PET study. Synapse. 2000;38:138–43. doi: 10.1002/1098-2396(200011)38:2<138::AID-SYN4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Christian BT, Lehrer DS, Shi B, Narayanan TK, Strohmeyer PS, Buchsbaum MS, et al. Measuring dopamine neuromodulation in the thalamus: using [F-18]fallypride PET to study dopamine release during a spatial attention task. Neuroimage. 2006;31:139–52. doi: 10.1016/j.neuroimage.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cropley VL, Fujita M, Bara-Jimenez W, Brown AK, Zhang XY, Sangare J, et al. Pre- and post-synaptic dopamine imaging and its relation with frontostriatal cognitive function in Parkinson disease: PET studies with [11C]NNC 112 and [18F]FDOPA. Psychiatry Res. 2008;163:171–82. doi: 10.1016/j.pscychresns.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry. 2008;65:378–85. doi: 10.1001/archpsyc.65.4.378. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, et al. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49:298–303. doi: 10.1002/ana.65.abs. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90:226–35. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993;13:350–6. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Dlabac-de Lange JJ, Knegtering R, Aleman A. Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry. 2010;71:411–8. doi: 10.4088/JCP.08r04808yel. [DOI] [PubMed] [Google Scholar]
- Egerton A, Mehta MA, Montgomery AJ, Lappin JM, Howes OD, Reeves SJ, et al. The dopaminergic basis of human behaviors: a review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33:1109–32. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi B, Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function—systematic review of controlled clinical trials. Mov Disord. 2009;24:357–63. doi: 10.1002/mds.22364. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Sillaber I, Welt T, Muller MB, Singewald N, Keck ME. Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology. 2004;29:2074–80. doi: 10.1038/sj.npp.1300493. [DOI] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. Repetitive transcranial magnetic stimulation and electroconvulsive therapy: complementary or competitive therapeutic options in depression? Australas Psychiatry. 2004;12:234–8. doi: 10.1080/j.1039-8562.2004.02113.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, De Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–8. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Floel A, Cohen LG. Recovery of function in humans: cortical stimulation and pharmacological treatments after stroke. Neurobiol Dis. 2010;37:243–51. doi: 10.1016/j.nbd.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Florio T, Di Loreto S, Cerrito F, Scarnati E. Influence of prelimbic and sensorimotor cortices on striatal neurons in the rat: electrophysiological evidence for converging inputs and the effects of 6-OHDA-induced degeneration of the substantia nigra. Brain Res. 1993;619:180–8. doi: 10.1016/0006-8993(93)91610-5. [DOI] [PubMed] [Google Scholar]
- Fujita M, Verhoeff NP, Varrone A, Zoghbi SS, Baldwin RM, Jatlow PA, et al. Imaging extrastriatal dopamine D(2) receptor occupancy by endogenous dopamine in healthy humans. Eur J Pharmacol. 2000;387:179–88. doi: 10.1016/s0014-2999(99)00817-1. [DOI] [PubMed] [Google Scholar]
- Funamizu H, Ogiue-Ikeda M, Mukai H, Kawato S, Ueno S. Acute repetitive transcranial magnetic stimulation reactivates dopaminergic system in lesion rats. Neurosci Lett. 2005;383:77–81. doi: 10.1016/j.neulet.2005.04.018. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, Steppel J, Pascual-Leone A, Basser P, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. J Neuropsychiatry Clin Neurosci. 1996;8:172–80. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, III, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grisaru N, Bruno R, Pridmore S. Effect on the emotions of healthy individuals of slow repetitive transcranial magnetic stimulation applied to the prefrontal cortex. J Ect. 2001;17:184–9. doi: 10.1097/00124509-200109000-00007. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–87. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm. 2010;117:105–22. doi: 10.1007/s00702-009-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagelberg N, Aalto S, Kajander J, Oikonen V, Hinkka S, Nagren K, et al. Alfentanil increases cortical dopamine D2/D3 receptor binding in healthy subjects. Pain. 2004;109:86–93. doi: 10.1016/j.pain.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hamani C, Moro E. Surgery for other movement disorders: dystonia, tics. Curr Opin Neurol. 2007;20:470–6. doi: 10.1097/WCO.0b013e32825ea69d. [DOI] [PubMed] [Google Scholar]
- Hesse S, Strecker K, Winkler D, Luthardt J, Scherfler C, Reupert A, et al. Effects of subthalamic nucleus stimulation on striatal dopaminergic transmission in patients with Parkinson’s disease within one-year follow-up. J Neurol. 2008;255:1059–66. doi: 10.1007/s00415-008-0849-z. [DOI] [PubMed] [Google Scholar]
- Hilker R, Voges J, Ghaemi M, Lehrke R, Rudolf J, Koulousakis A, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord. 2003;18:41–8. doi: 10.1002/mds.10297. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34:714–23. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Luthi M, et al. Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience. 2008;151:921–8. doi: 10.1016/j.neuroscience.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Jenkins J, Shajahan PM, Lappin JM, Ebmeier KP. Right and left prefrontal transcranial magnetic stimulation at 1 Hz does not affect mood in healthy volunteers. BMC Psychiatry. 2002;2:1. doi: 10.1186/1471-244X-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Oikonen V, Vilkman H, Farde L, et al. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology. 2000;54:1482–7. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kanno M, Matsumoto M, Togashi H, Yoshioka M, Mano Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J Neurol Sci. 2004;217:73–81. doi: 10.1016/j.jns.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–98. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Sillaber I, Ebner K, Welt T, Toschi N, Kaehler ST, et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur J Neurosci. 2000;12:3713–20. doi: 10.1046/j.1460-9568.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- Keck ME, Welt T, Muller MB, Erhardt A, Ohl F, Toschi N, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43:101–9. doi: 10.1016/s0028-3908(02)00069-2. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Sved AF, Zigmond MJ, Abercrombie ED. Stress-induced dopamine release in the neostriatum: evaluation of the role of action potentials in nigrostriatal dopamine neurons or local initiation by endogenous excitatory amino acids. J Neurochem. 1993;61:1943–52. doi: 10.1111/j.1471-4159.1993.tb09837.x. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Zigmond MJ, Abercrombie ED. Extracellular dopamine in striatum: influence of nerve impulse activity in medial forebrain bundle and local glutamatergic input. Neuroscience. 1992;47:325–32. doi: 10.1016/0306-4522(92)90248-z. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93:525–46. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Foly N, Hamdy A. Dopamine levels after repetitive transcranial magnetic stimulation of motor cortex in patients with Parkinson’s disease: preliminary results. Mov Disord. 2007;22:1046–50. doi: 10.1002/mds.21460. [DOI] [PubMed] [Google Scholar]
- Kim JY, Chung EJ, Lee WY, Shin HY, Lee GH, Choe YS, et al. Therapeutic effect of repetitive transcranial magnetic stimulation in Parkinson’s disease: analysis of [11C] raclopride PET study. Mov Disord. 2008;23:207–11. doi: 10.1002/mds.21787. [DOI] [PubMed] [Google Scholar]
- Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56:315–20. doi: 10.1001/archpsyc.56.4.315. [DOI] [PubMed] [Google Scholar]
- Knapp M, Romeo R, Mogg A, Eranti S, Pluck G, Purvis R, et al. Cost-effectiveness of transcranial magnetic stimulation vs. electroconvulsive therapy for severe depression: a multi-centre randomised controlled trial. J Affect Disord. 2008;109:273–85. doi: 10.1016/j.jad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–72. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci. 2008;28:2147–55. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex affects performance of the Wisconsin card sorting task during provision of feedback. Int J Biomed Imaging. 2008;2008:143238. doi: 10.1155/2008/143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Ptito A, Monchi O, Cho SS, Van Eimeren T, Pellecchia G, et al. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: a [11C]FLB 457 PET study. Neuroimage. 2009;46:516–21. doi: 10.1016/j.neuroimage.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Franca M, Mochizuki H, Marconi B, Caltagirone C, Rothwell JC. Interactions between pairs of transcranial magnetic stimuli over the human left dorsal premotor cortex differ from those seen in primary motor cortex. J Physiol. 2007;578:551–62. doi: 10.1113/jphysiol.2006.123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–8. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Konishi S, Hayashi T, Uchida I, Kikyo H, Takahashi E, Miyashita Y. Hemispheric asymmetry in human lateral prefrontal cortex during cognitive set shifting. Proc Natl Acad Sci U S A. 2002;99:7803–8. doi: 10.1073/pnas.122644899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia: an autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Motohashi N, Ito H, Ito S, Takano A, Nishikawa T, et al. Effects of repetitive transcranial magnetic stimulation on [11C]raclopride binding and cognitive function in patients with depression. J Affect Disord. 2006;95:35–42. doi: 10.1016/j.jad.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Landau AM, Chakravarty MM, Clark CM, Zis AP, Doudet DJ. Electroconvulsive therapy alters dopamine signaling in the striatum of non-human primates. Neuropsychopharmacology. 2011;36:511–8. doi: 10.1038/npp.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Houeto JL, Krack P, Kubu C, Lyons KE, Moro E, et al. Deep brain stimulation: preoperative issues. Mov Disord. 2006;21(Suppl 14):S171–96. doi: 10.1002/mds.20955. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–51. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse. 1997;25:1–14. doi: 10.1002/(SICI)1098-2396(199701)25:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Leff P. The two-state model of receptor activation. Trends Pharmacol Sci. 1995;16:89–97. doi: 10.1016/s0165-6147(00)88989-0. [DOI] [PubMed] [Google Scholar]
- Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology. 2010;35:70–85. doi: 10.1038/npp.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–49. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett. 1990;115:248–52. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Belmaker RH. Animal models of the mechanisms of action of repetitive transcranial magnetic stimulation (RTMS): comparisons with electroconvulsive shock (ECS) Depress Anxiety. 2000;12:178–87. doi: 10.1002/1520-6394(2000)12:3<178::AID-DA10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lockman J, Fisher RS. Therapeutic brain stimulation for epilepsy. Neurol Clin. 2009;27:1031–40. doi: 10.1016/j.ncl.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol. 2000;27:661–70. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- Logan J. A review of graphical methods for tracer studies and strategies to reduce bias. Nucl Med Biol. 2003;30:833–44. doi: 10.1016/s0969-8051(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Logan J, Alexoff D, Fowler JS. The use of alternative forms of graphical analysis to balance bias and precision in PET images. J Cereb Blood Flow Metab. 2011;31:535–46. doi: 10.1038/jcbfm.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Barbanoj MJ, Schlaepfer TE, Thompson E, Perez V, Kulisevsky J. Repetitive transcranial magnetic stimulation for the treatment of depression: systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–91. doi: 10.1192/bjp.182.6.480. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mian MK, Campos M, Sheth SA, Eskandar EN. Deep brain stimulation for obsessive-compulsive disorder: past, present, and future. Neurosurg Focus. 2010;29:E10. doi: 10.3171/2010.4.FOCUS10107. [DOI] [PubMed] [Google Scholar]
- Michael N, Gosling M, Reutemann M, Kersting A, Heindel W, Arolt V, et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17:2462–8. doi: 10.1046/j.1460-9568.2003.02683.x. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of brain lesions on card sorting. Arch Neurol. 1963;9:90–100. [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. Neuroimage. 2006;33:907–12. doi: 10.1016/j.neuroimage.2006.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson’s disease during executive processing depends on striatal involvement. Brain. 2007;130:233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–64. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 2007;27:369–77. doi: 10.1038/sj.jcbfm.9600339. [DOI] [PubMed] [Google Scholar]
- Moro E, Lang AE. Criteria for deep-brain stimulation in Parkinson’s disease: review and analysis. Expert Rev Neurother. 2006;6:1695–705. doi: 10.1586/14737175.6.11.1695. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Rihs TA, Engeler J, Fisch H, Schlaepfer TE. Mood effects of repetitive transcranial magnetic stimulation of left prefrontal cortex in healthy volunteers. Psychiatry Res. 2000;94:251–6. doi: 10.1016/s0165-1781(00)00146-3. [DOI] [PubMed] [Google Scholar]
- Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–6. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Nimura T, Yamaguchi K, Ando T, Itoh M, Yoshimoto T, et al. The impact of stereotactic pallidal surgery on the dopamine D2 receptor in Parkinson disease: a positron emission tomography study. J Neurosurg. 2003;98:57–63. doi: 10.3171/jns.2003.98.1.0057. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C] fallypride. Synapse. 2009;63:447–61. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci. 2006;23:1651–7. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- Nyffeler T, Wurtz P, Luscher HR, Hess CW, Senn W, Pflugshaupt T, et al. Repetitive TMS over the human oculomotor cortex: comparison of 1-Hz and theta burst stimulation. Neurosci Lett. 2006;409:57–60. doi: 10.1016/j.neulet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Hayashi T, Okabe S, Nonaka I, Matsuda H, Iida H, et al. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol Psychiatry. 2004;55:484–9. doi: 10.1016/j.biopsych.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Onn SP, West AR, Grace AA. Dopamine-mediated regulation of striatal neuronal and network interactions. Trends Neurosci. 2000;23:S48–56. doi: 10.1016/s1471-1931(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Catala MD, Pascual-Leone Pascual A. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology. 1996;46:499–502. doi: 10.1212/wnl.46.2.499. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorso-lateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–58. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Paus T, Castro-Alamancos MA, Petrides M. Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2001;14:1405–11. doi: 10.1046/j.0953-816x.2001.01757.x. [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–84. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Popperl G, Tatsch K, Jakob F, Mulert C, et al. Acute prefrontal rTMS increases striatal dopamine to a similar degree as D-amphetamine. Psychiatry Res. 2007;156:251–5. doi: 10.1016/j.pscychresns.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Pogarell O, Koch W, Popperl G, Tatsch K, Jakob F, Zwanzger P, et al. Striatal dopamine release after prefrontal repetitive transcranial magnetic stimulation in major depression: preliminary results of a dynamic [123I] IBZM SPECT study. J Psychiatr Res. 2006;40:307–14. doi: 10.1016/j.jpsychires.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology. 2006;31:1016–26. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–13. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer F, Decary A, Lapierre MF, Rouleau I, Bouvier G, Saint-Hilaire JM. Target detection deficits in frontal lobectomy. Brain Cogn. 1993;21:203–11. doi: 10.1006/brcg.1993.1016. [DOI] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11:165–80. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Sahakian BJ, Hodges JR, Polkey CE, Kennard C, Robbins TW. Dissociating executive mechanisms of task control following frontal lobe damage and Parkinson’s disease. Brain. 1998;121(Pt 5):815–42. doi: 10.1093/brain/121.5.815. [DOI] [PubMed] [Google Scholar]
- Rounis E, Lee L, Siebner HR, Rowe JB, Friston KJ, Rothwell JC, et al. Frequency specific changes in regional cerebral blood flow and motor system connectivity following rTMS to the primary motor cortex. Neuroimage. 2005;26:164–76. doi: 10.1016/j.neuroimage.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Saijo T, Takano A, Suhara T, Arakawa R, Okumura M, Ichimiya T, et al. Effect of electroconvulsive therapy on 5-HT1A receptor binding in patients with depression: a PET study with [11C]WAY 100635. Int J Neuropsychopharmacol. 2010;13:785–91. doi: 10.1017/S1461145709991209. [DOI] [PubMed] [Google Scholar]
- Sassi M, Porta M, Servello D. Deep brain stimulation therapy for treatment-refractory Tourette’s syndrome: a review. Acta Neurochir (Wien) 2010 doi: 10.1007/s00701-010-0803-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Niznik HB. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: implications for positron emission tomography of the human brain. Synapse. 1989;3:96–7. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catechol-amine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Shaul U, Ben-Shachar D, Karry R, Klein E. Modulation of frequency and duration of repetitive magnetic stimulation affects catecholamine levels and tyrosine hydroxylase activity in human neuroblastoma cells: implication for the antidepressant effect of rTMS. Int J Neuropsychopharmacol. 2003;6:233–41. doi: 10.1017/S1461145703003493. [DOI] [PubMed] [Google Scholar]
- Shimamoto H, Takasaki K, Shigemori M, Imaizumi T, Ayabe M, Shoji H. Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson’s disease. J Neurol. 2001;248(Suppl 3):III48–52. doi: 10.1007/pl00007826. [DOI] [PubMed] [Google Scholar]
- Sibon I, Strafella AP, Gravel P, Ko JH, Booij L, Soucy JP, et al. Acute prefrontal cortex TMS in healthy volunteers: effects on brain 11C-alphaMtrp trapping. Neuroimage. 2007;34:1658–64. doi: 10.1016/j.neuroimage.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Bergmann TO, Bestmann S, Massimini M, Johansen-Berg H, Mochizuki H, et al. Consensus paper: combining transcranial stimulation with neuroimaging. Brain Stimul. 2009;2:58–80. doi: 10.1016/j.brs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Hartwigsen G, Kassuba T, Rothwell JC. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009;45:1035–42. doi: 10.1016/j.cortex.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerg M, Liow JS, Seneca N, Hong J, Lu S, Thorsell A, et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: a PET study in a receptor internalization-deficient mouse model. Neuroimage. 2010;50:1402–7. doi: 10.1016/j.neuroimage.2010.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J, et al. Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64:350–62. doi: 10.1002/syn.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, Laruelle M. Models and methods for derivation of in vivo neuroreceptor parameters with PET and SPECT reversible radiotracers. Nucl Med Biol. 2001;28:595–608. doi: 10.1016/s0969-8051(01)00214-1. [DOI] [PubMed] [Google Scholar]
- Slifstein M, Narendran R, Hwang DR, Sudo Y, Talbot PS, Huang Y, et al. Effect of amphetamine on [(18)F] fallypride in vivo binding to D(2) receptors in striatal and extrastriatal regions of the primate brain: single bolus and bolus plus constant infusion studies. Synapse. 2004;54:46–63. doi: 10.1002/syn.20062. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EM, Repella DJ, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–41. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009;101:2872–7. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TD, Ko JH, Kideckel DM, Rusjan P, Houle S, Sandor P, et al. Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann Neurol. 2010;67:170–81. doi: 10.1002/ana.21809. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson’s disease: a rTMS/[11C]raclopride PET study. Eur J Neurosci. 2005;22:2946–52. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Monchi O. Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: the contribution of expectation. Neuroimage. 2006;31:1666–72. doi: 10.1016/j.neuroimage.2006.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–15. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–15. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–10. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Taber MT, Fibiger HC. Electrical stimulation of the pre-frontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–28. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Teneback CC, Nahas Z, Speer AM, Molloy M, Stallings LE, Spicer KM, et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11:426–35. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- Terney D, Bergmann I, Poreisz C, Chaieb L, Boros K, Nitsche MA, et al. Pergolide increases the efficacy of cathodal direct current stimulation to reduce the amplitude of laser-evoked potentials in humans. J Pain Symptom Manage. 2008;36:79–91. doi: 10.1016/j.jpainsymman.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Thobois S, Fraix V, Savasta M, Costes N, Pollak P, Mertens P, et al. Chronic subthalamic nucleus stimulation and striatal D2 dopamine receptors in Parkinson’s disease? A [(11)C]-raclopride PET study. J Neurol. 2003;250:1219–23. doi: 10.1007/s00415-003-0188-z. [DOI] [PubMed] [Google Scholar]
- Tokuno H, Inase M, Nambu A, Akazawa T, Miyachi S, Takada M. Corticostriatal projections from distal and proximal forelimb representations of the monkey primary motor cortex. Neurosci Lett. 1999;269:33–6. doi: 10.1016/s0304-3940(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007;17:466–74. doi: 10.1093/cercor/bhj163. [DOI] [PubMed] [Google Scholar]
- Vandehey NT, Garell PC, Hampel JA, Murali D, Smith EM, Davidson R, et al. PET measurement of changes in D2/D3 dopamine receptor binding in a nonhuman primate during chronic deep brain stimulation of the bed nucleus of the stria terminalis. J Neurosci Methods. 2009;176:129–35. doi: 10.1016/j.jneumeth.2008.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci. 2000;1:73–9. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- Whitworth RH, Jr, LeDoux MS, Gould HJ., III Topographic distribution of connections from the primary motor cortex to the corpus striatum in Aotus trivirgatus. J Comp Neurol. 1991;307:177–88. doi: 10.1002/cne.903070202. [DOI] [PubMed] [Google Scholar]
- Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, et al. High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry. 2006;59:389–94. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–45. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Yang JR, Liao CH, Pang CY, Huang LL, Lin YT, Chen YL, et al. Directed differentiation into neural lineages and therapeutic potential of porcine embryonic stem cells in rat Parkinson’s disease model. Cell Reprogram. 2010;12:447–61. doi: 10.1089/cell.2009.0078. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312:43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: a review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009;2:339–52. [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–5. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–6. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]