Abstract
A new family of heterobifunctional phenyl-substituted vinyl ether (PIVE) coupling agents with tunable acid-sensitivity has been developed. The PIVE compounds are designed to hydrolyze under acidic conditions with hydrolysis rates that can be varied by rational selection of the phenyl ring substituent. These reagents were incorporated within 2-methoxypoly(ethylene glycol) PEG-conjugated 1,3-dioctadecyl-rac-glycerol lipids to produce the acid-cleavable lipopolymers mPEG-[H-PIVE]-DOG, mPEG-[F-PIVE]-DOG, mPEG-[Me-PIVE]-DOG, and mPEG-[MeO-PIVE]-DOG. These lipopolymers were hydrolyzed under acidic conditions (pH 3.5 or 4.5) at rates that were dependent on the electron donating or withdrawing character of the α-phenyl vinyl ether substituent, while remaining stable at pH 7.4. Blending of these compounds with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) in a 10:90 mPEG-PIVE-Lipid:DOPE ratio produced stable liposomes at neutral pH; however, acidification of the solution led to dePEGylation and release of the liposomal cargo in a manner that correlated with the PIVE proton affinity. Specifically, we observed 70 % calcein release within 12 h from mPEG-[MeO-PIVE]-DOG-containing liposomes at pH 4.5, whereas only 22% calcein release was observed from mPEG-[F-PIVE]-DOG:DOPE liposomes over this same timescale and pH. These results indicate that dePEGylation following acidification is a triggering mechanism that can be rationally designed and controlled through the appropriate selection of PIVE moieties.
INTRODUCTION
Many important physiological and pathological processes such as endosomal processing, tumor growth and inflammation occur in acidic environments.1–2 For example, molecules internalized within cells via the endocytic pathway encounter a significant pH drop from neutral to pH 5.9–6.0 within 2 minutes after entry into this transient organelle.3 These compartments can experience further reduction in pH from 6.0 to 4.0 during progression from late endosomes to lysosomes.4 Solid tumors and inflammatory tissues are also known to be slightly acidic.5–6 Therefore, the design and development of materials that can respond to pH changes in a measured and a priori predictable way remains an unmet goal for the realization of drug delivery systems that are molecularly engineered to respond specific biological conditions and kinetic time scales.
The main challenge in the development of carrier systems with these characteristics is the need for the vehicle to remain stable at physiological pH, but rapidly release its contents when it is exposed to the decreased pH of the target site. Thus, there have been many reports of pH-responsive carrier systems based on formulation approaches,7 manipulation of surface charge,8–9 as well as a wide variety of pH-sensitive linkages including acetals,10–12 ortho esters,13–18 hydrazones,19–20 cis-aconityl,21–23 silyl ether,24 and vinyl ethers,8, 25–26 that have been developed for controlled drug release from macro- and nanoparticulate carriers.
Thompson and coworkers have studied plasmenylcholines27 and other types of vinyl ether lipids8, 25–26, 28 as potential vehicles for pH-sensitive drug delivery. Since the rate determining step of acid-catalyzed vinyl ether hydrolysis is protonation of the β-carbon atom, followed by rapid hydration of the resulting transient, resonance-stabilized carbocation, the hydrolysis rate of this motif can be controlled by varying the electronic character of the vinyl ether α-substituent.29–30 We have recently reported studies showing a correlation between the proton affinity of vinyl ether molecules determined by DFT calculations and their intrinsic second order hydrolysis rate constants.28 Our initial studies guided the development of a novel acid-labile PEG conjugated vinyl ether lipids (ST302, ST305, ST352, ST355) that have ability to accelerate acid-triggered contents release from DOPE unilamellar liposomes when used as the minor component in the membrane bilayer.8, 31 Calcein release rate data suggested that rate of contents release was affected by the molar ratio of acid-labile PEG-Lipid, the PEG molecular weight, and the proton affinity of the vinyl ether bond linking the PEG and lipid moieties.
This work describes a new class of heterobifunctional compounds that utilize an α-phenyl substituted vinyl ether (PIVE) bond as the modulating substituent for tuning the acid-sensitivity of the construct (Scheme 1). These bifunctional PIVEs allow for the rational design of cleavable linkers with predictable pH-sensitivity and facile synthesis of compounds with a diverse set of substituents with varying electronic character. These features enable their facile incorporation into a wide range of pH-sensitive materials, requiring effectively no major changes to the composition of the delivery vehicle formulation.
Scheme 1.
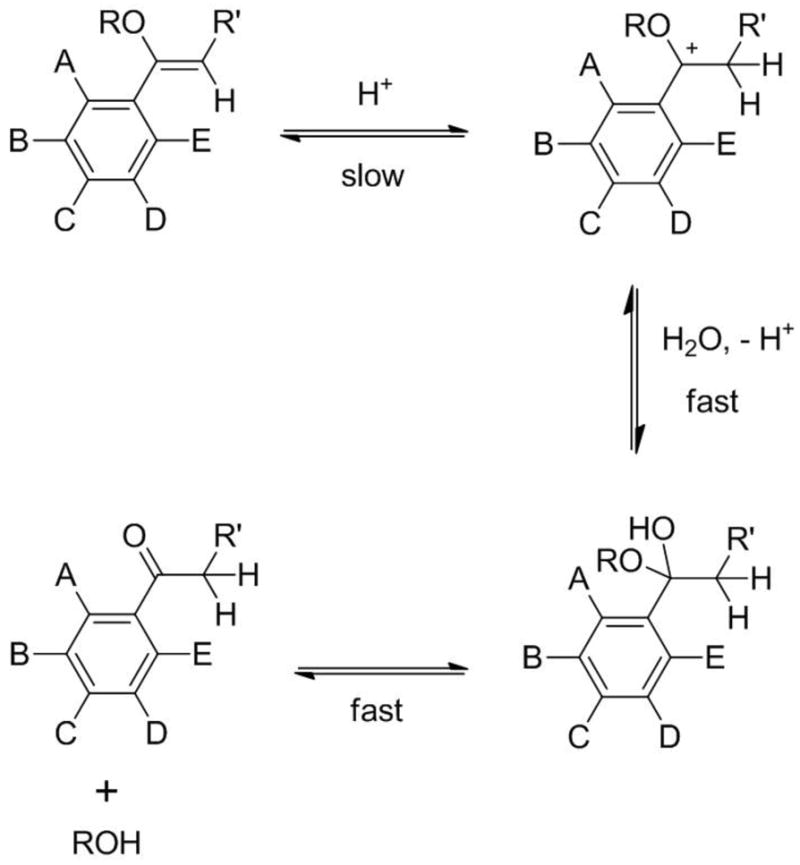
PIVE hydrolysis mechanism under acidic conditions in water. A – E: Electron donating or withdrawing substituents.
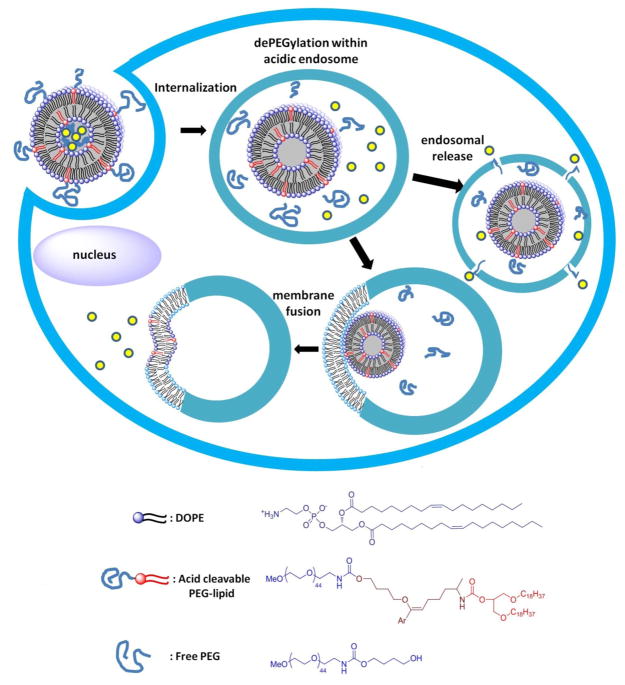
Four PIVE heterobifunctional linkers were prepared and used to couple the same mPEG2000 water-soluble headgroup and 1,3-dioctadecyl-rac-glycerol lipid anchor to provide a homologous series of lipopolymers for direct comparison of their activity as the critical components of pH-sensitive liposomes for intracellular drug delivery (Figure 1). Proton affinities of mPEG-[H-PIVE]-DOG, mPEG-[F-PIVE]-DOG, mPEG-[Me-PIVE]-DOG, and mPEG-[MeO-PIVE]-DOG containing different 4′-substituents on the α-phenyl vinyl ether bond were calculated using density functional theory (DFT) methods. These target compounds were then synthesized and their hydrolysis rates evaluated by HPLC analysis. Cargo release rates via dePEGylative activation of 10:90 mPEG-PIVE-Lipid:1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPE) liposomes also were tested using a fluorescence dequenching assay and the change in liposome morphology evaluated by cryogenic transmission electron microscopy (cryoTEM).
Figure 1.
Schematic of acid-catalyzed DePEGylative triggering from PEG-PIVE-Lipid:DOPE liposomes through lamellar (Lα) to hexagonal (HII) phase transition.
EXPERIMENTAL PROCEDURES
Materials
All chemicals were obtained from Aldrich Chemical Company, except DOPE that was obtained from Avanti Polar Lipids. Synthetic intermediates and final products were characterized by 300 MHz 1H NMR and MALDI MS using the Purdue University Center for Cancer Research’s Interdepartmental NMR Facility and Campus-Wide Mass Spectrometry Center, respectively. CryoTEM images of liposome samples were obtained via the Purdue University Biological Electron Microscopy Facility.
DFT Calculations
The proton affinity values of the vinyl ether series were determined by DFT calculations using Gaussian 03 and the B3LYP hybrid functional.32–33 Neutral and protonated structures were optimized using the 6–31G** basis set. Single point energies of the optimized structures were obtained with the cc-pVTZ basis set.34
Synthesis of mPEG-PIVE-Lipids
Detailed methods for the preparation of 1,3-bis(octadecyloxy)propan-2-yl(7-(((4-methoxypoly(ethylene glycol [MW2000])carbamoyl)oxy)butoxy)-7-phenylhept-6-en-2-yl)carbamate (mPEG-[H-PIVE]-DOG), 1,3-bis(octadecyloxy)propan-2-yl(7-(4-fluorophenyl)-7-(((4-methoxypoly(ethylene glycol [MW2000])carbamoyl)oxy)butoxy)hept-6-en-2-y)carbamate (mPEG-[F-PIVE]-DOG), 1,3-bis(octadecyloxy)propan-2-yl(7-(((4-methoxypoly(ethylene glycol[MW2000])carbamoyl)oxy)butoxy)-7-(4′-methylphenyl)hept-6-en-2-yl)carbamate (mPEG-[Me-PIVE]-DOG), and 1,3-bis(octadecyloxy)propan-2-yl(7-(((4-methoxypoly(ethylene glycol [MW2000])carbamoyl)oxy)butoxy)-7-(4′-methoxyphenyl)hept-6-en-2-y)carbamate (mPEG-[MeO-PIVE]-DOG) are described in Supplementary Information.
Hydrolysis Kinetics of mPEG-PIVE-Lipid Conjugates
Hydrolysis reactions were initiated by dissolving 0.2 mg of the mPEG-PIVE-Lipid conjugate in 10 mL of 20 mM HEPES (pH7.4), 20 mM citrate (pH 4.5), or 20 mM oxalate (pH 3.5) and incubating the mixture at 37 °C. Aliquots (200 μL) were periodically removed and the product mixture analyzed by HPLC at 40 °C (injection volume = 40 μL) on an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA) equipped with a Corona Charged Aerosol Detector and an Eclipse XDB-C8 column (4.6 × 150 mm, Agilent Technologies). Gradient elution was performed using a flow rate of 0.7 mL/min: 0–20 min, 1:9 MeOH:H2O → 7:3 MeOH:H2O; 20–35 min, 7:3 MeOH:H2O → 7:3 MeOH:CH3CN; 35–50 min, 7:3 MeOH:CH3CN → 1:9 MeOH:CH3CN; 50 min-60 min, 1:9 MeOH:CH3CN → 1:9 MeOH:H2O. The rate of hydrolysis was determined by monitoring the chromatographic peak area using mPEG-OH RT = 22.9 min and mPEG-PIVE-Lipid conjugates RT = 43.5 min. The mPEG-OH chromatographic peak was verified by comparison with the retention time determined for commercial available standard samples. The observed first-order rate constants were obtained using the half-life method; the intrinsic second order hydrolysis rate constants were obtained by correcting the observed first-order rate constants for the reactant in excess.
Liposome Preparation
Stock solutions (20 mg/mL) of DOPE and the pH-sensitive mPEG-PIVE-Lipid conjugates were prepared in 95:5 benzene:MeOH. The lipid stock solutions were combined at the desired ratios (10 μmol total lipid) and vortexed for 2 min before flash freezing in LN2 for 3 min and lyophilization (15 h) to produce a powder. The lyophilized lipid mixtures were then hydrated in 1 mL of 50 mM calcein solution (dissolved in 20 mM 4-(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid (HEPES), 150 mM NaCl, pH 7.4) via ten freeze-thaw-vortex cycles to produce multilamellar liposomes that were extruded ten times through 100 nm polycarbonate membranes filters at 50 °C with ~200–250 psi N2. The liposome solutions were passed through a 0.5 × 25 cm Sephadex G-50 column equilibrated with a 20 mM HEPES, 150 mM NaCl, pH 7.4 solution for removal of unentrapped calcein and the liposomes used immediately after isolation.
Dynamic Light Scattering
Liposome sizes were measured by dynamic light scattering using a Malvern Nano ZS-90 instrument and the distributions calculated using the manufacturer’s supplied software. Samples were prepared by diluting 50 μL liposome stock with 1 mL 18 MΩ H2O. Experiments were run in triplicate.
Calcein Release Assay
An aliquot of the liposome stock solution (100 μL) was added to 1.3 mL of 20 mM HEPES (pH7.4), 20 mM citrate (pH 4.5), or 20 mM oxalate (pH 3.5) containing 150 mM NaCl and the solutions maintained at 37 °C for the duration of the experiment. At each measurement point, 100 μL aliquots were taken and diluted with 2 mL HEPES-buffered saline.
The fluorescence (λex = 490 nm, λem = 518 nm) was then measured before and after the addition of 2 drops 10% Triton X-100 (solution gently shaken to ensure complete liposome disruption). The percent release was quantified using the ratio method: % release = 100 × [(T − T0)/(1 − T0)], where T = (fluorescence before Triton addition/fluorescence after Triton addition) at time T; T0=fluorescence ratio at T = 0 min.35
Cryogenic Transmission Electron Microscopy
A small drop of liposome solution was placed on a bare Quantifoil grid by pipet and the excess liquid blotted away using filter paper under humid environmental conditions to produce a thin (20–500 nm) sample film around the holes in the polymer grid. The sample film was vitrified by rapid freezing in a liquid ethane slush cooled by LN2 and transferred via cryotransfer device to a Philips CM200 transmission electron microscope equipped with a LN2-cooled stage and a field-emission gun operating at 200 kV accelerating voltage for observation. In order to minimize radiative sample damage, the collection of high-resolution cryoTEM images was made at 50,000x magnification under low dose conditions.
RESULTS AND DISCUSSION
PIVE Design Guided by DFT Calculations
A variety of groups have reported how electronic effects can affect pH-sensitive drug delivery systems,8,10,20,24,36–37 including a recent study describing a series of mPEG-Lipids linked by vinyl ethers with both electron donating and electron withdrawing groups.28 The proton affinities were calculated using DFT methods, producing a reasonably good correlation between predicted proton affinities and observed second order hydrolysis rate constants. Unfortunately, these compounds were limited by the fact that a unique synthetic pathway was required for each member of the vinyl ether-linked PEG-lipid series. Furthermore, those pathways restricted the scope of the methodology to the synthesis of lipid-type structures. These limitations led us to seek a family of heterobifunctional molecules with tunable hydrolysis rates that could all be produced via a common reaction pathway to afford derivatives that could easily be incorporated into many pharmacologically interesting constructs.
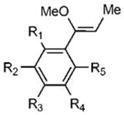
DFT (B3LYP) calculations were initially performed to evaluate whether a broad range of proton affinities could be expected from PIVEs using different substituents on the phenyl ring. Neutral vinyl ether precursors and protonated intermediates were first optimized with the 6–31G** basis set.32–33 The single point energies of the optimized structures then were obtained with the cc-pVTZ basis set at the DFT level.34 Comparison of these parameters (Table 1) with the intrinsic second order rate constants of similar vinyl ethers28 were then used to select targets for synthesis that should display easily detected changes in reaction rate constants.
Table 1.
Proton affinities determined by DFT (CC-PVTZ) calculations and measured intrinsic second order hydrolysis rate constants for a related series of α-phenyl vinyl ether (PIVE) derivatives.
| ||
|---|---|---|
| Substitution | ΔHP.A. (kcal/m) | Measured k2 (M−1s−1) |
| R1,3,5 = OMe; R2,4 = H | −249.2 | n.d. |
| R1,3,5 = CF3; R2,4 = H | −243.3 | n.d. |
| R3 = OMe; R1,2,4,5 = H | −235.7 | 2.8 |
| R2,4 = OMe; R1,3,5 = H | −233.9 | n.d. |
| R3 = Me; R1,2,4,5 = H | −233.7 | 1.4 |
| R3 = H; R1,2,4,5 = H | −230.1 | 1.0 |
| R3 = F; R1,2,4,5 = H | −227.9 | 0.68 |
| R3 = CF3; R1,2,4,5 = H | −222.6 | n.d. |
| R2,4 = CF3; R1,3,5 = H | −217.2 | n.d. |
Synthesis of Acid Labile PEG-PIVE-Lipid Conjugates
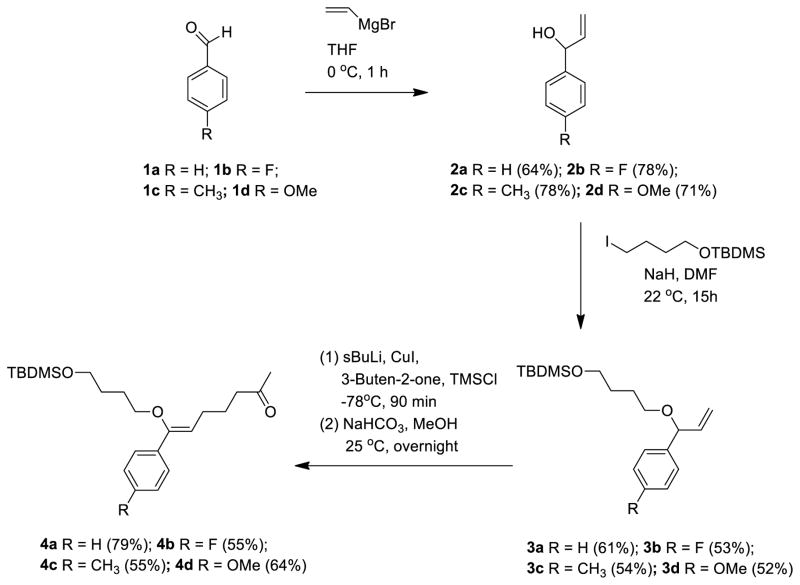
Based on the findings of the DFT calculations, we designed a series of pH-sensitive heterobifunctional PIVE reagents to investigate the effect of α-phenyl ring substitution on acid-catalyzed hydrolysis rates. Four different heterobifunctional PIVE crosslinking reagents (Scheme 2) were prepared to effect the synthesis of the mPEG-PIVE-Lipid conjugates mPEG-[F-PIVE]-DOG, mPEG-[H-PIVE]-DOG, mPEG-[Me-PIVE]-DOG, and mPEG-[MeO-PIVE]-DOG (Scheme 3).
Scheme 2.
General synthesis pathway for the preparation of heterobifunctional PIVE coupling reagents.
Scheme 3.
Synthesis pathway for pH-sensitive mPEG-PIVE-Lipid conjugates from heterobifunctional PIVE reagents.
The heterobifunctional PIVE reagents were prepared from their corresponding benzaldehydes in three steps as shown in Scheme 2. Para-substituted α-vinyl-benzyl alcohols were prepared through a Grignard reaction of the benzaldehydes 2 with vinyl magnesium bromide in THF at 22 °C. Williamson etherification with t-butyl(4-iodobutoxy)dimethylsilane in the presence of NaH resulted in the allyl ethers 3 in 52–61% yield. The allyl ethers were treated with sec-BuLi converting them to their corresponding lithioalkoxyallyl intermediates, which were transmetallated using CuI in DMS. These organocopper reagents improved the coupling efficiency with methyl vinyl ketone in the presence of trimethylsilyl chloride as previously described by Berrien et al.,38 without the detectable formation of α-alkylation sideproducts. Deprotection of TMS with NaHCO3 in MeOH produced the PIVE linkers (4) in 55–79% yield. Overall yields for the synthesis of F-PIVE, H-PIVE, Me-PIVE, and MeO-PIVE from their corresponding benzaldehyde precursors were 22.7%, 30.8%, 23.2%, and 23.6%.
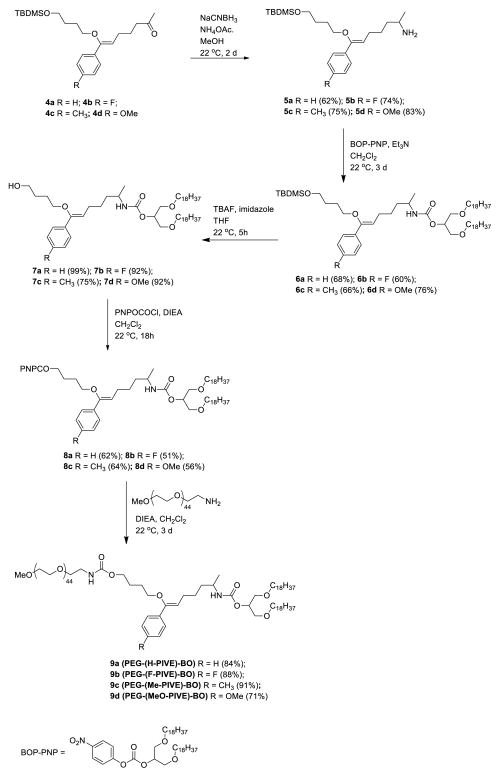
Ketones 4 then were converted to primary amines via reductive animation in 62–83% yields (Scheme 3) to produce intermediates that enable facile incorporation into a wide variety of pH-sensitive constructs. To generate the target series of pH-sensitive mPEG-PIVE-Lipids, Intermediates 5 were treated with the 4-nitrophenylcarbonate-activated 1,3-dioctadecyl-rac-glycerol intermediate, BOP-PNP, in DCM at 22 °C to give 6, followed by TBDMS deprotection to give alcohols 7. Treatment of this intermediate with 4-nitrophenyl chloroformate in DCM at 22 °C for 18 h gave activated carbonate 8 that was subsequently coupled with mPEG2000-NH2 for 3 d at 22 °C to afford the desired mPEG-PIVE-Lipids 9 in 71–91 % yield.
Hydrolysis of mPEG-PIVE-Lipid Conjugates
The hydrolysis kinetics of mPEG-PIVE-Lipid derivatives were evaluated as a function of pH at 37 °C via HPLC-CCAD by monitoring both the disappearance of the starting materials and the appearance of the hydrolysis product, 1-methoxypoly(ethylene glycol), MW2000 (mPEG-OH). The extent of lipid hydrolysis was determined by integrating the areas under the chromatographic peaks for mPEG-PIVE-Lipid and mPEG-OH. Under all hydrolysis conditions, a decrease in mPEG-PIVE-lipid peak area was accompanied by a proportional increase in the peak area of the degradation product, mPEG-OH, which was verified by injection of a commercial standard.
The HPLC data (Figure 2) indicated that mPEG-PIVE-lipid hydrolysis rates are strongly pH-dependent. Substituent effects on mPEG-PIVE-Lipid hydrolysis rates were also compared for electron-donating (4′-methyl, 4′-methoxy) and electron-withdrawing (4′-fluoro) substituents on the phenyl ring relative to the unsubstituted PIVE (Table 1). The hydrolysis of PEG-[H-PIVE]-DOG after 2 h was 19 % at pH 4.5 with t1/2 = 5.46 h, whereas negligible hydrolysis was observed after 3 d at pH 7.4. The hydrolysis rate increased slightly for mPEG-[Me-PIVE]-DOG (24% hydrolysis after 2 h at pH 4.5; t1/2 = 4.04 h), but much more for mPEG-[MeO-PIVE]-DOG (42% hydrolysis after 2 h at pH 4.5; t1/2 = 2.64 h). Conversely, the hydrolysis rate of the electron deficient PEG-[F-PIVE]-DOG congener was significantly slower at 37 °C (16 % hydrolysis after 2 h at pH 4.5; t1/2= 7.5 h). These results are consistent with the hypothesis that the rate of PIVE hydrolysis can be influenced by varying the electronics of the α-phenyl ring.
Figure 2.
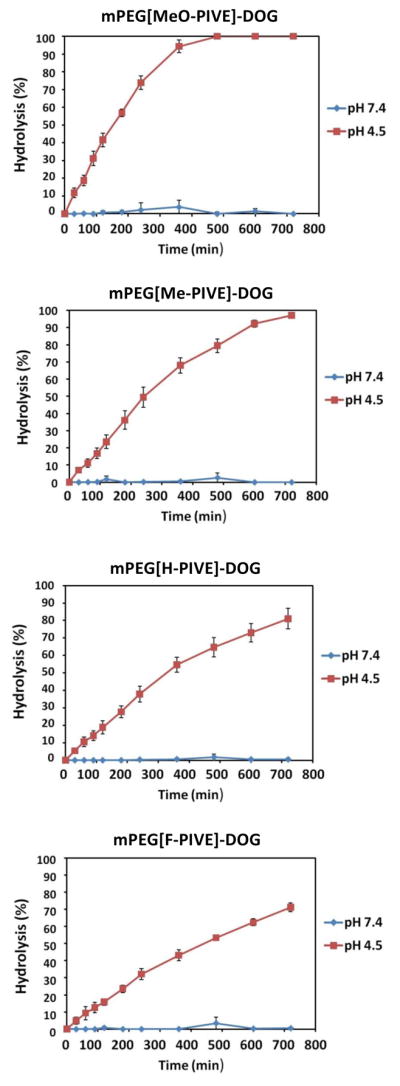
Hydrolysis rates of mPEG-PIVE-Lipids at pH 4.5 and pH 7.4, 37 °C. Aliquots were taken at each time point and the degree of hydrolysis was analyzed by HPLC-CCAD.
Liposome Preparation and Analysis of Acid-Triggered Calcein Release from mPEG-PIVE-Lipid:DOPE Dispersions
Liposomes were prepared via the thin film extrusion method using mPEG-PIVE-lipid:DOPE ratios of 5:95 and 10:90. These dispersions had average diameters of ~170 nm as measured by dynamic light scattering and were stable for at least one week.
Content release rates of mPEG-PIVE-Lipid:DOPE liposomes as a function of pH were conducted by using a calcein fluorescence dequenching assay. As calcein encapsulated at self-quenching concentration leaks into the extraliposomal solution, fluorescence dequenching occurs to produce an increase in calcein fluorescence.35 Liposomes containing calcein (50 mM) were incubated in buffer solutions of either pH 3.5, 4.5, or 7.4 at 37 °C and the time-dependent change in calcein fluorescence monitored in HEPES buffer at 518 nm. The calcein release percentage at each time point was calculated as a ratio versus 100% release as determined by addition of Triton X-100 to the liposome sample.
Figure 3 shows the calcein release kinetics at 37 °C of 10:90 mPEG-[H-PIVE]-DOG:DOPE liposomes at pH 4.5 and 7.4, with observed release rates of 36% at pH 4.5 after 12 h and less than 10% over 24 h at pH 7.4. These data show that faster calcein release can be achieved at lower pH values; we infer from these results that the rate of mPEG-PIVE-lipid hydrolysis controls the rate of calcein release. Consistent with this interpretation is the observation that increased rates of calcein release occur under acidic conditions when the membrane composition of mPEG-[H-PIVE]-DOG is reduced from 10 to 5 mol% (Supplemental Information).
Figure 3.
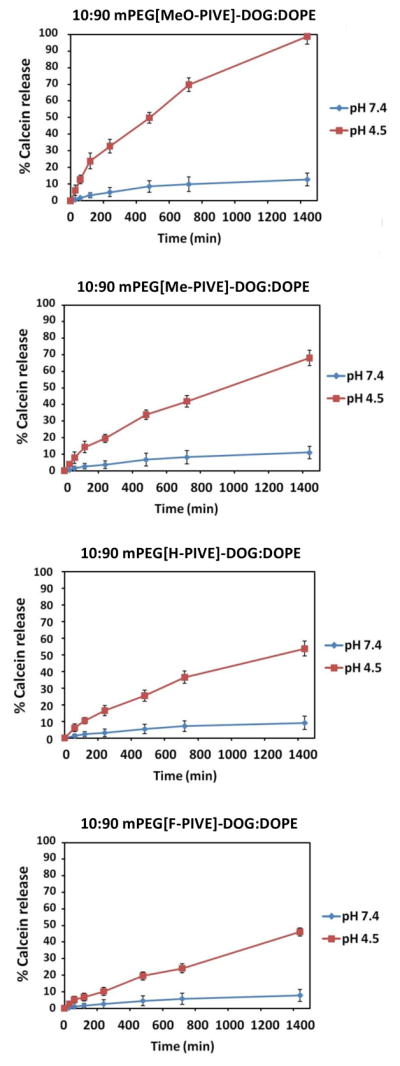
Calcein release kinetics from 10:90 mPEG-PIVE-Lipid:DOPE liposomes at pH 4.5 and pH 7.4 at 37 °C. Aliquots were taken at each time point and the fluorescence (λex = 490 nm, λem = 518 nm) was then measured before and after the addition of 2 drops 10% Triton X-100.
Calcein release rates also were found to depend on the electronic properties of the PIVE conjugate. Liposomes composed of 10:90 mPEG-[Me-PIVE]-DOG:DOPE produced 42 % calcein release at pH 4.5 within 24 h at 37 °C, whereas mPEG-[MeO-PIVE]-DOG-containing DOPE liposomes at the same molar ratio liberated 86% of their calcein cargo under the same conditions. Notably slower was the 10:90 PEG-[F-PIVE]-DOG:DOPE liposome dispersion, that released only 24 % of the encapsulated calcein within 12 h at pH 4.5 (37 °C). All mPEG-PIVE-Lipid:DOPE liposomes dispersions were stable (i.e., < 10% release over 2 d at pH 7.4) under normal physiological conditions. These results parallel the findings of the mPEG-PIVE-Lipid hydrolysis studies in that the electronic character of the 4′-substituent on the phenyl group also produces a marked influence on calcein release rates, via modulation of the PIVE hydrolysis rate and accompanying rate of liposome dePEGylation.
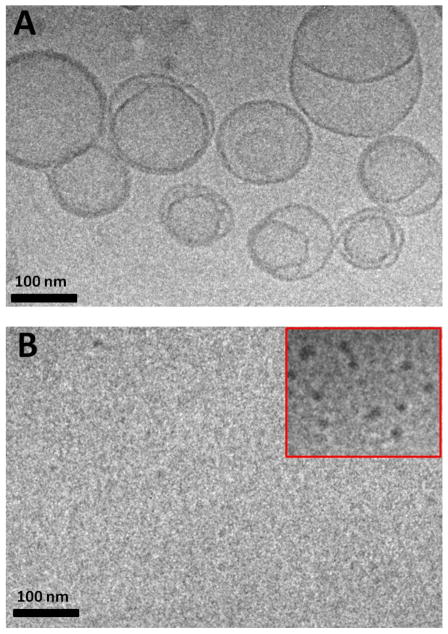
The structural consequences of PIVE linker hydrolysis on mPEG-PIVE-Lipid:DOPE liposomes was evaluated using cryoTEM. Dispersions comprised of 10:90 mPEG-[H-PIVE]-DOG:DOPE were stable liposomes at pH 7.4, appearing as spherical vesicle-like structures; however, after acidification to pH 4.5, dense collapsed structures and small vesicle fragments were observed. We infer from these results that the individual mPEG-PIVE-BO:DOPE liposomes had collapsed into small HII phase lipidic structures upon exposure to pH 4.5, due to a Lα - HII phase transition that is induced by acid-catalyzed dePEGylation of the mPEG-PIVE-Lipid:DOPE liposome dispersion. We infer from these observations that hydrolyzed mPEG may be weakly adsorbed onto the small HII phase clusters, thus preventing the contact and coalescence to form larger HII phase aggregates, in a manner similar to that reported for hydrolyzed mPEG-vinyl ether-cholesterol:DOPE dispersions at low lipid concentrations.26
CONCLUSIONS
A new class of heterobifunctional reagents with tunable acid-sensitivity that can be used to couple two different functional components in a drug delivery formulation been developed. These compounds are based on a phenyl substituted vinyl ether (PIVE) motif that can provide rational control over the design of pH-sensitive drug delivery systems based on the electronic effects exerted on the phenyl substituent(s) of the PIVE using DFT calculations. A short, facile synthetic pathway was developed for this new family of vinyl ether derivatives. Degradative analysis showed that these linkers hydrolyzed under acidic conditions while remaining stable at pH 7.4. As anticipated by the DFT calculations, the rates of acid-catalyzed hydrolysis were dependent on the electron donating or withdrawing character of the PIVE substituents. Taken together, these results suggest that it is possible to design pH-responsive delivery vehicles on an a priori basis to provide a level of functional control that is difficult to achieve with standard formulation methodology. These results also suggest that PIVE linkers can be readily and reliably incorporated into a variety of materials to enable more precisely controllable acid-triggered drug delivery.
Supplementary Material
Figure 4.
Cryogenic transmission electron microscopy images of 10:90 PEG-[H-PIVE]-DOG:DOPE liposomes before (A) and after (B) 24 h exposure at pH 4.5. Liposomes had converted to small dark fragments after dePEGylation at pH 4.5.
Acknowledgments
We gratefully acknowledge financial support by National Institutes of Health (GM087016). We would like to thank Valorie Bowman (Purdue University, Department of Biological Sciences) for her assistance in the collection of cryoTEM images of the liposome dispersions and the Purdue University Center for Cancer Research Interdepartmental NMR Facilities and Campus-Wide Mass Spectrometry Center (supported by NCI CCSG CA23168 to Purdue University Center for Cancer Research). We also thank Prof. Shin-ichi Fujiwara for fruitful discussions and initial assistance with the proton affinity calculations.
Footnotes
Supporting Information. DFT calculations, detailed synthetic procedures of PIVE and mPEG-PIVE-Lipid conjugates, 1H NMR and 13C NMR spectra, and calcein release kinetics from 5:95 mPEG-PIVE-Lipid:DOPE liposomes. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 2.Gerweck LE. Tumor pH: Implications for treatment and novel drug design. Semin Radiat Oncol. 1998;8:176–182. doi: 10.1016/s1053-4296(98)80043-x. [DOI] [PubMed] [Google Scholar]
- 3.Watson P, Jones AT, Stephens DJ. Intracellular trafficking pathways and drug delivery: fluorescence imaging of living and fixed cells. Adv Drug Deliv Rev. 2005;57:43–61. doi: 10.1016/j.addr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Benjaminsen RV, Sun HH, Henriksen JR, Christensen NM, Almdal K, Andresen TL. Evaluating nanoparticle sensor design for intracellular pH measurements. ACS Nano. 2011;5:5864–5873. doi: 10.1021/nn201643f. [DOI] [PubMed] [Google Scholar]
- 5.Brune K, Graf P. Non-steroid anti-inflammatory drugs - influence of extracellular pH on biodistribution and pharmacological effects. Biochem Pharmacol. 1978;27:525–530. doi: 10.1016/0006-2952(78)90388-x. [DOI] [PubMed] [Google Scholar]
- 6.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 7.Yatvin MB, Kreutz W, Horwitz BA, Shinitzky M. pH-Sensitive liposomes: possible clinical implications. Science. 1980;210:1253–1255. doi: 10.1126/science.7434025. [DOI] [PubMed] [Google Scholar]
- 8.Shin J, Shum P, Thompson DH. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipids. J Controlled Release. 2003;91:187–200. doi: 10.1016/s0168-3659(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 9.Yeo Y, Xu PS, Bajaj G, Shugg T, Van Alstine WG. Zwitterionic chitosan derivatives for pH-sensitive stealth coating. Biomacromolecules. 2010;11:2352–2358. doi: 10.1021/bm100481r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies ER, Goodwin AP, Frechet JMJ. Acetals as pH-sensitive linkages for drug delivery. Bioconjugate Chem. 2004;15:1254–1283. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]
- 11.Knorr V, Allmendinger L, Walker GF, Paintner FF, Wagner E. An acetal-based PEGylation reagent for pH-sensitive shielding of DNA polyplexes. Bioconjugate Chem. 2007;18:1218–1225. doi: 10.1021/bc060327a. [DOI] [PubMed] [Google Scholar]
- 12.Knorr V, Russ V, Allmendinger L, Ogris M, Wagner E. Acetal linked oligoethyleneimines for use as pH-sensitive gene carriers. Bioconjugate Chem. 2008;19:1625–1634. doi: 10.1021/bc8001858. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Szoka FC. Steric stabilization of fusogenic liposomes by a low-pH sensitive PEG-diortho ester-lipid conjugate. Bioconjugate Chem. 2001;12:291–300. doi: 10.1021/bc000110v. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, MacKay JA, Szoka FC. Mechanism of pH-triggered collapse of phosphatidylethanolamine liposomes stabilized by an ortho ester polyethyleneglycol lipid. Biophys J. 2003;84:1784–1795. doi: 10.1016/s0006-3495(03)74986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masson C, Garinot M, Mignet N, Wetzer B, Mailhe P, Scherman D, Bessodes M. pH-Sensitive PEG lipids containing orthoester linkers: new potential tools for nonviral gene delivery. J Controlled Release. 2004;99:423–434. doi: 10.1016/j.jconrel.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Huang ZH, Guo X, Li WJ, MacKay JA, Szoka FC. Acid-triggered transformation of diortho ester phosphocholine liposome. J Am Chem Soc. 2006;128:60–61. doi: 10.1021/ja057024w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HG, Zhang HZ, McCallum CM, Szoka FC, Guo X. Unsaturated cationic ortho esters for endosome permeation in gene delivery. J Med Chem. 2007;50:4269–4278. doi: 10.1021/jm060128c. [DOI] [PubMed] [Google Scholar]
- 18.Bruyere H, Westwell AD, Jones AT. Tuning the pH sensitivities of orthoester based compounds for drug delivery applications by simple chemical modification. Bioorg Med Chem Lett. 2010;20:2200–2203. doi: 10.1016/j.bmcl.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: Double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjugate Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the pH stability of PEG-PE conjugates. Bioconjugate Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen WC, Ryser HJP. Cis-aconityl spacer between daunomycin and macromolecular carriers - a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Comm. 1981;102:1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- 22.Alshamkhani A, Duncan R. Synthesis, controlled release properties and antitumor activity of alginate-cis-aconityl-daunomycin conjugates. Int J Pharm. 1995;122:107–119. [Google Scholar]
- 23.Remenyi J, Balazs B, Toth S, Falus A, Toth G, Hudecz F. Isomer-dependent daunomycin release and in vitro antitumour effect of cis-aconityl-daunomycin. Biochem Biophys Res Comm. 2003;303:556–561. doi: 10.1016/s0006-291x(03)00394-2. [DOI] [PubMed] [Google Scholar]
- 24.Parrott MC, Luft JC, Byrne JD, Fain JH, Napier ME, DeSimone JM. Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials. J Am Chem Soc. 2010;132:17928–17932. doi: 10.1021/ja108568g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boomer JA, Inerowicz HD, Zhang ZY, Bergstrand N, Edwards K, Kim JM, Thompson DH. Acid-triggered release from sterically-stabilized fusogenic vesicles via a hydrolytic dePEGylation strategy. Langmuir. 2003;19:6408–6415. [Google Scholar]
- 26.Boomer JA, Qualls MM, Inerowicz HD, Haynes RH, Patri GVS, Kim JM, Thompson DH. Cytoplasmic delivery of liposomal contents mediated by an acid-labile cholesterol-vinyl ether-PEG conjugate. Bioconjugate Chem. 2009;20:47–59. doi: 10.1021/bc800239b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerasimov O, Schwan A, Thompson DH. Acid-catalyzed plasmenylcholine hydrolysis and its effect on bilayer permeability: a quantitative study. Biochim Biophys Acta. 1997;1324:200–214. doi: 10.1016/s0005-2736(96)00220-9. [DOI] [PubMed] [Google Scholar]
- 28.Shin J, Shum P, Grey J, Fujiwara S, Malhotra GS, González-Bonet AM, Hyun SH, Moase E, Allen TM, Thompson DH. 2012 doi: 10.1021/mp300326z. unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kresge AJ. Unusual reactivity of prostacyclin: rational drug design through physical organic chemistry. Acc Chem Res. 1987;20:364–370. [Google Scholar]
- 30.Keeffe JR, Kresge AJ. In: The Chemistry of Enols. Rappoport Z, editor. John Wiley & Sons; New York, NY: 1990. p. 437. [Google Scholar]
- 31.Shin J. PhD Thesis. 2002. Synthesis and Acid-Triggered Release Activity of PEG Lipid Conjugates in DOPE Liposomes. [Google Scholar]
- 32.Becke AD. Density-functional thermochemistry. 3 The role of exact exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 33.Lee CT, Yang WT, Parr RG. Development of the Colle-Salvetti correlation energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 34.Kendall RA, Dunning TH, Harrison RJ. Electron affinities of the 1st row atoms revisited - systematic basis sets and wave functions. J Chem Phys. 1992;96:6796–6806. [Google Scholar]
- 35.Allen TM, Hong K, Papahadjopoulos D. Membrane contact, fusion and hexagonal (HII) transitions in phosphatidylethanolamine liposomes. Biochemistry. 1990;29:2976–2985. doi: 10.1021/bi00464a013. [DOI] [PubMed] [Google Scholar]
- 36.Patel VF, Hardin JN, Mastro JM, Law KL, Zimmermann JL, Ehlhardt WJ, Woodland JM, Starling JJ. Novel acid labile COL1 trityl-linked difluoronucleoside immunoconjugates: Synthesis, characterization, and biological activity. Bioconjugate Chem. 1996:497–510. doi: 10.1021/bc960038u. [DOI] [PubMed] [Google Scholar]
- 37.Kong SD, Luong A, Manorek G, Howell SB, Yang J. Acidic hydrolysis of N-ethoxybenzylimidazoles (NEBIs): potential applications as pH-sensitive linkers for drug delivery. Bioconjugate Chem. 2007;18:293–296. doi: 10.1021/bc060224s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berrien JF, Raymond MN, Moskowitz H, Mayrargue J. Selective gamma-1,4 addition of methoxyallylcopper to enone. Tetrahedron Lett. 1999;40:1313–1314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.