Abstract
Most plants have the ability to respond to fluctuations in light to minimize damage to the photosynthetic apparatus. A proteolytic activity has been discovered that is involved in the degradation of the major light-harvesting chlorophyll a/b-binding protein of photosystem II (LHCII) when the antenna size of photosystem II is reduced upon acclimation of plants from low to high light intensities. This ATP-dependent proteolytic activity is of the serine or cysteine type and is associated with the outer membrane surface of the stroma-exposed thylakoid regions. The identity of the protease is not known, but it does not correspond to the recently identified chloroplast ATP-dependent proteases Clp and FtsH, which are homologs to bacterial enzymes. The acclimative response shows a delay of 2 d after transfer of the leaves to high light. This lag period was shown to be attributed to expression or activation of the responsible protease. Furthermore, the LHCII degradation was found to be regulated at the substrate level. The degradation process involves lateral migration of LHCII from the appressed to the nonappressed thylakoid regions, which is the location for the responsible protease. Phosphorylated LHCII was found to be a poor substrate for degradation in comparison with the unphosphorylated form of the protein. The relationship between LHCII degradation and other regulatory proteolytic processes in the thylakoid membrane, such as D1-protein degradation, is discussed.
The main focus of photosynthesis research has been the functional and structural aspects behind the energy-transducing process. The progress has been significant, and the photosynthetic protein complexes can now be described at a very refined molecular level (Barber and Andersson, 1994). Conversely, relatively little is known about the acclimating, regulatory, and protective processes that maintain high photosynthetic efficiency during ever-fluctuating and even stressful environmental conditions. The identification of such auxiliary processes associated with thylakoid membranes and the characterization of the enzymes involved are therefore central tasks of current photosynthesis research. Examples of auxiliary enzymes or components are kinases and phosphatases responsible for reversible protein phosphorylation (Gal et al., 1997), desaturases controlling the lipid composition and dynamics of thylakoid membranes (Wada et al., 1990), stress-induced proteins such as the ELIPs (Adamska, 1997), and several enzymatic activities involved in various forms of regulatory proteolysis (Adam, 1996; Andersson and Aro, 1997). In the latter case, D1-protein turnover during repair of damage caused by photoinhibition is the most illustrative example (Prasil et al., 1992; Andersson and Aro, 1997). The knowledge of chloroplast proteases involved in regulatory proteolysis is still very limited. The presence of homologs to bacterial proteases in chloroplasts, such as the Clp and FtsH proteins, has provided new insights and possibilities (Gray et al., 1990; Moore and Keegstra, 1993; Adam, 1996; Lindahl et al., 1996; Ostersetzer et al., 1996).
In a previous study we discovered a regulatory proteolytic activity involved in the degradation of LHCII during acclimation of spinach (Spinacia oleracea) leaves from low- to high-intensity light (Lindahl et al., 1995). LHCII is the most abundant protein in the thylakoid membrane of higher plants (Jansson, 1994). It is not only a key component for light harvesting, but is also essential for regulation and distribution of excitation energy within the photosynthetic apparatus and responds to both short- and long-term fluctuations in light intensity and quality (Anderson and Andersson, 1988; Melis, 1991, 1996). This acclimation of the light-harvesting apparatus is generally thought to optimize photosynthetic efficiency and minimize light stress and photoinhibition to PSII (Andersson and Barber, 1996; Melis, 1996; Park et al., 1997).
The identified proteolytic activity was associated with degradation of the so-called “outer” or “peripheral” pool of LHCII enriched in the rapidly phosphorylated 25-kD subunit (Larsson et al., 1987a, 1987b; Anderson and Andersson, 1988), thereby reducing the antenna size of PSII in spinach leaves by 20% to 30% in response to elevated light intensities (Lindahl et al., 1995). The protease involved was found to be of the Ser or Cys type and extrinsically bound at the outer surface of the stroma-exposed regions of thylakoid membranes. Notably, the proteolytic process did not occur in the absence of ATP; therefore, it can be added to the list of the few known examples of ATP-dependent proteolysis associated with the thylakoid membrane (Adam, 1996). Another feature of the acclimative proteolysis of LHCII is that it requires a relatively long period of induction; therefore, no change in the size of the PSII antenna was seen during the first 2 d after transfer of spinach plants to increased light intensities (Lindahl et al., 1995).
To gain an understanding of the mechanism and regulation of the acclimative reduction of the LHCII antenna, we studied the induction of LHCII degradation and investigated the activation/deactivation pattern of the proteolytic process after transfer of spinach plants from low- to high-intensity light. The results revealed a light-dependent induction at the enzyme level and a regulatory control at the substrate level. Evidence was also provided that the newly discovered chloroplast ATP-dependent proteases Clp and FtsH were not responsible for the acclimative LHCII degradation.
MATERIALS AND METHODS
Growth and Acclimation of Plants
Spinach (Spinacia oleracea) plants were grown for 4 weeks at an intensity of 30 μE m−2 s−1 (low-intensity light). The light/dark cycle was 10 h of light followed by 14 h of darkness. The mature, low-light-grown spinach plants were transferred to an intensity of 600 to 800 μE m−2 s−1 (high-intensity light) for studies of the acclimative responses to high light.
Isolation and Subfractionation of Thylakoid Membranes
Thylakoid membranes were isolated according to the method of Andersson et al. (1976) from control plants grown at low light and from acclimating plants after the indicated times at the high-light regime. The isolated thylakoids were suspended in 15 mm Tricine-NaOH, pH 7.8, 15 mm NaCl, 3 mm MgCl2, 100 mm sorbitol, 1 mm ascorbate, and 0.1% (w/v) BSA (incubation buffer). Chlorophyll concentration and the chlorophyll a/b ratio were determined spectrophotometrically according to the method of Lichtenthaler and Wellburn (1983).
Digitonin fractionation of isolated thylakoid membranes (Carlberg et al., 1992) was performed at a chlorophyll concentration of 0.2 mg mL−1, and a final digitonin concentration of 0.1% (w/v) at room temperature for 30 s. The solubilization was terminated by a 10-fold dilution with ice-cold incubation buffer. Nonappressed thylakoid membranes (stromal lamellae vesicles) were isolated by differential centrifugation. The supernatant remaining after centrifugation at 40,000g for 30 min was centrifuged at 100,000g for 1 h. The final pellet containing the nonappressed membranes was resuspended in incubation buffer and stored at −80°C.
High-Salt Washings of Thylakoid Membranes
For studies of enzyme induction in vivo, thylakoid membranes were isolated from control leaves and from leaves at various stages in the acclimation process. Isolated thylakoid samples were washed three times in incubation medium supplemented with 0.5 m NaCl to release the extrinsically bound proteolytic activity from the membrane (Lindahl et al., 1995). Control washings were made in incubation buffer without the addition of extra NaCl. The wash supernatants were concentrated, dialyzed against incubation buffer, and used for assays of proteolytic activities and in reconstitution experiments. Protein concentration was determined according to the method of Lowry et al. (1951).
Reconstitution of LHCII Proteolysis in Situ
In situ degradation of LHCII in low- and high-light-acclimating thylakoid membranes was followed after high-salt washing and homologous or heterologous reconstitution with an appropriate desalted supernatant. The reaction mixture contained washed thylakoids (1 mg of chlorophyll) resuspended in incubation buffer supplemented with 0.5 mm ATP. A concentrated and dialyzed wash supernatant obtained from thylakoids corresponding to 1 mg of chlorophyll was added in darkness at 22°C. As a control, unwashed thylakoids were incubated under the same conditions. Aliquots were frozen in liquid nitrogen and kept at −80°C before analysis of LHCII degradation by SDS-PAGE and western blotting.
Effect of Phosphorylation on LHCII Degradation
Thylakoids were incubated at 1 mg chlorophyll mL−1 in incubation buffer in the presence of 12.5 μCi [γ-32P]ATP mL−1 and 0.5 mm unlabeled ATP. Different extents of phosphorylation of thylakoid membranes were induced in vitro by illumination at 160 μE m−2 s−1 at 25°C for various lengths of time. Residual radioactive ATP was removed by centrifugation, and the thylakoids were resuspended in fresh incubation buffer containing 0.5 mm ATP and 10 mm NaF to a concentration of 2 mg chlorophyll mL−1. Thylakoids were then incubated in the dark at 25°C, and samples were removed after 0, 2, 4, and 6 h. LHCII degradation was analyzed by two-dimensional gel electrophoresis (Larsson and Andersson, 1985; Lindahl et al., 1995). The relative protein phosphorylation was determined by autoradiography.
Assay of Proteolytic Activity Using Isolated LHCII or Fluorescein Thiocarbamoyl-Casein as a Substrate
LHCII was isolated from high-light-grown spinach according to the method of Burke et al. (1978). The purified LHCII was added to the wash supernatants in incubation buffer in the presence of 0.1% (w/v) Triton X-100. The ratio of LHCII protein to supernatant protein was 1:5. The samples were incubated at 25°C for 2 h, and then assayed for LHCII degradation by SDS-PAGE and western blotting. The antibody used was raised against both the LHCII-27 and LHCII-25 subunits.
Proteolysis of fluorescein thiocarbamoyl-casein was monitored by fluorescence emission spectra (500–600 nm) using an excitation wavelength of 495 nm (Twining, 1984).
Separation and Quantification of the LHCII-27 and LHCII-25 Polypeptides
Thylakoid proteins were separated either by two-dimensional SDS-PAGE, as described previously (Larsson and Andersson, 1985; Lindahl et al., 1995), or by SDS-PAGE according to the method of Laemmli (1970) using a 12% to 22.5% acrylamide gradient. Quantification of the LHCII-27 and LHCII-25 polypeptides was performed by Coomassie blue staining of the second-dimension gel or western blot, followed by laser-densitometry scanning.
Analyses of Clp and FtsH Proteins
Intact chloroplasts were isolated according to the method of Bartlett et al. (1982) and thylakoid membranes were prepared from them. The Clp protease was detected by western-blot analysis using antibodies raised against the rice clpP and pea clpC gene products expressed in Escherichia coli (Ostersetzer and Adam, 1996; Ostersetzer et al., 1996).
For FtsH analysis isolated thylakoid membranes were washed in either 2 m NaBr or 0.1 m NaOH; the suspensions were left on ice for 30 min, and then centrifuged at 10,000g for 5 min. Membrane preparations and wash supernatants were assayed for the presence of FtsH by western-blot analysis using an antibody raised against the E. coli enzyme, as described by Lindahl et al. (1996).
RESULTS
Regulation of LHCII Degradation at the Enzymatic Level
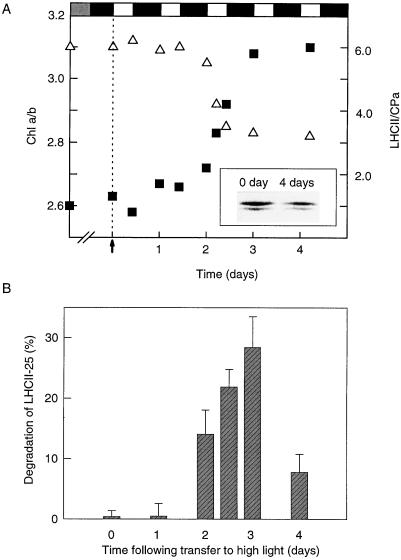
The acclimative reduction of the LHCII antenna is not an immediate response after transfer of low-light-grown spinach plants to high light (Lindahl et al., 1995). As illustrated in Figure 1A, no or very little decrease in the LHCII antenna occurred during the first 2 d of the high-light regime (including two night periods), as judged by the relatively constant chlorophyll a/b ratio and the unchanged proportion between LHCII and the PSII core (CPa). Only during d 3 to 4 was the reduction of the LHCII antenna complete, as revealed by a decrease of the LHCII/CPa ratio from 6.0 to 3.5 (Fig. 1A) and by the reduced level and changed proportion between the LHCII-27 and LHCII-25 subunits (Fig. 1A, inset). There was also an increase in the chlorophyll a/b ratio from 2.7 to 3.1.
Figure 1.
Induction of LHCII degradation and activation/deactivation of the LHCII protease during acclimation of spinach plants from low- to high-intensity light. Spinach plants grown at low light (30 μE m−2 s−1) were transferred to high-intensity light (600–800 μE m−2 s−1). The light/dark cycle was 10 h of light followed by 14 h of darkness. A, Thylakoid membranes were isolated during the acclimation period, and the reduction of the PSII antenna was followed by measurements of the chlorophyll a/b ratio (▪) and the LHCII/PSII core (CPa) ratio (▵), as determined by mild SDS-PAGE. Each point is the mean of four to six separate experiments. The arrow indicates the time when the plants were transferred to high light. The inset shows the relative amounts of the LHCII-27 and LHCII-25 polypeptides before (0 day) and after (4 days) high-light acclimation, as analyzed by Coomassie blue-stained two-dimensional SDS-PAGE. B, Thylakoid membranes isolated from leaves at various stages of acclimation were washed with a high-salt buffer to release the protease extrinsically bound to the outer membrane surface. The desalted and concentrated wash supernatants were tested for proteolytic activity by addition to washed high-light thylakoids. The degradation of LHCII was analyzed with SDS-PAGE and western blotting. Results are the means of three independent experiments + se. Chl, Chlorophyll.
The lag period of the acclimative response suggests that the proteolysis of LHCII requires some mechanism of induction. Such an induction could be controlled at the enzyme level and/or at the substrate level. To discriminate between these possibilities we took advantage of the fact that the LHCII protease is extrinsically located at the outer membrane surface of the stroma-exposed thylakoids and can be readily washed off with high-salt buffers (Lindahl et al., 1995). The wash supernatants were obtained from thylakoid membranes isolated from control low-light leaves and from leaves at various stages of the acclimation process, and used for different assays of proteolytic activities.
As a first approach, washed thylakoid membranes isolated from acclimating spinach leaves were used as a substrate. As illustrated in Figure 1B, the different supernatants showed various abilities to catalyze LHCII degradation. As judged by immunodetection, no proteolysis could be detected using the supernatant obtained from thylakoids derived from low-light-acclimated leaves. Only after 2 d (including dark periods) at the elevated light regime did the wash supernatant possess a proteolytic activity toward LHCII, particularly LHCII-25. The wash supernatant with maximal proteolytic activity (28% degradation of LHCII-25) was obtained after 3 d of exposure to high light (Fig. 1B).
After activation the proteolytic activity could either remain active or be deactivated once the new reduced level of the LHCII antenna size had been reached. The presence of proteolytic activity in the wash supernatant was therefore investigated after the amount of LHCII had reached a new stable value at the reduced level. The results revealed a rapid reduction in proteolysis during d 4 (Fig. 1B), suggesting a deactivation of the enzymatic activity.
These in situ degradations were complemented with experiments in which isolated LHCII was used as a substrate for the various wash supernatants. As judged by immunodetection, no or very little degradation occurred during the first 27 h after transfer of the dark-adapted leaves to the high-intensity light (not shown). Only after approximately 2 d could a significant degradation be observed, particularly for LHCII-25. Using the artificial protease substrate fluorescein thiocarbamoyl-casein (Twining, 1984), a similar increase of proteolytic activity was found for the various wash supernatants (not shown).
These data imply that LHCII degradation is regulated at the enzymatic level, which could involve expression and degradation of the protease (turnover) or a posttranslational activation/deactivation process.
Regulation at the Substrate Level
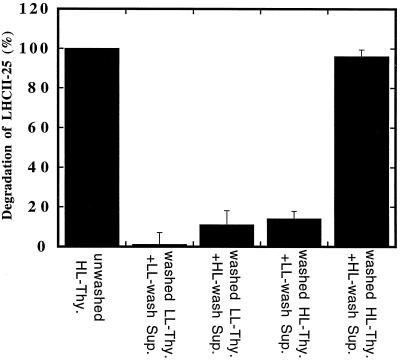
The fact that the initiation of the LHCII degradation involves a regulation at the enzymatic level does not exclude activation at the substrate level. For this reason, a series of reconstitution experiments was designed in which high-salt-wash supernatants from low-light controls or high-light-acclimating leaves were homologously or heterologously added to washed low- or high-light thylakoid membranes (Fig. 2). After these reconstitutions the proteolytic activity was determined in situ by immunoblotting and compared with the LHCII degradation occurring in unwashed thylakoid membranes. The wash supernatant derived from the high-light thylakoids induced a pronounced degree of degradation when it was homologously readded to the washed high-light thylakoids (Fig. 2), corroborating the high reconstitution ability of the proteolytic system (Lindahl et al., 1995). In contrast, when the wash supernatant derived from low-light thylakoids was heterologously reconstituted with washed high-light thylakoids, a very poor degree of degradation was obtained. Moreover, as expected, no degradation was obtained when the low-light supernatant was added to its washed low-light thylakoid counterpart. These observations are consistent with the requirement for an enzymatic induction of the proteolytic process as discussed above. A crucial observation was made in the fourth reconstitution experiment, when the high-light-wash supernatant was added to the washed low-light control thylakoids (Fig. 2). This heterologous reconstitution resulted in only about 15% of the maximal LHCII degradation. Therefore, even though the high-light-wash supernatant contained the active protease, it could not degrade LHCII in the low-light thylakoids.
Figure 2.
Homologous and heterologous reconstitution of LHCII proteolysis using high-light (HL)/low-light (LL) thylakoids (Thy.) and their corresponding high-light/low-light wash supernatants (Sup.). The degradation of LHCII was followed by SDS-PAGE and western-blot analyses. Results are the means ± se of four independent experiments.
The homologous and heterologous reconstitution experiments provide evidence that induction of the enzyme is not sufficient for the acclimative LHCII degradation, but that some additional activation at the substrate level is required. In the search for the molecular basis of such a substrate activation, two previous observations with respect to the long-term acclimation of LHCII have been considered. First, there is a lateral segregation between the LHCII substrate and the responsible protease along the stacked thylakoid membrane. The outer pool of LHCII is normally associated with the PSII centers located in the appressed thylakoid regions (Larsson et al., 1987a, 1987b; Mäenpää and Andersson, 1989; Melis, 1991), whereas the proteolytic activity is confined to the nonappressed thylakoid regions (Lindahl et al., 1995). Second, the outer pool of LHCII is not only a substrate for proteolysis during long-term acclimation (Lindahl et al., 1995), but is also readily subjected to protein phosphorylation (particularly the 25-kD subunit) and rapid lateral migration from the appressed to the nonappressed thylakoid regions (Kyle et al., 1983; Larsson and Andersson, 1985; Larsson et al., 1987b). The latter events are of significance for the so-called state transitions involved in the short-term regulation of the photosynthetic antenna (Gal et al., 1997). Consequently, the substrate activation could involve protein phosphorylation and lateral migration of phospho-LHCII from its original location in the appressed thylakoid regions to the nonappressed thylakoid regions, where it would come in contact with the protease.
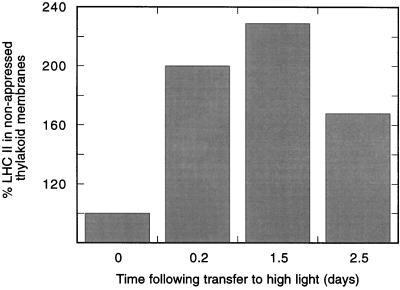
The relative content of LHCII in the stroma-exposed regions of thylakoids isolated from leaves at high light intensity was therefore followed during the acclimation period in vivo (Fig. 3). A transient increase of the steady-state level of LHCII in the nonappressed thylakoid regions was observed during the acclimation period, as judged by SDS-PAGE analyses. After 5 h in high light, there was almost a doubling of LHCII in the stromal thylakoid fraction. This indicates a migration of LHCII from the appressed to the nonappressed membrane regions similar to that observed after thylakoid protein phosphorylation in vitro (Kyle et al., 1983; Larsson et al., 1987b). The relative amount of LHCII in the stromal thylakoids increased further during the high-light conditions and reached a maximal level after 1.5 d (including a dark period). After 2.5 d, however, there was a decrease in the relative level of LHCII (Fig. 3) and a concomitant overall decrease in the amount of LHCII in the unfractionated thylakoids by approximately 30% (Fig. 1A). We therefore interpret the transient appearance of LHCII in the stromal thylakoids as a migration of LHCII during the lag period to the stromal thylakoids, where proteolysis will occur once an activated protease is present.
Figure 3.
The relative amount of LHCII in the stroma-exposed regions of thylakoid membranes isolated from spinach leaves during their acclimation from low- to high-intensity light. Stromal lamellae vesicles were isolated by digitonin incubation of thylakoid membranes at different stages in the acclimation process, followed by differential centrifugation. The relative amounts of LHCII were determined by mild SDS-PAGE. The light/dark cycle was 10 h of light followed by 14 h of darkness. The 100% value represents the initial amount of LHCII in the stromal thylakoids before exposure of plants to high light. Results are the means of three separate experiments.
The requirement for lateral migration during the proteolysis of LHCII could indicate a role for protein phosphorylation in the regulatory process. This possibility was also considered in view of evidence for the regulation of proteolysis via protein phosphorylation in several biological systems (Molinari et al., 1995; King et al., 1996). In chloroplasts this has been demonstrated for D1- and D2-protein degradation during photoinhibitory conditions (Elich et al., 1992; Koivuniemi et al., 1995). During the onset of light stress the two PSII reaction center proteins become phosphorylated but the actual proteolysis is preceded by dephosphorylation (Rintamäki et al., 1996).
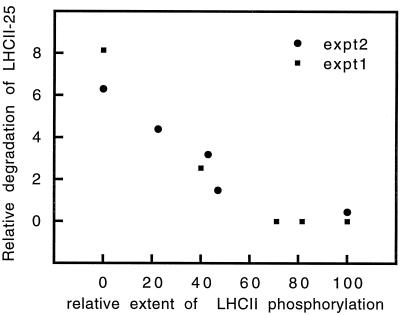
In view of these considerations and observations, thylakoid membranes isolated from leaves in the acclimating phase were phosphorylated in vitro to different extents in the presence of [γ-32P]ATP. The extent of degradation of LHCII-25 in the thylakoids was then monitored by two-dimensional gel electrophoresis during a 6-h period. When the degradation of LHCII-25 was plotted against the relative extent of phosphorylation of this polypeptide (Fig. 4), a clear correlation could be seen. As the level of phosphorylation increased, the rate of degradation of LHCII-25 decreased, and at about 70% phosphorylation no degradation could be seen. Thus, it can be concluded that the protease appears to be specific for the nonphosphorylated form of LHCII, so any role for protein phosphorylation during the process of LHCII degradation must be indirect (see Discussion).
Figure 4.
Correlation between LHCII phosphorylation and degradation. Thylakoid membranes were labeled using [γ-32P]ATP. The maximal amount of phospho-LHCII obtained at 160 μE m−2 s−1 for 15 min was taken as 100% phosphorylation. The extent of degradation was determined as a decrease in the LHCII-25/LHC-27 ratio during a 6-h period. The analysis was with two-dimensional SDS-PAGE combined with autoradiography and Coomassie blue staining. Results are from two independent experiments (expt1 and expt2).
Possible Relation between LHCII Degradation and the Clp and FtsH Proteases
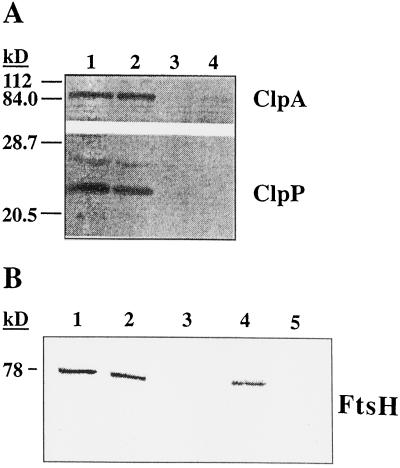
Chloroplast homologs to bacterial proteases have recently been identified in higher plants (Adam, 1996). One of these proteins, Clp, is an ATP-dependent Ser-type protease composed of two subunits, ClpA and ClpP. Because the proteolytic activity degrading LHCII is both ATP dependent and of the Ser type (Lindahl et al., 1995), this prompted us to test whether Clp could be involved in this particular process. In Figure 5A, the presence of the Clp subunits in chloroplasts isolated from acclimating as well as low-light control spinach leaves is shown by immunoblotting using ClpA and ClpP antisera. Quantification revealed no changes in the relative levels of the Clp subunits between the two chloroplast samples. Furthermore, the two Clp subunits were absent from thylakoid membranes isolated from both of these particular chloroplast samples and were recovered in the soluble stromal fraction. This demonstrates that the Clp protease does not associate with the membrane under the applied physiological conditions and that it cannot be responsible for the acclimative proteolysis of LHCII, since this enzymatic activity remained bound to the thylakoid membrane unless it was removed by high-salt treatment.
Figure 5.
Analysis of the chloroplast proteases Clp and FtsH in connection with LHCII degradation. A, The presence of ClpA and ClpP in chloroplasts (lanes 1 and 2) and thylakoids (lanes 3 and 4) isolated from high-light (lanes 2 and 4) and low-light (lanes 1 and 3) spinach leaves was analyzed by SDS-PAGE and western blotting. B, Association of FtsH with thylakoid membranes during acclimation was investigated by washings with either NaBr or NaOH. Analysis was with SDS-PAGE and western blotting. Lane 1, Unwashed thylakoids; lane 2, NaBr pellet; lane 3, NaBr-wash supernatant; lane 4, NaOH pellet; lane 5, NaOH-wash supernatant.
Another recently identified ATP-dependent protease of higher plant chloroplasts homologous to a bacterial protein is the FtsH protease (Lindahl et al., 1996). To find a relationship between this protease and the LHCII-degrading activity, we subjected the isolated thylakoid membranes to high-salt and alkaline washings, after which the presence of FtsH in thylakoid pellets and wash supernatants was probed by immunoblotting against a heterologous antiserum. As shown in Figure 5B, FtsH could not be released from the membranes upon washing with either NaBr or NaOH. Therefore, the firm association of FtsH with the thylakoid membrane excludes any possible involvement of this protease in the process of acclimative LHCII degradation.
DISCUSSION
The overall changes of the PSII antenna in response to variations in the light environment for different plant species have been thoroughly described (Osmond, 1994; Melis, 1996). Despite the wealth of physiological information, the underlying molecular mechanisms for the differences in antenna composition and the dynamic acclimations are still not understood. The expression of the nuclear cab genes encoding LHCII is light regulated at both the transcriptional and translational levels (Green and Salter, 1996), and the hierarchy of Cab protein assembly during biogenesis has been characterized (Melis, 1996). Aside from the recently discovered proteolytic process (Lindahl et al., 1995; Lindahl, 1997), the process of reduction of the LHCII antenna during acclimation to elevated light intensities is basically unknown. An important feature of this acclimation is that it shows a pronounced lag in its response, so a major aim of the present investigation was to address the molecular control of this process.
It can be demonstrated that initiation of the acclimative LHCII proteolysis requires regulation at both the enzyme and the substrate levels. The lag period in the physiological response could be attributed mainly to an expression or activation of the responsible protease at high light. We have not been able to discriminate between these two mechanistic alternatives. In the case of a posttranslational activation, redox control via plastoquinone or regulation via thiol groups, as has been shown to occur for the enzymes involved in reversible thylakoid protein phosphorylation (Gal et al., 1997; Vener et al., 1998), are interesting possibilities for future experimental investigations.
What is the mechanism that triggers LHCII degradation at the substrate level? We conclude from the thylakoid-subfractionation experiments that the induction of degradation involves a lateral movement of the outer pool of LHCII, which is enriched in the rapidly phosphorylated 25-kD subunit, from the appressed to the nonappressed thylakoid regions where the protease is located (Lindahl et al., 1995). It is known that phosphorylation of LHCII is a driving force behind its lateral migration during the state-1 to state-2 transitions of the short-term acclimation of light harvesting (Barber, 1982; Gal et al., 1997). Therefore, it seems reasonable to assume that the lateral migration of LHCII seen under the present long-term acclimation to increased irradiance (Fig. 3) also involves protein phosphorylation. If that is the case, a likely mechanism for the transformation of the short-term, reversible reduction of the LHCII antenna (state transition) to the long-term, irreversible reduction of the LHCII antenna (degradation) would be the induction of proteolytic activity. This enzyme induction will occur only after the plants are exposed for a prolonged period to a high light intensity, as shown by the observed lag phase in the physiological response (Fig. 1).
Such a simple mechanistic connection between short- and long-term acclimation of the PSII antenna would be consistent with the fact that both responses are targeted mainly to LHCII-25 (Larsson et al., 1987a, 1987b). However, it should be stressed that a role for protein phosphorylation during LHCII degradation can only be indirect, because it brings the protein substrate and enzyme together in the thylakoid membrane system. We found that phospho-LHCII is a poor substrate for the degradation compared with the unphosphorylated form (Fig. 4). LHCII degradation, therefore, may be more similar to D1-protein degradation during photoinhibitory conditions, in which the phosphorylated form of the photodamaged protein is protected from proteolysis and no degradation occurs in the absence of dephosphorylation (Koivuniemi et al., 1995; Ebbert and Godde, 1996; Rintamäki et al., 1996).
It should also be considered that high light intensities can lead to kinase inactivation (Schuster et al., 1986; Horton and Hague, 1988; Walters and Horton, 1991), which could complicate a potential physiological role of protein phosphorylation during long-term acclimation to high light. However, at the rather moderate nonphotoinhibitory irradiance applied in our present acclimation studies (600 μE m−2 s−1), no reduction in the kinase activity took place (not shown). Kinase inactivation readily occurred at higher light intensities (>1000 μE m−2 s−1), in accordance with previous studies (Schuster et al., 1986; Horton and Hague, 1988; Walters and Horton, 1991).
Degradation of LHCII should potentially release chlorophyll molecules that would be highly toxic to the photosynthetic apparatus if they were not rapidly degraded or quenched by some other mechanism. ELIPs may be involved in the binding of liberated chlorophyll molecules, thereby providing quenching of any toxic effects during LHCII degradation. This suggestion would be in accordance with the function proposed for ELIPs as “transient pigment-binding proteins” (Adamska, 1997) and with the experimental finding that ELIPs bind both chlorophyll and lutein (I. Adamska, unpublished data). ELIPs in spinach leaves were recently shown to have unusually high expression under conditions of LHCII degradation (Lindahl et al., 1997).
In our efforts to identify the LHCII protease, we have at least ruled out that it could be related to the chloroplast Clp or FtsH proteases. On the other hand, it has previously been shown that a detergent-activated proteolytic activity against LHCII is present in isolated bean thylakoids (Anastassiou and Argyroudi-Akoyunoglou, 1995). A comparison of the proteases responsible for acclimative proteolysis of LHCII and the detergent-induced degradation reveals similarities as well as discrepancies. Both enzymes are of the Ser type and probably contain sulfhydryl groups essential for the catalytic activity. The detergent-induced proteolytic activity of LHCII is, in contrast to the acclimative degradation of LHCII, independent of ATP. The relationship between the two proteases is still uncertain, because additional information concerning the localization and the nature of the association with the thylakoid membrane of the detergent-activated protease is lacking.
Recently, a proteolytic activity involved in low-light-induced degradation of ELIPs, which are homologous to the Cab proteins, has been characterized in vivo and in vitro and the protease has been partially purified (Adamska et al., 1996). This ELIP protease was also found to be of the Ser type and extrinsically located at the stroma-exposed thylakoid surfaces. However, the fact that its activity does not require ATP argues against an involvement of this protease in the acclimative degradation of LHCII. The identity of the LHCII protease, therefore, remains unknown, and its identification and characterization provide a central challenge for gaining further knowledge of the light acclimation of PSII and its light-harvesting antenna.
ACKNOWLEDGMENTS
We gratefully acknowledge Drs. I. Ohad and I. Carlberg for their constructive suggestions and discussions of this work, as well as K. Svennersjö for her help typing the manuscript.
Abbreviations:
- ELIP
early light-inducible protein
- LHCII
major light-harvesting chlorophyll a/b-binding protein of PSII
- LHCII-25 and LHCII-27
25- and 27-kD subunits, respectively, of LHCII
Footnotes
This work was supported by the Swedish Natural Science Research Council, the Carl Trygger Foundation, and a network grant from the European Commission Human, Capital, and Mobility program (contract no. ERB CHRX CT 940619).
LITERATURE CITED
- Adam Z. Protein stability and degradation in chloroplasts. Plant Mol Biol. 1996;32:773–783. doi: 10.1007/BF00020476. [DOI] [PubMed] [Google Scholar]
- Adamska I. ELIPs: light induced stress proteins. Physiol Plant. 1997;100:794–805. [Google Scholar]
- Adamska I, Lindahl M, Roobol-Bóza M, Andersson B. Degradation of the light-stress protein is mediated by an ATP-independent, serine-type protease under low-light conditions. Eur J Biochem. 1996;236:591–599. doi: 10.1111/j.1432-1033.1996.00591.x. [DOI] [PubMed] [Google Scholar]
- Anastassiou R, Argyroudi-Akoyunoglou JH. Thylakoid-bound proteolytic activity against LHCII apoprotein in bean. Photosynth Res. 1995;43:241–250. doi: 10.1007/BF00029937. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Andersson B. The dynamic photosynthetic membrane and regulation of solar energy conservation. Trends Biochem Sci. 1988;13:351–355. doi: 10.1016/0968-0004(88)90106-5. [DOI] [PubMed] [Google Scholar]
- Andersson B, Åkerlund H-E, Albertsson P-Å. Separation of subchloroplast membrane particles by counter-current distribution. Biochim Biophys Acta. 1976;423:122–132. doi: 10.1016/0005-2728(76)90106-7. [DOI] [PubMed] [Google Scholar]
- Andersson B, Aro E-M. Proteolytic activities and proteases of plant chloroplasts. Physiol Plant. 1997;100:780–793. [Google Scholar]
- Andersson B, Barber J. Mechanisms of photodamage and protein degradation during photoinhibition of photosystem II. In: Baker N, editor. Advances in Photosynthesis, Vol 5. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 101–121. [Google Scholar]
- Barber J. Influence of surface charges on thylakoid structure and function. Annu Rev Plant Physiol. 1982;33:261–295. [Google Scholar]
- Barber J, Andersson B. Revealing the blueprint of photosynthesis. Nature. 1994;370:31–34. [Google Scholar]
- Bartlett SG, Grossman AR, Chua N-H (1982) In vitro synthesis and uptake of cytoplasmically-synthesized chloroplast proteins. In M Edelman, RB Hallick, N-H Chua, eds, Methods in Chloroplast Molecular Biology. Elsevier Biomedical Press, Amsterdam, pp 1081–1092
- Burke JJ, Ditto CL, Arntzen CJ. Involvement of the light-harvesting complex in cation regulation of excitation energy distribution in chloroplasts. Arch Biochem Biophys. 1978;187:252–263. doi: 10.1016/0003-9861(78)90031-0. [DOI] [PubMed] [Google Scholar]
- Carlberg I, Bingsmark S, Vennigerholz F, Larsson UK, Andersson B. Low temperature effects on thylakoid protein phosphorylation and membrane dynamics. Biochim Biophys Acta. 1992;1099:111–117. [Google Scholar]
- Ebbert V, Godde D. Phosphorylation of PS II polypeptides inhibits D1 protein-degradation and increases PS II stability. Photosynth Res. 1996;50:257–269. doi: 10.1007/BF00033124. [DOI] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Identification, characterization and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction centre protein. J Biol Chem. 1992;267:3523–3529. [PubMed] [Google Scholar]
- Gal A, Zer H, Ohad I. Redox-controlled thylakoid protein phosphorylation: news and views. Physiol Plant. 1997;100:869–885. [Google Scholar]
- Gray JC, Hird SM, Dyer TA. Nucleotide sequence of a wheat chloroplast gene encoding the proteolytic subunit of an ATP-dependent protease. Plant Mol Biol. 1990;15:947–950. doi: 10.1007/BF00039435. [DOI] [PubMed] [Google Scholar]
- Green BR, Salter AH, (1996) Light regulation of nuclear-encoded thylakoid proteins. In B Andersson, AH Salter, J Barber, eds, Molecular Genetics of Photosynthesis. Oxford University Press, Oxford, UK, pp 75–103
- Horton P, Hague A. Studies on the induction of chlorophyll fluorescence in isolated barley protoplasts. IV. Resolution of non-photochemical quenching. Biochim Biophys Acta. 1988;932:107–115. [Google Scholar]
- Jansson S. The light-harvesting chlorophyll a/b-binding proteins. Biochim Biophys Acta. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters JM, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Koivuniemi A, Aro E-M, Andersson B. Degradation of the D1- and D2-proteins of photosystem II in higher plants is regulated by reversible phosphorylation. Biochemistry. 1995;34:16022–16029. doi: 10.1021/bi00049a016. [DOI] [PubMed] [Google Scholar]
- Kyle DJ, Staehelin LA, Arntzen CJ. Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochem Biophys. 1983;222:527–541. doi: 10.1016/0003-9861(83)90551-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson UK, Anderson JM, Andersson B. Variations in the relative content of the peripheral and tightly bound LHCII subpopulations during thylakoid light adaptation and development. Biochim Biophys Acta. 1987a;894:69–75. [Google Scholar]
- Larsson UK, Andersson B. Different degrees of phosphorylation and lateral mobility of two polypeptides belonging to the light-harvesting complex of photosystem II. Biochim Biophys Acta. 1985;809:396–402. [Google Scholar]
- Larsson UK, Sundby C, Andersson B. Characterisation of two different subpopulations of spinach light-harvesting chlorophyll a/b-protein complex. Biochim Biophys Acta. 1987b;894:59–68. [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans. 1983;11:591–592. [Google Scholar]
- Lindahl M (1997) Regulatory proteolysis of higher plant thylakoid membranes: the significance of protein degradation in light acclimation of the photosynthetic apparatus. PhD thesis. Stockholm University, Sweden
- Lindahl M, Funk C, Webster J, Bingsmark S, Adamska I, Andersson B. Expression of ELIPs and PSII-S protein in spinach during acclimative reduction of the photosystem II antenna in response to increased light intensities. Photosynth Res. 1997;54:227–236. [Google Scholar]
- Lindahl M, Tabak S, Cseke L, Pichersky E, Andersson B, Adam Z. Identification, characterization and molecular cloning of a homologue of the bacterial FtsH protease in chloroplasts of higher plants. J Biol Chem. 1996;271:29329–29334. doi: 10.1074/jbc.271.46.29329. [DOI] [PubMed] [Google Scholar]
- Lindahl M, Yang D-H, Andersson B. Regulatory proteolysis of the major light-harvesting chlorophyll a/b protein of photosystem II by a light-induced membrane-associated enzymic system. Eur J Biochem. 1995;231:503–509. doi: 10.1111/j.1432-1033.1995.tb20725.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AI, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mäenpää P, Andersson B. Photosystem II heterogeneity and long-term acclimation of light-harvesting. Z Naturforsch. 1989;44C:403–406. [Google Scholar]
- Melis A. Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta. 1991;1058:87–106. [Google Scholar]
- Melis A. Excitation energy transfer: functional and dynamic aspects of Lhc (cab) proteins. In: Ort DR, Yocum CF, editors. Advances in Photosynthesis, Vol 4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 523–538. [Google Scholar]
- Molinari M, Anagli J, Carafoli E. PEST sequences do not influence substrate susceptibility to calpain proteolysis. J Biol Chem. 1995;270:2032–2035. doi: 10.1074/jbc.270.5.2032. [DOI] [PubMed] [Google Scholar]
- Moore T, Keegstra K. Characterization of a cDNA clone encoding a chloroplast targeted Clp homologue. Plant Mol Biol. 1993;21:525–537. doi: 10.1007/BF00028809. [DOI] [PubMed] [Google Scholar]
- Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In NR Baker, JR Bowyer, eds, Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Bioscientific Publishers, Oxford, UK, pp 1–24
- Ostersetzer O, Adam Z. Effects of light and temperature on expression of ClpC, the regulatory subunit of chloroplastic Clp protease, in pea seedlings. Plant Mol Biol. 1996;31:673–676. doi: 10.1007/BF00042238. [DOI] [PubMed] [Google Scholar]
- Ostersetzer O, Tabak S, Yarden O, Shapira R, Adam Z. Immunological detection of proteins similar to bacterial proteases in higher plant chloroplasts. Eur J Biochem. 1996;236:932–936. doi: 10.1111/j.1432-1033.1996.00932.x. [DOI] [PubMed] [Google Scholar]
- Park YI, Chow WS, Anderson JM. Antenna size dependency of photoinactivation of photosystem II in light-acclimated pea leaves. Plant Physiol. 1997;115:151–157. doi: 10.1104/pp.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II: mechanism of photoinhibition and recovery processes. In: Barber J, editor. Topics in Photosynthesis, Vol 11. Amsterdam: Elsevier; 1992. pp. 295–348. [Google Scholar]
- Rintamäki E, Kettunen R, Aro E-M. Differential D1-phosphorylation in functional and photodamaged photosystem II centers. J Biol Chem. 1996;271:14970–14975. doi: 10.1074/jbc.271.25.14870. [DOI] [PubMed] [Google Scholar]
- Schuster G, Dewit M, Staehelin LA, Ohad I. Transient inactivation of the thylakoid photosystem II light-harvesting protein kinase system and concomitant changes in intramembrane particle size during photoinhibition of Chlamydomonas reinhardtii. J Cell Biol. 1986;103:71–80. doi: 10.1083/jcb.103.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining SS. Fluorescein isothiocyanate-labelled casein assay for proteolytic enzymes. Anal Biochem. 1984;143:30–34. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- Vener AV, Ohad I, Andersson B. Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr Opinions Plant Biol. 1998;1:217–223. doi: 10.1016/s1369-5266(98)80107-6. [DOI] [PubMed] [Google Scholar]
- Wada H, Gombos Z, Murata N. Enhancement of chilling tolerance of a cyanobacterium by genetic manipulation of fatty acid desaturation. Nature. 1990;347:200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- Walters RG, Horton P. Resolution of components of non-photochemical chlorophyll fluorescence quenching in barley leaves. Photosynth Res. 1991;27:121–133. doi: 10.1007/BF00033251. [DOI] [PubMed] [Google Scholar]