Abstract
Experiments have shown that homologous Ras proteins containing different lipid-modification, which is required for membrane binding, form non-overlapping nanoclusters on the plasma membrane. However, the physical basis for clustering and lateral organization remained poorly understood. We have begun to tackle this issue using coarse-grained molecular dynamics simulations of the H-ras lipid anchor (tH), a triply lipid-modified heptapeptide embedded in a domain-forming mixed bilayer [Janosi L. et al., Proc. Natl. Acad. Sci. U. S. A. 2012 109:8097]. Here we use the same simulation approach to investigate the effect of peptide concentration and bilayer composition on the clustering and lateral distribution of tH. We found no major difference in the clustering behavior of tH above a certain concentration. However, the simulations predict the existence of a critical concentration below which tH does not form nanoclusters. Moreover, our data demonstrate that cholesterol enhances the stability of tH nanoclusters but is not required for their formation. Finally, analyses of peptide distributions and partition free energies allowed us to quantitatively describe how clustering facilitates the accumulation of tH at the interface between ordered and disordered domains of the simulated bilayer systems. These thermodynamic insights represent some of the key elements for a comprehensive understanding of the molecular basis for the formation and stability of Ras signaling platforms.
Keywords: Ras, nanocluster, lipid anchor, membrane domain, Molecular Dynamics, partitioning
Introduction
Ras proteins are guanine triphosphate (GTP)-hydrolyzing enzymes that act as molecular switches to regulate cell proliferation, differentiation and development1. H-, N- and K-ras proteins are ubiquitously expressed in humans, and somatic mutations on these proteins are associated with ~30% of all human cancers1. Ras proteins share a nearly identical catalytic machinery but differ in their C-terminal hypervariable (HVR) region, which contains a lipid-modified motif required for binding to specific membrane micro-domains1,2. The minimal membrane-binding motif, also called the lipid anchor, of Ras proteins is well characterized3-5. The mechanism by which individual Ras lipid anchors attach to cellular or model membranes has also been investigated experimentally (for reviews see refs. 6,7) and computationally8-14. As a result, the atomic interactions responsible for the insertion of Ras lipid-anchors into fluid-phase phospholipid bilayers are relatively well established15-17. However, this knowledge alone is not sufficient to explain recent observations about the specific spatiotemporal organization of multiple Ras proteins on bilayer surfaces2,18,19.
To better understand the process of assembly and lateral organization of Ras proteins on membrane surfaces, numerous biophysical and cell-biological experiments have been carried out on the full-length protein3,4,20-22 as well as on simplified model peptides representing different Ras lipid-anchors5,23-25. An emerging consensus is that different Ras lipid anchors have different preferences for raft-like liquid-ordered (Lo) and non-raft liquid-disordered (Ld) membrane domains2. Specifically, the farnesylated and dually palmitoylated lipid-anchor of H-ras prefers cholesterol-enriched Lo domains18; the farnesylated and singly palmitoylated lipid-anchor of N-ras predominantly localizes at the boundary between Lo and Ld domains26; and the farnesylated and polycationic lipid anchor of K-ras resides at disordered Ld domains22. The concept of chain packing27-29 in phospholipid bilayers, which states that optimal packing occurs between lipids of the same chain length and saturation, offers an appealing institutive explanation for these observations. According to this theory, proteins (or peptides) modified by saturated palmitoyl lipids likely partition into Lo domains rich with saturated lipid species whereas those modified by unsaturated and branched prenyl lipids likely partition into Ld domains enriched with unsaturated lipid. However, this does not readily predict the domain-preference of proteins that are modified by multiple lipid types, such as N- and H-ras, because the balance of forces associated with different lipid modifications is hard to predict without a direct quantitative measure. Aiming at filling this gap, recent theoretical studies have begun to shed light on the thermodynamic basis for domain-specific partitioning of individual lipid-modified peptides and hybrid lipids29-37. For instance, Uline et al.29 used a mean-field approach to calculate the Lo/Ld partition coefficients of several lipid chain anchors. They found that the chain length, degree of saturation and architecture of the anchor, as well as the composition of the membrane, modulate the domain preference of the lipid anchors. Similar observations have been made in molecular dynamics (MD) simulations of hybrid lipids and the H-ras lipid anchor30,37. However, it is still unclear how lipid-modified peptides and proteins self-assemble and then laterally-organize in membrane domains or how clustering and lateral segregation might be coupled. The present work focuses on this issue.
Coarse-grained molecular dynamics (CGMD) simulations, which allow sampling of phase space in larger length and time scale than is possible by fully atomistic models, have been successfully used to characterize the aggregation behavior of a variety of surface-bound and trans-membrane proteins38-42. Recently, we have used this approach to study the clustering and lateral segregation behavior of the H-ras lipid anchor (tH) in a bilayer of co-existing Lo and Ld domains37. We found that 30-40% of the tH molecules form dynamic nanoclusters that exist in equilibrium with the non-clustered fraction. Similarly, both de-palmitoylated and de-farnesylated tH variants self-assemble into transient clusters of 4-10 molecules, but they exhibit dramatically different preferences for Lo and Ld domains. The current work provides insights into the interplay between clustering and domain partitioning based on new simulations in which concentrations of tH and cholesterol were systematically varied.
Methods
Model systems and simulation setup
The starting configuration for the current simulations is a previously reported37 bilayer system composed of 960 DPPC, 576 DLiPC and 384 (20%) cholesterol in which 64 tH peptides were inserted into the lower leaflet (system S64,20). A detailed description of the models and protocols for system preparation, simulation setup and other relevant information can be found in our recent report37. Here we briefly describe the setup of the simulations with variable tH and cholesterol content.
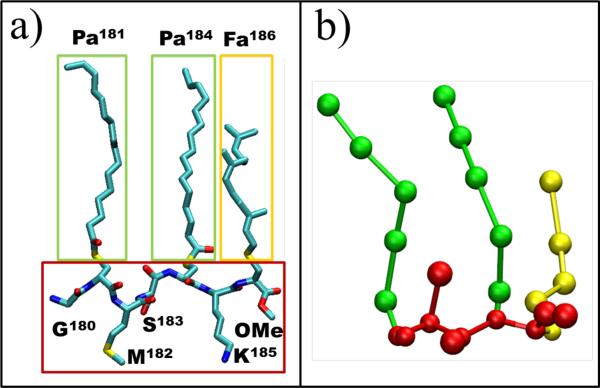
We carried out seven simulations using GROMACS 4.343. The simulations involved a fully solvated mixed bilayer made up of dipalmitoylphosphatydilcholine (DPPC) and dilinoleaylphosphatydilcholine (DLiPC) lipids, plus a variable number of tH and cholesterol. DPPC, DLiPC, cholesterol, water and ions were modeled by the MARTINI version 2.0 coarse-grained (CG) force field44,45. A MARTINI-compatible CG model of tH was built as described previously37 and in the supporting information. Fig. 1 shows both the atomic and CG representations of tH, a seven-residue peptide comprising Gly180, Pa181, Met182, Ser183, Pa184, Lys185, Fa186 and a carboxymethylated C-terminus, where Pa and Fa represent Cys residues posttranslationally modified by palmitoyl and farnesyl lipids, respectively.
Fig. 1.
Structure of the tH peptide. The amino acid sequence of tH is labeled based on residue numbering in the full-length H-ras protein. (a) All atom representation with carbon in cyan, oxygen in red, nitrogen in blue and sulfur in yellow. Hydrogen atoms are not shown. (b) A MARTINI-based CG representation of tH with Gly, Met, Ser and Lys side chains as well as the peptide backbone shown in red, palmitoyl (Pa) in green and farnesyl (Fa) in yellow, as shown by the correspondingly-colored boxes in (a).
To generate systems with 16, 32 and 48 tH molecules (S16,20, S32,20 and S48,20) 48, 32 and 16 tHs were randomly deleted from S64,20 along with the corresponding number of chloride ions to maintain total charge neutrality. Note that in this set of simulations the cholesterol content was kept fixed to the original value of 20%. In another set of simulations, the fraction of cholesterol in the bilayer was set to 0, 11 or 27% by removing or adding the appropriate number of cholesterol molecules from or to S64,20. Specifically, all of the cholesterol molecules in S64,20 were removed to generate system S64,00, 192 cholesterols were removed in the case of S64,11, and 192 cholesterols were added (equally distributed in each leaflet) to generate system S64,27. It is worth mentioning that because increasing the cholesterol content from 0 to 27% expanded the simulation box area only by ~3%, the number of peptides per unit area is comparable in all four of these systems. Each system was then energy minimized, equilibrated and simulated for at least 40 μs at 28°C and 1 atm. Table 1 summarizes the composition, simulation length, and the potential of each system to form co-existing lipid domains. Note that all simulation times in table 1 and the rest of this report are effective times, which is 4 times the real simulation time because the water diffusion coefficent in this CG model is around 4 times faster than in an atomistic model44.
Table 1.
Summary of the CGMD simulations analyzed in this study*.
| Sa,b | Np (tH/lipid) | Nc (%) | Length [μs] | lipid domains | tH clusters | Used for analysis [μs] |
|---|---|---|---|---|---|---|
| S16,20 | 16 (0.008) | 384 (20) | 40 | yes | no | 16 |
| S32,20 | 32 (0.017) | 384 (20) | 48 | yes | yes | 16 |
| S48,20 | 48 (0.025) | 384 (20) | 40 | yes | yes | 16 |
| S64,20 | 64 (0.033) | 384 (20) | 40 | yes | yes | 16 |
| S64,00 | 64 (0.042) | 0 (0) | 80 | no | yes | 16 |
| S64,11 | 64 (0.037) | 192 (11) | 40 | dynamic | yes | 16 |
| S64,27 | 64 (0.030) | 576 (27) | 40 | yes | yes | 16 |
Sa,b represents the name of the system, where ‘a’ is the number of tH molecules and ‘b’ is the fraction of cholesterol in the bilayer. Np is the number of tH molecules while tH/lipid refers to peptide-to-lipid ratio. Nc is the number of cholesterol molecules in the system, with its percentage relative to the total number of lipids given in brackets. The number of DPPC lipids is 960, and that of DLiPC is 576 in all simulations. Each simulation was run for at least 40μs, but only the equilibrated 24-40μs data was used for analysis of equilibrium properties.
Analysis
Trajectories were analyzed using a combination of GROMACS tools and in-house tcl scripts in conjunction with VMD46. Analysis of the time evolution of the bilayer structural properties and tH clustering (discussed later) confirmed that full equilibration was achieved within 24 μs of each simulation. Therefore, the bilayer and the tH nanoclusters were analyzed based on the next 16 μs data (i. e., from 24 μs to 40 μs).
The composition and size of individual nanoclusters at equilibrium were monitored to characterize the dynamics of the nanoclusters. In this analysis, the molecular indices and cluster size of a nanocluster at a given time t’, which represents the time point at which we begin to follow the cluster, were recorded and then monitored with time. The identity of a nanocluster at any time t remains unchanged until more than half of its constituent molecules have left. The evolution of cluster composition with time is monitored using a molecular expulsion autocorrelation function f(t)37,47. In this function, all tH molecules in a given cluster (a cluster is defined here as an aggregate of four or more peptides) of specific size were marked as “native” at t’. A “native” peptide is unmarked if it leaves the cluster either as a monomer or as part of another aggregate. f(t) was then calculated as:
| (1) |
where M(t’) is the cluster size at t’ and Mleave (t) is the accumulating number of “native” peptides that are no longer part of the cluster at time t. The cluster size at t, N(t), was also recorded to monitor the cluster size evolution with time.
Peptide density profiles at equilibrium were calculated by evenly dividing the simulation box into 88 bins of width ~0.25nm. For each bin, the time-averaged peptide density, ρ, was calculated as a function of position along the direction perpendicular to the domain boundary. The distribution profile of peptides in different domains was then calculated by normalizing the density profile, which was then used to estimate the probability of finding a peptide at a specific position on the bilayer surface. The free energy of partition of tH at the domain boundary (int) relative to the Lo and Ld domains, ΔG, was estimated as:
| (2) |
where R is the gas constant, T is the temperature in Kelvin, ρint and ρb are the average densities of tH at the boundary and the bulk region of a domain, respectively. Equation 2 relies on the inversion of the probability density distribution profile (ρ) based on the relationship:
| (3) |
where Gp is an intermediate variable. ΔG can then be readily calculated from the difference between the average Gp at the interface and in the bulk region of a domain. For the purpose of these calculations, ρint was calculated for a ~2 nm wide region centered at the peak of the tH density profile. The average density of peptides at the Lo domain, ρo, was calculated as the average density of a ~4 nm wide region in the middle of the Lo domain. The average density of peptides at the Ld domain, ρd, was calculated similarly after defining an Ld domain as the DLiPC-dominated region of size ~2nm. The smaller size of the Ld domain reflects the lower percentage of DLiPC lipids in the simulations. Error bars for ΔG were estimated by propagating the standard deviation of Gp in different regions.
Results and Discussion
We have shown recently that wild-type tH and its partially de-lipidated counterparts form clusters of comparable size37. Depending on the nature of the lipid modification, these clusters segregate to either the ordered or disordered lipid domains or the boundary between them. In the subsequent sections of this paper, we describe how clustering, dynamics and lateral organization behaviors of the triply lipidated wild-type tH are modulated by peptide concentration and membrane environment relative to data from a S64,20 system reported before37.
Lipid domain formation and tH clustering
The time evolutions of the bilayer structural properties (Fig. 2a), and the profile of tH cluster formation (Fig. 2b), show that all of the simulations are well equilibrated. Moreover, two stable, striped lipid domains have formed in all but two of the simulations (Table 1). Since lipid de-mixing was limited in the 0% and 11% cholesterol systems (systems S64,00 and S64,11) no stable lipid domains were observed (Fig. 3). To check if domain formation is limited by the length of of the simulations, S32, 20 and S64,00 were extended to 48μs and 80 μs, respectively. We found no major changes in the domain behavior of the bilayer or the clustering behavior of tH. The lack of striped domains in S64,00 (Fig. 3a) is consistent with the fact that cholesterol is required for phase separation at the current simulation temperature of 28°C37. That the lipid domains were found to be small and unstable in the presence of 11% cholesterol (Fig. 3b) suggests this cholesterol concentration is below the threshold needed for an extensive DPPC/DLiPC segregation. To our knowledge, the phase-diagram for a DPPC/DLiPC/cholesterol mixture is not known. Judging from the corresponding phase-diagram of a similar mixture containing dioleoylhoshpahtydilcholine instead of DLiPC48, we surmise that 11% cholesterol would be below the concentration range for a Lo/Ld domain co-existence. Therefore, our models accurately captured the expected domain behavior of our lipid mixtures under the simulation conditions used here.
Fig. 2.
Time evolution of the bilayer structure and the average cluster size for systems containing different number of tH (Np=32, 48, 64). (a) Time evolution of the incompatible contact ratio between DPPC and DLiPC in the lower leaflet calculated as the fraction of DLiPC (dashed line) and DPPC (solid line) lipids that are within 7.5 Å of a central DPPC (DLiPC) molecule, respectively. (b) Time evolution of the number-averaged cluster size (Nn). Similar profiles were obtained for the rest of the simulations.
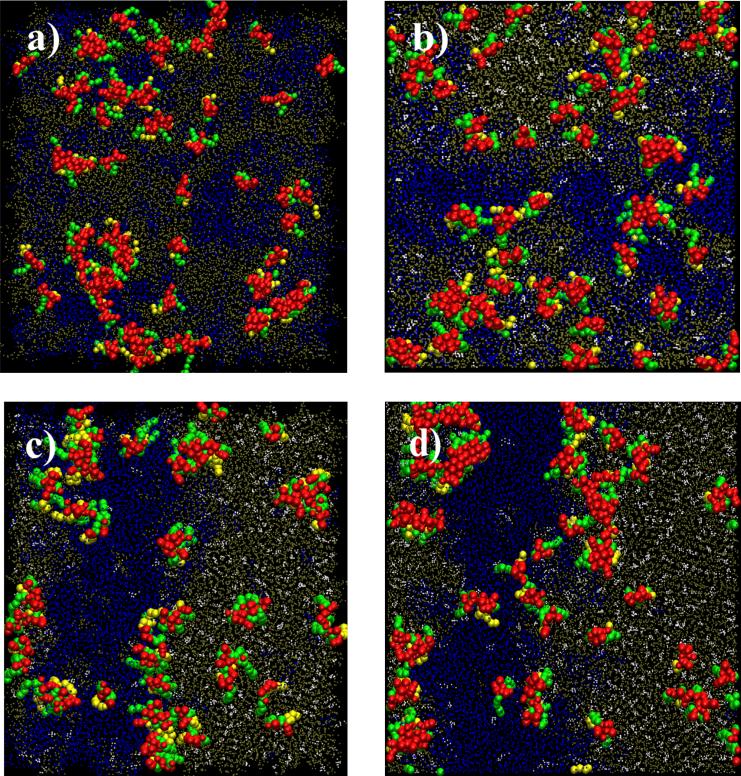
Fig. 3.
Simulation snapshots at t=24μs for systems with different cholesterol content. (a) S64,00: no cholesterol; (b) S64,11: 192 cholesterol (11%); (c) S64,20: 384 cholesterol (20%); and (d) S64,27: 576 cholesterol (27%). Color codes: DPPC in tan, DLiPC in blue, cholesterol in white, peptide backbone and non-lipidated side chains in red and palmitoyl and farnesyl in green and yellow, respectively. All snapshots were rendered by VMD46.
Consistent with our previous observation37, the presence of tH did not significantly affect the phase behavior of the bilayer while analyses of aggregation numbers (Table 1, Fig. 4) indicate that tH clustering has occurred in all but one (S16,20) of the simulations. Clustering was always fast, being complete within the first 6μs (Fig. 2b) in all simulations with Np =32 or higher. However, no significant clustering took place in system S16,20 within the time scale of the simulation. The following section focuses on the physical basis for this observation and its implication for future experiments. We will then turn to the role of cholesterol on tH clustering.
Fig. 4.
tH cluster size distributions from simulations at various concentrations of tH and cholesterol. (a) Weighted cluster size probability distribution of systems containing 16 (black), 32 (red), 48 (blue) and 64 (cyan) tH peptides derived from simulations S16,20, S32,20, S48,20, and S64,20, respectively. The grey dashed line represents the distribution for 64 tH peptides that lack Pa and Fa (with all tH lipid tails replaced by the parent Cys). Inset: the non-weighted cluster size distribution in a semi-logarithmic scale. (b) Weighted cluster size distribution of 64 tH molecules on a bilayer whose cholesterol content was 0% (black), 11% (red), 20% (cyan), and 27% (blue) obtained from simulations S64,00, S64,11, S64,20, and S64,27, respectively. Inset: The non-weighted cluster size distribution for the system with 27% cholesterol (one of the “interacting” systems) fitted with a double-exponential decay function (blue) and for a non-interacting system derived from a numerical simulation of 64 inert particles fitted with a single exponential decay function. Except for the inset of panel (b), lines are for eye guide only.
Effect of protein concentration on the formation and dynamics of tH nanoclusters
1) Cluster size
Fig. 4a displays the distribution of tH monomers and aggregates of various sizes derived from simulations in which the number of peptides (Np) was 16, 32, 48 and 64 with a fixed cholesterol content of 20%. One can readily see that most of the profiles are similar, but there are some crucial differences that warrant a closer look. First, the tH cluster distribution in S16,20 is almost exactly identical to that of a peptide without lipid modification (where each of the palmitoyls and farnesyl were mutated to the parent cysteine, gray in Fig. 4a). Both of these distributions, in turn, are almost identical to the distribution of an idealized non-interacting particle system with complete spatial randomness37. On average, 9 out of 16 tHs in S16,20 remain monomer while the other 7 form dimers or trimers. Defining nanoclusters as aggregates of four or more tH molecules37, we conclude that tH does not form nanoclusters at this concentration within the time scale of the simulations.
Second, whereas the number of tH in clusters of size four or larger (the clustered fraction) is zero in S16,20, increasing Np by 2-, 3- or 4-fold (systems S32,20, S48,20 and S64,20) increases the clustered fraction to 14, 28 and 34%, respectively (Fig. 4a). The corresponding total numbers of monomers, dimers and trimers (i. e., the non-clustered fraction) decrease by similar magnitudes. Notice that the clustered fraction in systems S48,20 and S64,20 is not significantly different and roughly falls within the 30-40% range estimated from cell-based experiments18. The absence of nanoclusters at Np=16, coupled with the doubling of the clustered fraction between Np=32 and Np=48 and the smaller increase when Np is further increased to 64, suggest the existence of two critical concentrations: (i) a minimum, below which nanoclustering does not occur, and (ii) a maximum, at which tH nanoclustering reaches an eventual saturation.
2) Dynamics and internal interaction
In addition to the aforementioned effect of peptide concentration on the formation of nanoclusters, there are variations in the dynamics and internal interaction of the tH aggregates derived from the four simulations. Illustrating this point, the inset in Fig. 4a shows that the cluster size distribution in S16,20 can be fitted to a single-exponential function, as are the distributions of the non-lipidated and non-interacting particle systems. In contrast, the distribution exhibits a double-exponential decay for the two systems with the highest tH concentration (S48,20 and S64,20). The distribution in S32,20 is somewhat different and lies between these two extremes. The single exponential decay indicates the lack of strong interaction within small aggregates, which appear to be formed via random collision. In the case of the bi-phasic distribution, the first phase suggests sharp differences between the average number of monomers, dimers and trimers whereas the slow second phase suggests a similar average number of larger clusters. The bi-phasic distribution is also related to the generally more dynamic nature of small aggregates than nanoclusters, and to the fact that dimers and trimers are held together by weak non-specific interactions while cumulative interactions in large clusters produce tighter packing. This is consistent with our previous observation that tH within clusters adopts a specific conformation and organizes in a manner that allows for maximum inter-tH and tH-lipid interactions37.
3) Predicting a critical cluster concentration for tH
A number of important lessons can be drawn from the data described above. In the absence of lipid modification, the peptides partition to solvent where nanoclusters cannot form. In contrast, even at a relatively low concentration (e.g., Np=32), a fraction of the lipid-modified membrane-bound tH quickly assembles into clusters. This can be explained, in part, by the reduced dimensionality in the 2D surface of the bilayer and therefore the lower entropic cost of association. The extra degree of freedom in the 3D environment of the bulk solvent favors monomers or small aggregates even at a high tH concentration of Np=64. Thus, tH nanoclustering is not a random event facilitated by high concentration but rather a consequence of lipid modification and hence membrane binding.
However, in the same manner as many surfactants do not form micelles below a certain critical concentration49, the amphipathic tH does not form nanoclusters below a “critical cluster concentration” (ccc). Unfortunately, the published cell-based experiments18 do not provide any clear indication as to what tH's ccc might be. This is perhaps related to the difficulty of systematically varying the peptide-to-lipid ratio (p/l) in cell membranes, which is required to monitor clustering as a function of peptide concentration. Our simulations predict that a p/l ≈ 0.01 may represent the ccc for tH. This value is roughly between 0.008 in S16,20 where no clustering was observed and 0.017 in S32,20 where partial clustering has occurred. The amount of the clustered fraction changes little at p/l values of 0.03 or higher, such as in systems S48,20 and S64,20. Though this appears to be in qualitative agreement with the experimentally observed fixed clustered fraction at different expression levels of Ras18, it is not conclusive because extrinsic factors in cells, such as the actin cytoskeleton and integral membrane proteins, are also likely to play a role18. Therefore, verification of our prediction awaits a suitable experimental technique in an in vitro setting.
The role of cholesterol in tH clustering and dynamics
1) Cholesterol is required for the formation of striped lipid domains
To evaluate the influence of lipid domain stability on tH clustering, we carried out three additional simulations in which the peptide concentration was fixed to Np=64 while the cholesterol content of the bilayer was set to 0, 11, and 27% (simulations S64,00, S64,11, and S64,27). We reiterate that these simulations were conducted to study the effect of lipid phase behavior on tH clustering as cholesterol is known to facilitate phase separation and stabilize membrane domains. Thus, as mentioned above, systems S64,00 and S64,11 were not expected to result in phase separation whereas S64,27 should lead to more stable lipid domains than the reference system S64,20. This means that Lo/Ld domains were expected to co-exist only in S64,20 and S64,27. Consistent with these expectations, extensive lipid segregation occurred in S64,20 and S64,27 while no striped domains were observed in systems S64,00 and S64,11 (Fig. 3).
2) Cluster size and dynamics
The cluster size distributions are similar in all four of these simulations, except for a slight increase in the fraction of large clusters at high cholesterol content (Fig. 4b). Clearly, tH clustering exhibits negligible dependence on the cholesterol content of the bilayer. At first glance, this finding appears to contradict earlier cell-based experiments that suggested cholesterol dependence of tH clustering18. In order to resolve this apparent contradiction, we looked at the dynamics of the nanoclusters in each system based on the time-dependent auto-correlation function (ACF) of molecular expulsion f(t) (Fig. 5, first column) and the time evolution of individual clusters N(t) (Fig. 5, second column) (see Methods). The molecular expulsion ACF f(t) reflects how quickly a nanocluster loses its “native” components either by single molecule or sub-cluster expulsion. In contrast, the size evolution of an individual cluster, N(t), tells us how the size of an existing cluster changes with time through the joint effect of molecular expulsion and addition. Since the clusters in all systems are polydisperse in size, i. e., contain clusters of different size (Fig. 4), we calculated these quantities for three representative cluster sizes: 4, 6 and 8. As shown in the first four plots of each column in Fig. 5, both f(t) and N(t) are always dependent on the initial cluster size. Within the same system, large clusters (size 6 and 8) lose their “native” components more quickly than small clusters (size 4) (Fig. 5, a, b, c, d). In addition, the size of large clusters always drops quickly to the optimal cluster size (which is reflected by the convergence of N(t) to a single value from different initial sizes) (Fig. 5, f, g, h, i). This trend indicates that de-clustering forces originating from the thermodynamic fluctuation and conformational entropic penalty prevent nanoclusters from growing indefinitely. By comparing clusters of the same size derived from simulations with different cholesterol content (Fig. 5 e, j, also see supporting information), we found that the larger the cholesterol content, the slower the rate at which the cluster loses its “native” components. Furthermore, the optimal cluster size increases with increasing cholesterol content (Fig. 5e, j).
Fig. 5.
Nanocluster dynamics for systems with different cholesterol content. The first column (a, b, c, d, e) are the molecular expulsion ACF (f(t)), and the second column (f, g, h, i, j) are the time evolution of the size of individual clusters (N(t)). The first four figures in each column represent the f(t) and N(t) for systems with 0% (a, f), 11% (b, g), 20% (c, h), 27% (d, i) cholesterol and intial cluster size of 4 (black), 6 (red) and 8 (blue). Panels e and j show f(t) and N(t) of clusters with initial size 8 for systems with 0% (black), 11% (red), 20% (cyan), 27% (blue) cholesterol.
Overall, the larger the cholesterol content the more stable is the cluster in terms of both composition and size, consistent with the fact that cholesterol enhances lipid packing and reduces bilayer fluidity50,51. Moreover, for the two systems with the largest cholesterol content, the formation of lipid domains increases the effective concentration of tH at the domain boundaries and thereby drives the dynamic equilibrium towards clustering. This relationship between lipid domain stability and cluster dynamics is in agreement with the temperature-dependence of tH clustering, where we have shown that nanocluster stability increases with decreasing temperature and hence increasing domain stability37. It follows that clustering is an intrinsic property of lipidated Ras peptides that does not require cholesterol, but cholesterol facilitates lipid phase separation and thereby increases nanocluster stability.
3) Implication for Ras signaling
It is tempting to speculate that the reported18 absence of tH nanoclusters in cholesterol depleted cell membranes might be a consequence of the limited time-resolution of the spectroscopic techniques rather than tH de-clustering. We predict that future experiments with sub-microsecond or better resolution might capture fast-exchanging nanoclusters in mixed bilayers lacking cholesterol, or even in pure bilayers that do not form microdomains. From a functional perspective, it stands to reason that the potential to form nanoclusters under various membrane environments is encoded in the sequence and structure of Ras proteins. However, the clusters have to be stabilized by lipid domains to remain intact long enough for signaling events to occur. In this manner, cells can regulate the lateral organization of Ras and its interaction partners by reconfiguring the local composition of the bilayer, possibly in an energy dependent manner.
Insights into the lateral organization and domain-partitioning of tH nanoclusters
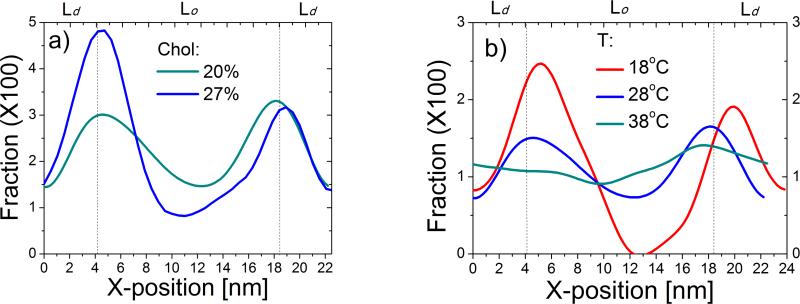
Comparison of the wild-type tH with its de-palmitoylated and de-farnesylated counterparts demonstrated that the palmitoyl tails tend to pull the peptide toward the Lo domain while farnesyl drives it toward the Ld domain37. This antagonistic action of the two lipid moieties leads to the accumulation of tH nanoclusters at the boundary between the Lo and Ld domains (see Fig. 3b,c in ref. 37). Similar observations have been made by others for hybrid lipids with one saturated tail and one unsaturated tail30,33-36. This holds true for each of the current simulations where stable Lo/Ld domains are formed. As an example, Fig. 6a shows the distribution of tH across the bilayer (and perpendicular to the line of the phase separation) for simulations S64,20 and S64,27. It is clear that tH accumulates in the region between the Lo and Ld domains, and preference for the domain boundary is highest for the system with the largest cholesterol content. Since cholesterol stabilizes the Lo domain, this data demonstrates once again that tH's preference for the domain boundary increases with increasing lipid domain stability.
Fig. 6.
tH distribution profiles in membrane domains. (a) The probability distribution of tH along the x dimension of the simulation box (i.e., perpendicular to the inter-domain line) from S64,20 (blue) and S64,27 (green) simulated at 28°C. (b) Same as in (a) but for a 64 tH system containing 20% cholesterol simulated at 18, 28 and 38°C. Dashed lines indicate the approximate center of the domain boundary derived from bilayer composition analysis.
To further examine this issue, we compared the tH distribution profiles in S64,20, which was carried out at 28°C, with two other previously reported37 simulations performed at 18 °C and 38 °C (Fig. 6b). The preference for the domain boundary is much higher at 18°C where the lipid domains are most stable. There is negligible preference for any region of the bilayer at 38°C where no clearly defined striped domain exists. This provides another example to the fact that tH preference for the domain boundary is a function of lipid segregation and domain stability. The data in Fig. 6 also show that tH is more efficiently excluded from the Lo domain in simulations where lipid packing was enhanced by the effect of high cholesterol concentration or low temperature. Therefore, despite its two saturated lipid tails, tH is less compatible with the tightly packed DPPC lipids in the Lo domain than with the more flexible DLiPC lipids in the Ld domain. We conclude that tH lateral segregation is primarily a function of packing deficiency, and that the same fundamental forces that underlie clustering also dictate lateral organization52.
Finally, in an initial effort to evaluate the affinity of tH for the domain boundary, we estimated the free energy of tH partitioning from the Lo and Ld domains to the interface (see Methods). Oligomerization has been shown to amplify the partitioning preference of lipidated proteins to specific membrane domains53, consistent with the high concentration of nanoclusters of tH and its variants in different lipid domains37 and the additive effect of chain anchors on partition coefficient29. It is therefore interesting to evaluate the cluster size dependence of ΔGint/Lo and ΔGint/Ld assuming an uncorrelated partitioning behavior for tH aggregates of different size. Table 2 lists the weighted average aggregation number (<s>w) grouped into monomers, dimers/trimers, and larger aggregates along with the corresponding values of ΔGint/Lo and ΔGint/Ld. The result indicates that partitioning to the interface is energetically favored at all tH concentrations and cluster sizes, and re-emphasizes the preference of tH for the interface and the enhancement of this preference by cluster growth.
Table 2.
Estimation of the partition free energy (in kJ/mol) of tH at the interface relative to the Ld and Lo domains*.
| Np | 16 | 32 | 48 | 64 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <sw> | 1 | 2.4 | - | 1 | 2.3 | 4.6 | 1 | 2.4 | 5.3 | 1 | 2.4 | 6.6 |
| ΔGint/Ld | -2.0 (0.4) | -4.5 (1.0) | - | -2.2 (0.3) | -3.7 (0.2) | -4.2 (0.6) | -1.0 (0.1) | -2.4 (0.1) | -1.6 (0.6) | -1.0 (0.1) | -1.8 (0.1) | -2.7 (0.3) |
| ΔGint/Lo | -1.8 (0.4) | -5.1 (1.1) | - | -0.9 (0.2) | -2.7 (0.2) | -5.3 (0.2) | -0.7 (0.1) | -1.8 (0.1) | -3.3 (0.6) | -0.5 (0.1) | -0.9 (0.1) | -5.5 (0.3) |
<s w> is the weighted average cluster size for monomer, dimer/trimer, and large clusters. ΔGint/Ld is the partition free energy between the interface and the Ld domain and ΔGint/Lo is the corresponding partition free energy between the interface and the Lo domain. Standared errors are indicated in brackets.
Conclusions
In this work, we have examined the effect of peptide concentration and membrane composition on the clustering and domain-preference of the H-ras lipid anchor (tH) using a coarse-grained molecular dynamics simulation approach. We have shown that tH molecules self-assemble into nanoclusters (with at least 4 molecules in each cluster) only above a certain threshold peptide concentration, which we call “critical cluster concentration” or “ccc”. We have estimated the ccc for tH to be approximately 1 peptide per 100 lipids at 28°C. Above its ccc, the fraction of tH in clusters was found to stabilize at around 30%. Moreover, as the concentration of tH increases the cluster size distribution changes from a single-exponential decay to a double-exponential one, indicating that the driving force for clustering is relatively weak and that nanoclusters are dynamic in nature. We have also shown that tH cluster size distribution is only slightly dependent on cholesterol but cholesterol increases the stability of the nanoclusters. Furthermore, in all of our simulations where lipid domains co-exist, tH and especially large clusters of tH localize predominantly at the domain boundary.
More broadly, our simulations revealed the crucial role of the C-terminus of Ras proteins and membrane environment for nanocluster formation, and provided detailed insights into the conditions required for Ras nanocluster formation. Our data also demonstrated the reversible nature of Ras nanoclustering and the coupling between Ras clustering and partitioning in membrane domains. These results therefore compliment the available macroscale experimental data by providing molecular insights and a thermodynamic foundation for the lateral organization of Ras on the plasma membrane, and highlight the power of simulations to provide information that is extremely difficult to obtain by current experimental techniques. This information is crucial for a better understanding of Ras signaling platforms, which are potential targets to inhibit abnornal Ras signaling in cancer.
Supplementary Material
Acknowledgment
This work was supported in part by the National Institutes of Health (Grant number:1R01GM10078). We that the Texas Advanced Computing Center for computational resources and members of the Gorfe laboratory for fruitful discussions.
Footnotes
Supporting information
Supporting information about the CG model of tH and nanocluster dynamics are available. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Karnoub AE, Weinberg RA. Nat Rev Mol Cell Biol. 2008;9:517. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abankwa D, Gorfe AA, Hancock JF. Semin Cell Dev Biol. 2007;18:599. doi: 10.1016/j.semcdb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotblat B, Prior IA, Muncke C, Parton RG, Kloog Y, Henis YI, Hancock JF. Mol Cell Biol. 2004;24:6799. doi: 10.1128/MCB.24.15.6799-6810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abankwa D, Gorfe AA, Inder K, Hancock JF. Proc Natl Acad Sci U S A. 2010;107:1130. doi: 10.1073/pnas.0903907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TY, Leventis R, Silvius JR. Biochemistry. 2001;30:13031. doi: 10.1021/bi0112311. [DOI] [PubMed] [Google Scholar]
- 6.Brunsveld L, Waldmann H, Huster D. Biochim Biophys Acta. 2009;1788:273. doi: 10.1016/j.bbamem.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Gorfe AA. Curr Med Chem. 2010;17:1. doi: 10.2174/092986710789957832. [DOI] [PubMed] [Google Scholar]
- 8.Vogel A, Reuther G, Roark MB, Tan KT, Waldmann H, Feller SE, Huster D. Biochim Biophys Acta. 2010;1798:275. doi: 10.1016/j.bbamem.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 9.Vogel A, Tan KT, Waldmann H, Feller SE, Brown MF, Huster D. Biophys J. 2007;93:2697. doi: 10.1529/biophysj.107.104562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorfe AA, Pellarin R, Caflisch A. J Am Chem Soc. 2004;126:15277. doi: 10.1021/ja046607n. [DOI] [PubMed] [Google Scholar]
- 11.Gorfe AA, Babakhani A, McCammon JA. Angew Chem Int Ed Engl. 2007;46:8234. doi: 10.1002/anie.200702379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorfe AA, Babakhani A, McCammon JA. J Am Chem Soc. 2007;129:12280. doi: 10.1021/ja073949v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorfe AA, Hanzal-Bayer M, Abankwa D, Hancock JF, McCammon JA. J Med Chem. 2007;50:674. doi: 10.1021/jm061053f. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MO, Mouritsen OG, Peters GH. Biophys J. 2004;86:3556. doi: 10.1529/biophysj.103.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorfe AA, McCammon JA. J Am Chem Soc. 2008;130:12624. doi: 10.1021/ja805110q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abankwa D, Gorfe AA, Hancock JF. Cell Cycle. 2008;7:2667. doi: 10.4161/cc.7.17.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorfe AA, Baron R, McCammon JA. Biophys J. 2008;95:3269. doi: 10.1529/biophysj.108.136481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plowman SJ, Muncke C, Parton RG, Hancock JF. Proc Natl Acad Sci U S A. 2005;102:15500. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock JF. Nat Rev Mol Cell Biol. 2006;7:456. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prior IA, Muncke C, Parton RG, Hancock JF. J Cell Biol. 2003;160:165. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weise K, Triola G, Janosch S, Waldmann H, Winter R. BBA-Biomembranes. 2010;1798:1409. doi: 10.1016/j.bbamem.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Weise K, Kapoor S, Denter C, Nikolaus J, Opitz N, Koch S, Triola G, Herrmann A, Waldmann H, Winter R. J Am Chem Soc. 2011;133:880. doi: 10.1021/ja107532q. [DOI] [PubMed] [Google Scholar]
- 23.Huster D, Vogel A, Katzka C, Scheidt HA, Binder H, Dante S, Gutberlet T, Zschörnig O, Waldmann H, Arnold K. J Am Chem Soc. 2003;125:4070. doi: 10.1021/ja0289245. [DOI] [PubMed] [Google Scholar]
- 24.Vogel A, Reuther G, Weise K, Triola G, Nikolaus J, Tan KT, Nowak C, Herrmann A, Waldmann H, Winter R. Angew Chem Int Ed Engl. 2009;48:8784. doi: 10.1002/anie.200903396. [DOI] [PubMed] [Google Scholar]
- 25.Abankwa D, Hanzal-Bayer M, Ariotti N, Plowman SJ, Gorfe AA, Parton RG, McCammon JA, Hancock JF. EMBO J. 2008;27:727. doi: 10.1038/emboj.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolini C, Baranski J, Schlummer S, Palomo J, Lumbierres-Burgues M, Kahms M, Kuhlmann J, Sanchez S, Gratton E, Waldmann H, Winter R. J Am Chem Soc. 2006;128:192. doi: 10.1021/ja055779x. [DOI] [PubMed] [Google Scholar]
- 27.Elliott R, Szleifer I, Schick M. Phys Rev Lett. 2006;96:98101. doi: 10.1103/PhysRevLett.96.098101. [DOI] [PubMed] [Google Scholar]
- 28.Garbès Putzel G, Schick M. Biophys J. 2008;95:4756. doi: 10.1529/biophysj.108.136317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uline MJ, Longo GS, Schick M, Szleifer I. Biophys J. 2010;98:1883. doi: 10.1016/j.bpj.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schäfer LV, Marrink SJ. Biophys J. 2010;99:L91. doi: 10.1016/j.bpj.2010.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schäfer LV, de Jong DH, Holt A, Rzepiela AJ, de Vries AH, Poolman B, Killian JA, Marrink SJ. Proc Natl Acad Sci U S A. 2011;108:1343. doi: 10.1073/pnas.1009362108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jong DH, Lopez C, Marrink SJ. Farad. Discuss. 2012 doi: 10.1039/c2fd20086d. (in press) [DOI] [PubMed] [Google Scholar]
- 33.Brewster R, Pincus P, Safran S. Biophys J. 2009;97:1087. doi: 10.1016/j.bpj.2009.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewster R, Safran SA. Biophys J. 2010;98:L21. doi: 10.1016/j.bpj.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto T, Brewster R, Safran S. Europhys Lett. 2010;91:28002. [Google Scholar]
- 36.Yamamoto T, Safran SA. Soft Matter. 2011;7:7021. [Google Scholar]
- 37.Janosi L, Li Z, Hancock JF, Gorfe AA. Proc Natl Acad Sci U S A. 2012;109:8097. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parton DL, Klingelhoefer JW, Sansom MSP. Biophys J. 2011;101:691. doi: 10.1016/j.bpj.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Periole X, Huber T, Marrink SJ, Sakmar TP. J Am Chem Soc. 2007;129:10126. doi: 10.1021/ja0706246. [DOI] [PubMed] [Google Scholar]
- 40.Prakash A, Janosi L, Doxastakis M. Biophys J. 2010;99:3657. doi: 10.1016/j.bpj.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janosi L, Prakash A, Doxastakis M. Biophys J. 2010;99:284. doi: 10.1016/j.bpj.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Domanski J, Marrink SJ, Schafer LV. Biochim Biophys Acta. 2012;1818:984. doi: 10.1016/j.bbamem.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Hess B, Kutzner C, Van Der Spoel D, Lindahl E. J Chem Theory Comput. 2008;4:435. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 44.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, De Vries AH. J Phys Chem B. 2007;111:7812. doi: 10.1021/jp071097f. [DOI] [PubMed] [Google Scholar]
- 45.Monticelli L, Kandasamy SK, Periole X, Larson RG, Tieleman DP, Marrink SJ. J Chem Theory Comput. 2008;4:819. doi: 10.1021/ct700324x. [DOI] [PubMed] [Google Scholar]
- 46.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Dormidontova EE. Soft Matter. 2011;7:4179. [Google Scholar]
- 48.Davis JH, Clair JJ, Juhasz J. Biophys J. 2009;96:521. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosen MJ. John Wiley & Son Inc; Hoboken, New Jersey: 2012. [Google Scholar]
- 50.Mitchell DC, Litman BJ. Biophys J. 1998;75:896. doi: 10.1016/S0006-3495(98)77578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofsäß C, Lindahl E, Edholm O. Biophys J. 2003;84:2192. doi: 10.1016/S0006-3495(03)75025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Z, Gorfe AA. Small GTPases. 2012;3(1) doi: 10.4161/sgtp.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levental I, Lingwood D, Grzybek M, Coskun Ü, Simons K. Proc Natl Acad Sci U S A. 2010;107:22050. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.